Abstract
Three-dimensional (3D) organization of transcription in the nucleus and mechanisms controlling the global chromatin folding including spatial interactions between the genes, non-coding genome elements, epigenetic and transcription machinery are essential for the establishment of lineage-specific gene expression programs during cell differentiation. Spatial chromatin interactions in the nucleus involving gene promoters and distal regulatory elements are currently considered as one of the major forces that drive cell differentiation and the genome evolution in general, while such interactions are substantially re-organized during many pathological conditions. During terminal differentiation of the epidermal keratinocytes, the nucleus undergoes programmed transformation from highly active status, associated with execution of the genetic program of epidermal barrier formation, to fully inactive condition and finally becomes a part of the keratinized cells of the cornified epidermal layer. This transition is accompanied by marked remodeling of the 3D nuclear organization and micro-anatomy including changes in the spatial arrangement of lineage-specific genes, nuclear bodies and heterochromatin. This mini-review highlights the important milestones in accumulation of the current knowledge on three-dimensional organization of the nucleus, spatial arrangement of the genes and their distal regulatory elements, and provides an update on the mechanisms that control higher-order chromatin remodeling in the context of epidermal keratinocyte differentiation in the skin.
Three-dimensional (3D) organization of transcription in the nucleus and mechanisms controlling the global chromatin folding including spatial interactions between the genes, non-coding genome elements, epigenetic and transcription machinery are essential for the establishment of lineage-specific gene expression programs during cell differentiation (Bickmore, 2013; Chakalova and Fraser, 2010; Cremer et al., 2015; Gomez-Diaz and Corces, 2014; Sequeira-Mendes and Gutierrez, 2015). During the last decade, a tremendous progress has been achieved in understanding of the functional micro-anatomy of the nucleus as a dynamic structure, in which actively transcribed or repressed genes are spatially compartmentalized into the distinct domains and frequently form preferential intra- and inter-chromosomal interactomes, which provide functional and structural frameworks for cell-specific transcription (Dekker et al., 2013; Schoenfelder et al., 2010; Sexton and Cavalli, 2015).
The cell nucleus is highly complex organelle that consists of the nuclear membrane, individual chromosomes occupying the distinct territories, as well as of a number of nuclear bodies (nucleoli, Cajal bodies, promyelocytic leukaemia (PML) bodies, nuclear speckles, Polycomb bodies, etc.) facilitating an execution of gene expression programs and other nuclear functions (Hubner and Spector, 2010; Lanctot et al., 2007; Mao et al., 2011; Misteli, 2007; Pederson, 2011). Serving as a central hub in the establishing adaptive cell behavior, the nucleus integrates the signals coming from the extracellular space and transforms them into specific gene expression programs to assist cells to survive and generate an appropriate response to changes in the microenvironment.
During terminal differentiation of the epidermal keratinocytes, the nucleus undergoes programmed transformation from highly active status, associated with execution of the genetic program of epidermal barrier formation, to fully inactive condition and finally becomes a part of the keratinized cells of the cornified epidermal layer. This transition is accompanied by marked remodeling of the 3D nuclear organization and micro-anatomy including changes in the spatial arrangement of lineage-specific genes, nuclear bodies and heterochromatin (Gdula et al., 2013). This mini-review highlights the important milestones in accumulation of the current knowledge on three-dimensional organization of the nucleus and provides an update on the mechanisms that control higher-order chromatin remodeling in the context of epidermal keratinocyte differentiation in the skin.
Chromosomes and chromosomal territories
Chromosomes are the largest units of the genome organization occupying distinct territories in the interphase nucleus (Cremer et al., 2001; Cremer and Cremer, 2011; Cremer et al., 2015) (Fig. 1a). In the chromosomes, DNA is compacted up to several thousand fold and organized into DNA-protein complex (chromatin) that allow the genome to be transcribed, replicated and repaired (Hemberger et al., 2009; Ho and Crabtree, 2010; Sequeira-Mendes and Gutierrez, 2015). Each chromosome contains a centromer (pericentromeric chromatin enriched in α-satellite repetitive sequences), chromosome arms containing the gene-rich and gene-poor domains enriched in the GC- and AT-sequences and visualized as the light and dark bands by Gimsa staining, respectively, as well as the telomeres (Fukui, 2009). Chromosomes are visualized by three-dimensional fluorescence hybridization (3D-FISH) technique with specific paints that allow defining their positioning in the nucleus (Cremer and Cremer, 2001; Solovei and Cremer, 2010).
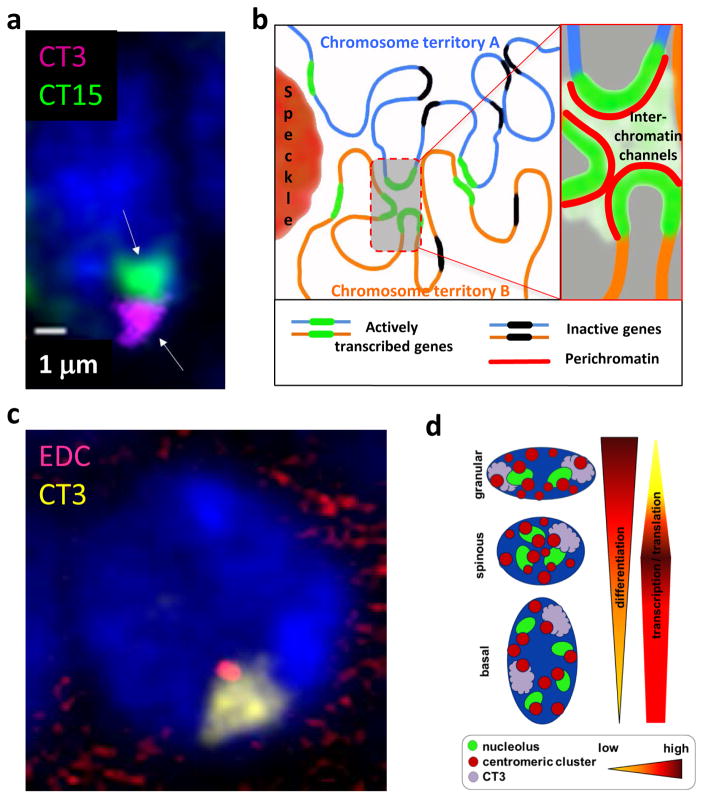
Figure 1. Changes in the spatial organization of the keratinocyte nucleus during epidermal development and differentiation.
A – 3D-FISH image of the nucleus of murine basal epidermal keratinocyte showing the positioning of the chromosomes 3 and 15 (arrows). Chromosome territory 3 (CT3, pink/violet) occupy more peripheral positioning in the nucleus, while the chromosome territory 15 (CT15, green) show more central positioning (courtesy of I. Malashchuk).
B - Chromosomes occupy distinct territories, in which distinct chromatin domains are permeated by interchromatin channels connected with a network of larger channels and lacunas separating distinct chromosomes and harboring different nuclear bodies including speckles (see Cremer et al., 2015, for details).
C – 3D-FISH image of the nucleus of murine basal epidermal keratinocyte showing the chromosome territory 3 (CT3, yellow) with EDC locus located at the internal part of the CT3 (red) (courtesy of I. Malashchuk).
D – Scheme illustrating the remodeling of 3D nuclear organization during terminal keratinocyte differentiation in the epidermis (see Gdula et al., 2013, for details).
The term “chromosomal territory” was first introduced by Theodor Boveri in 1909 (reviewed in (Cremer and Cremer, 2006a, b). Research in Thomas Cremer’s laboratory performed during last three decades has brought a tremendous progress into our understanding of the spatial organization of the genes and chromosomes in the interphase nucleus [for reviews, see (Cremer and Cremer, 2001; Cremer and Cremer, 2011; Cremer et al., 2015)]. Confocal microscopy analyses of tissue sections or isolated cells by using the whole chromosome 3D-FISH probes demonstrated that in interphase nucleus the relative positioning of the chromosomes within 3D nuclear space is not random and depends on many factors including the cell type, differentiation stage, chromosome size and their gene-rich or gene-poor status (Cremer and Cremer, 2010). Data obtained from the mouse skin in situ show that in basal epidermal keratinocytes, the chromosome 3 harboring the Epidermal Differentiation Complex (EDC) locus is always located at the nuclear periphery (Fig. 1a, c), and its positioning does not change during embryonic and post-natal development, as well as during terminal differentiation and keratinocyte transition to the spinous and granular epidermal layers (Fessing et al., 2011; Gdula et al., 2013; Mardaryev et al., 2014). However, the chromosomes 11 and 15 harboring the Keratin type I and type II loci, respectively, occupy predominantly central positions in the keratinocyte nuclei (Botchkarev et al., 2012).
In the interphase nucleus, positioning of the chromosomes is controlled through several mechanisms that include the interactions between specialized lamina-associated domains (LADs) and nuclear lamina, as well as through association of the chromosomes bearing the nucleolar-organizing region domains with nucleoli [reviewed in (Joffe et al., 2010; Kind and van Steensel, 2014; McKeown and Shaw, 2009; Misteli, 2007)]. Distinct chromosomes may be arranged in the nuclei of differentiated cells in a cell lineage-specific manner, which explain an increased frequency of translocations between the distinct chromosomal parts in the corresponding tumors (Brianna Caddle et al., 2007; Khalil et al., 2007; Parada et al., 2004; Roix et al., 2003). However, it is unclear whether genes from neighboring chromosomes may share common regulatory mechanisms required for their transcription (Cremer and Cremer, 2011).
Introduction of the super-resolution confocal microscopy allowed improving the resolution of the fluorescence images up to the 20–100 nm and served as an important next step in the analyses of the nuclear architecture (Cremer et al., 2015; Schermelleh et al., 2010). Super-resolution confocal microscopy revealed that each chromosome territory resembles a sponge-like structure and consists of the chromatin domains permeated by interchromatin channels connected with a network of larger channels and lacunas separating distinct chromosomes and harboring a number of nuclear bodies (Markaki et al., 2011). Inter-chromatin channels are also serving as a reservoir for macromolecular complexes, transcription factors, regulators of splicing, replication, and repair, as well as for exporting the mRNA-containing ribonucleoprotein complexes (Cremer et al., 2015). The network of interchromatin channels starts at nuclear pores and expands throughout the nuclear space, while chromatin domains in each territory are separated from the interchromatin channels by a 100–200-nm layer of decondensed chromatin, called the perichromatin and enriched by nascent DNA and RNA, RNA polymerase II (RNA Pol II), as well as by the H3K4me3 histone modification specific for transcriptionally active chromatin (Cremer et al., 2015; Markaki et al., 2011).
These observations were further developed into a model that suggests a presence of the active and inactive nuclear compartments inside of each chromosome territory that harbor trancriptionally active or inactive genes, respectively (Cremer et al., 2015) (Fig. 1b). This model also suggests a large degree of flexibility in the positioning of distinct chromatin domains inside of each chromosome territory, which is correspond well to the fact that some gene loci (IFN-γ and TH2 cytokine loci in TH lymphocytes, globin genes in erythroid cells, Nanog locus in iPS cells) may change their positioning relatively to other loci or the corresponding chromosomal territories associated with either gene activation or silencing (Spilianakis et al., 2005; Schoenfelder et al., 2010; Jost et al., 2011). During epidermal morphogenesis and differentiation of the basal epidermal progenitor cells, the lineage-specific EDC locus shows marked remodeling of its higher-order chromatin structure and relocates away from the peripheral part of the chromosomal territory 3 towards its internal part, which is associated with an increase in the transcriptional activity of genes involved in the control of terminal keratinocyte differentiation and epidermal barrier formation (Mardaryev et al., 2014) (Fig. 1c). Such developmentally-regulated relocation of the EDC towards the nuclear interior is a keratinocyte-specific event, which does not occur in dermal cells, and it is maintained during adulthood despite the many cycles of cell division that occur in this rapidly proliferating and self-renewing epithelial tissue (Mardaryev et al., 2014).
These data are generally consistent with previous observations showing the looping out from chromosome territory 1 of the EDC locus in cultured human keratinocytes, which suggest that the positioning of this genomic domain within the nucleus is quite flexible (Williams et al., 2002). Developmentally-regulated relocation of the EDC locus into the nuclear interior is associated with an increase in the number of SC-35 nuclear speckles present within the vicinity of the EDC, suggesting that this nuclear compartment may provide a “permissive environment” for the efficient transcription and maintenance of the high expression levels among genes activated during keratinocyte differentiation (Mardaryev et al., 2014).
Nuclear speckles are considered to be the sites of association between active genes within the nucleus (Brown et al., 2008; Mao et al., 2011; Popken et al., 2014; Spector and Lamond, 2011; Szczerbal and Bridger, 2010) and contain important constituents of the pre-mRNA processing machinery, such as polyadenylation and splicing factors including small nuclear ribonuclear proteins (snRNPs), as well as poly-A+ RNA and other splicing-related proteins (Spector and Lamond, 2011). Many of these factors are either recruited to transcription sites from the speckles or are involved in mRNA processing in the speckles (Spector and Lamond, 2011). Nuclear speckles are also considered as the sites of accumulation of the non-coding RNAs including MALAT1, which interacts with RNA-binding proteins and target pre-mRNAs at sites of active transcription (Engreitz et al., 2014). However, the impact of distinct speckle components in the control of gene expression within the EDC and other keratinocyte-specific gene loci remains to be further determined.
Systematic analyses of the remodelling of nuclear architecture during terminal keratinocyte differentiation in mouse epidermis demonstrate that terminally differentiated keratinocytes show marked differences in micro-anatomical organization of the nucleus compared to basal epidermal cells including: i) Decrease of the nuclear volume; ii) Decrease in expression of the markers of transcriptionally-active chromatin; iii) Internalization and decrease in the number of nucleoli; iv) Increase in the number of pericentromeric heterochromatic clusters; v) Increase in the frequency of associations between nucleoli, pericentromeric clusters and chromosomal territory 3 (Gdula et al., 2013) (Fig. 1b). These changes are likely to contribute to the global changes in the transcriptional landscape in terminally differentiating keratinocytes and transition of the keratinocyte nucleus from a metabolically active status to an inactive condition (Gdula et al., 2013). These data also suggest the nucleoli and pericentromeric clusters as important elements of the nuclear architecture which may control the local “transcriptional micro-environment” of the distinct chromatin domains by modulating the processes of chromosome tethering and regulating their positioning, folding and/or orientation.
Spatial proximity of the genes and chromosomes in the nucleus play an important role in the occurrence of chromosomal translocations during neoplastic transformation: neighboring chromosomes show higher frequencies of translocations compared to distal chromosomes, and translocations are formed predominantly between proximal chromosome breaks (Roukos and Misteli, 2014). In basal cell carcinoma, SHH gene shows translocation between chromosomes 7 and Y, which might contribute to its abnormal activation in the absence of the PTCH1 and SMO mutations (Gomez-Ospina et al., 2012). Thus, it appears to be important to carefully dissect how topological organization of the genome in keratinocytes is changed in pathological skin conditions including epidermal tumors or the disorders of epidermal differentiation (such as psoriasis), and how such changes contribute to the alterations in the transcriptional landscape of keratinocytes underlying these diseases.
Chromatin conformation capture analyses of 3D genome organization
Chromatin conformation capture (3C and its variations 4C, 5C and Hi-C) technologies were developed by Job Dekker and his laboratory (Dekker et al., 2002) and are based on the formaldehyde-mediated cross-linking between the closely located chromatin domains and multi-protein complexes followed by the DNA digestion with the restriction enzymes and the ligation at high dilution to facilitate the formation of intra-molecular but not inter-molecular products (Dekker et al., 2013; Lajoie et al., 2015). These techniques allowed defining the chromatin interactions between two distinct genomic sites (3C or “one-versus-one”) or between the genomic site of interest and the genome globally (4C or “one-versus-all”), as well as assessing the complex interactions within the distinct genomic locus (5C or “many-versus-many”) or global interactions within the whole genome (Hi-C or “all-versus-all”) (de Laat and Dekker, 2012).
Hi-C analyses of the global chromatin interactions revealed that the genes and chromatin domains from the same chromosomes show the higher frequency of interactions compared to the genes from other chromosomes, which confirmed the presence of chromosome territories on the molecular levels (Lieberman-Aiden et al., 2009). Furthermore, these analyses demonstrated an existence of at least two types of sub-chromosomal compartments, in which actively transcribed or transcriptionally silenced chromatin domains are segregated (Lieberman-Aiden et al., 2009). Such sub-chromosomal compartments were subsequently identified by applying the 3D structural illumination microscopy that revealed presence of the active and inactive sub-chromosomal compartments enriched either by the elongating form of PolII and H3K4me3 or by the H3K9me3 histone modifications, respectively (Popken et al., 2014).
Most importantly, Hi-C analyses also revealed an existence of another level of chromatin folding and presence of the Topologically Associating Domains (TADs) on each interphase chromosome, which size varies from several hundred Kb to 1–2 Mb (Dekker and Heard, 2015). TADs are characterized by much higher interaction frequencies between the distinct elements within the TAD (intra-TAD interactions) compared to the interactions between different TADs (inter-TAD interactions) (Dekker and Heard, 2015). Interestingly, TAD’s borders are conserved between the humans and mice and are not changed during cell differentiation, while TADs are lost alongside the inactive X-chromosome, as well as during the mitosis (Dixon et al., 2012; Naumova et al., 2013; Nora et al., 2012).
TAD borders in the mammalian genome are enriched in the binding sites for a number of architectural proteins including the CTCF and cohesin (Dixon et al., 2012; Gomez-Diaz and Corces, 2014). CTCF and cohesin binding sites also exist within TADs, in which CTCF is involved in organizing the smaller-sized (100–200 kb) intra-TAD chromatin loops (Rao et al., 2014) and in mediating the enhancer-promoter contacts (Dekker and Heard, 2015). Satb1 is another chromatin architectural protein that binds specialized DNA regions with an ATC-sequence context and folds chromatin into loops involving tissue-specific gene loci (TH2-cytokine and MHC class I loci, globin locus, etc.) (Cai et al., 2003; Cai et al., 2006; Kohwi-Shigematsu et al., 2012). Satb1 also targets chromatin remodelers/transcription factors to gene loci and plays a unique role in the execution of lineage-specific gene expression programs by integrating high-order chromatin organization with regulation of gene expression (Kohwi-Shigematsu et al., 2012; Kohwi-Shigematsu et al., 2013).
Chromatin conformation capture analyses allowed substantiating our knowledge on the enhancer-promoter interactions as a major driving force facilitating execution of lineage-specific differentiation programs (Dowen et al., 2014). Enhancers are the sequence modules that are preferentially located in the non-coding part of the genome at various distances from their target genes or even at different chromosomes (de Laat and Duboule, 2013). In normal differentiating cells, interactions between the gene promoters and their enhancers occurring via chromatin looping are very important for execution of lineage-specific gene expression programs (de Laat and Duboule, 2013; Dowen et al., 2014). In keratinocytes, an epidermal-specific regulatory enhancer 923 is present within the EDC locus and interacts with multiple EDC gene promoters, while some of these interactions are regulated by AP-1 transcription factor (Oh et al., 2014). Also, calcium stimulation in differentiating keratinocytes results in increased physical proximity of the enhancer and the promoter regions of the peptidylarginine deiminase 3 gene that control metabolism of the filaggrin (Adoue et al., 2008). However, the role of CTCF, cohesin, Satb1 and other chromatin architectural proteins in regulation of the enhancer-promoter interactions during establishment and maintenance of epidermal differentiation program in keratinocytes remain to be clarified.
Higher-order chromatin remodeling and the control of gene expression in keratinocytes
Establishment of the functional epidermal barrier is one of the major goals of the epidermal differentiation program, which includes a tightly regulated process of keratinocyte proliferation, terminal differentiation, apoptosis and shedding. The program of epidermal development and keratinocyte differentiation is governed by coordinated involvement of several transcription factors (p63, AP-1, Klf4, Arnt, etc.), signalling pathways (Wnt, Bmp, Hedgehog, EGF, Notch, FGF, etc.) and epigenetic regulators (DNA/histone-modifying enzymes, Polycomb genes, higher-order and ATP-dependent chromatin remodelers, non-coding RNAs) that control expression of lineage-specific genes [reviewed in (Botchkarev et al., 2012; Fessing, 2014; Frye and Benitah, 2012; Perdigoto et al., 2014)].
Epigenetic regulators exhibit both activating and repressive effects on chromatin in KCs: histone demethylase Jmjd3, ATP-dependent chromatin remodeler Brg1 and genome organizer Satb1 promote terminal KC differentiation, while DNA methyltransferase DNMT1, histone deacetylases HDAC1/2, Polycomp components Bmi1 and Ezh1/2 stimulate proliferation of progenitor cells via repression of the genes encoding cell-cycle inhibitors, as well as inhibit premature activation of terminal differentiation-associated genes (reviewed in (Benitah and Frye, 2012; Botchkarev et al., 2012; Fessing, 2014; Perdigoto et al., 2014).
Our recent studies revealed that transcription factor-dependent and epigenetic regulatory mechanisms in keratinocytes are highly connected, and p63 transcripton factor, operating as a master regulator of epidermal development (Koster and Roop, 2007; Kouwenhoven et al., 2015b; Vanbokhoven et al., 2011, Botchkarev, 2014 #2114), plays a hitherto unrecognized role in the higher-order chromatin remodeling of the EDC locus via direct control of the genome organizer Satb1 and ATP-dependent chromatin remodeler Brg1 (Fessing et al., 2011; Mardaryev et al., 2014). Satb1 is expressed in basal epidermal KCs and promotes cell differentiation via establishment of specific conformation of the EDC locus, while its ablation in mice results in the marked elongation of the EDC central domain associated with alterations in expression of the EDC genes and in epidermal morphology (Fessing et al., 2011).
ATP-dependent chromatin remodeler Brg1, on the other hand, promotes developmentally-regulated relocation of the EDC locus from the nuclear periphery towards nuclear interior into the compartment enriched by nuclear speckles, which is associated with marked increase in expression of the EDC genes (Mardaryev et al., 2014). Importantly, conditional ablation of Brg1 in the epidermis results in failure to form a functional barrier, thus partially resembling phenotype of p63 KO mice (Indra et al., 2005). These data suggest that chromatin remodeling genes represent a novel cohort of p63 targets that mediate its effects on execution of lineage-specific gene expression program in KCs (Botchkarev et al., 2012; Fessing, 2014).
Recent data revealed that in human keratinocytes, about 50% of the p63 binding sites are co-localized with H3K27ac histone modification specific for active enhancers (Kouwenhoven et al., 2015a). Interestingly, p63 binding alone was not sufficient for the regulation of gene transcription, while the gene expression dynamics correlated better with the H3K27ac signal at p63 binding sites than with p63 binding itself (Kouwenhoven et al., 2015a). Apparently, other co-regulators, such as RUNX1, are involved in the control of expression of p63 target genes (Kouwenhoven et al., 2015a). These data suggest that p63-mediated regulation of the epidermal differentiation program is far more complex than previously appreciated and include the control of enhancer-promoter interactions of the p63 target genes (Kouwenhoven et al., 2015b).
Conclusions
Spatial chromatin interactions in the nucleus involving gene promoters and distal regulatory elements located in the non-coding genomic domains are currently considered as one of the major forces that drive evolution of the mammalian genome (de Laat and Duboule, 2013). Genome-wide association studies (GWAS) demonstrate that many human diseases show the single nucleotide polymorphisms (SNPs) in the intergenic regions and suggest that such defects might perturb normal gene expression programs by affecting the activity of distal gene regulatory elements (Maurano et al., 2012). Furthermore, the global chromatin landscape and spatial arrangements between different genes and their regulatory elements are substantially re-organized in malignant cells and are functionally important for their growth (Gondor, 2013; Kohwi-Shigematsu et al., 2013; Zane et al., 2014).
Clearly, at present, we have only a limited knowledge of the mechanisms that control the spatial folding of the genome in keratinocytes in healthy and diseased skin (Fessing, 2014), while additional efforts are required to fully understand the complexity of interactions between distinct transcription factors and epigenetic regulatory machinery in the control of epidermal development, regeneration and stem cell activity. Recently, a number of molecules that are capable of modulating distinct components of the epigenetic machinery have been developed, and some of them are already approved for treatment of the distinct neoplastic conditions or under clinical trials (Tough et al., 2014). Thus, understanding of the complexity of spatial genome organization as a part of epigenetic regulatory program controlling epidermal differentiation and skin stem cell activity and their alterations in different pathological skin conditions will help to further progress in this exciting area of research towards the development of a novel cohort of epigenetic drugs for the management of skin disorders.
Acknowledgments
Author thank all current and former members of his laboratory including Drs. M. Fessing, M. Gdula, I. Malashchuk, A. Mardaryev, K. Poterloiwicz, V. Rapisarda, A. Sharov, T. Sharova, J. Yarker for their invaluable contribution to this work.
References
- Adoue V, Chavanas S, Coudane F, Mechin MC, Caubet C, Ying S, et al. Long-range enhancer differentially regulated by c-Jun and JunD controls peptidylarginine deiminase-3 gene in keratinocytes. J Mol Biol. 2008;384:1048–57. doi: 10.1016/j.jmb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Frye M. Stem cells in ectodermal development. J Mol Med (Berl) 2012;90:783–90. doi: 10.1007/s00109-012-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore WA. The spatial organization of the human genome. Annu Rev Genomics Hum Genet. 2013;14:67–84. doi: 10.1146/annurev-genom-091212-153515. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Gdula MR, Mardaryev AN, Sharov AA, Fessing MY. Epigenetic regulation of gene expression in keratinocytes. J Invest Dermatol. 2012;132:2505–21. doi: 10.1038/jid.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianna Caddle L, Grant JL, Szatkiewicz J, van Hase J, Shirley BJ, Bewersdorf J, et al. Chromosome neighborhood composition determines translocation outcomes after exposure to high-dose radiation in primary cells. Chromosome Res. 2007;15:1061–73. doi: 10.1007/s10577-007-1181-7. [DOI] [PubMed] [Google Scholar]
- Brown JM, Green J, das Neves RP, Wallace HA, Smith AJ, Hughes J, et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J Cell Biol. 2008;182:1083–97. doi: 10.1083/jcb.200803174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Han HJ, Kohwi-Shigematsu T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat Genet. 2003;34:42–51. doi: 10.1038/ng1146. [DOI] [PubMed] [Google Scholar]
- Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–88. doi: 10.1038/ng1913. Epub 2006 Oct 22. [DOI] [PubMed] [Google Scholar]
- Chakalova L, Fraser P. Organization of transcription. Cold Spring Harb Perspect Biol. 2010;2:a000729. doi: 10.1101/cshperspect.a000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer M, von Hase J, Volm T, Brero A, Kreth G, Walter J, et al. Non-random radial higher-order chromatin arrangements in nuclei of diploid human cells. Chromosome Res. 2001;9:541–67. doi: 10.1023/a:1012495201697. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C. Rise, fall and resurrection of chromosome territories: a historical perspective. Part I. The rise of chromosome territories. Eur J Histochem. 2006a;50:161–76. [PubMed] [Google Scholar]
- Cremer T, Cremer C. Rise, fall and resurrection of chromosome territories: a historical perspective. Part II. Fall and resurrection of chromosome territories during the 1950s to 1980s. Part III. Chromosome territories and the functional nuclear architecture: experiments and models from the 1990s to the present. Eur J Histochem. 2006b;50:223–72. [PubMed] [Google Scholar]
- Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010;2:a003889. doi: 10.1101/cshperspect.a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T, Cremer M. Chromosome territories. In: Misteli T, Spector DL, editors. The Nucleus. Cold-Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2011. pp. 93–114. [Google Scholar]
- Cremer T, Cremer M, Hubner B, Strickfaden H, Smeets D, Popken J, et al. The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments. FEBS Lett. 2015 doi: 10.1016/j.febslet.2015.05.037. [DOI] [PubMed] [Google Scholar]
- de Laat W, Dekker J. 3C-based technologies to study the shape of the genome. Methods. 2012;58:189–91. doi: 10.1016/j.ymeth.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat W, Duboule D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature. 2013;502:499–506. doi: 10.1038/nature12753. [DOI] [PubMed] [Google Scholar]
- Dekker J, Heard E. Structural and functional diversity of Topologically Associating Domains. FEBS Lett. 2015 doi: 10.1016/j.febslet.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet. 2013;14:390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–11. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowen JM, Fan ZP, Hnisz D, Ren G, Abraham BJ, Zhang LN, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159:374–87. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Sirokman K, McDonel P, Shishkin AA, Surka C, Russell P, et al. RNA-RNA interactions enable specific targeting of noncoding RNAs to nascent Pre-mRNAs and chromatin sites. Cell. 2014;159:188–99. doi: 10.1016/j.cell.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessing MY. Gene regulation at a distance: higher-order chromatin folding and the coordinated control of gene transcription at the epidermal differentiation complex locus. J Invest Dermatol. 2014;134:2307–10. doi: 10.1038/jid.2014.247. [DOI] [PubMed] [Google Scholar]
- Fessing MY, Mardaryev AN, Gdula MR, Sharov AA, Sharova TY, Rapisarda V, et al. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol. 2011;194:825–39. doi: 10.1083/jcb.201101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Benitah SA. Chromatin regulators in mammalian epidermis. Semin Cell Dev Biol. 2012;23:897–905. doi: 10.1016/j.semcdb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Fukui K. Structural analyses of chromosomes and their constituent proteins. Cytogenet Genome Res. 2009;124:215–27. doi: 10.1159/000218127. [DOI] [PubMed] [Google Scholar]
- Gdula MR, Poterlowicz K, Mardaryev AN, Sharov AA, Peng Y, Fessing MY, et al. Remodelling of Three-Dimensional Organization of the Nucleus During Terminal Keratinocyte Differentiation in the Epidermis. J Invest Dermatol. 2013;133:2191–2001. doi: 10.1038/jid.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina N, Chang AL, Qu K, Oro AE. Translocation affecting sonic hedgehog genes in basal-cell carcinoma. N Engl J Med. 2012;366:2233–4. doi: 10.1056/NEJMc1115123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz E, Corces VG. Architectural proteins: regulators of 3D genome organization in cell fate. Trends Cell Biol. 2014;24:703–11. doi: 10.1016/j.tcb.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondor A. Dynamic chromatin loops bridge health and disease in the nuclear landscape. Semin Cancer Biol. 2013;23:90–8. doi: 10.1016/j.semcancer.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10:526–37. doi: 10.1038/nrm2727. Epub 2009 Jul 15. [DOI] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner MR, Spector DL. Chromatin dynamics. Annu Rev Biophys. 2010;39:471–89. doi: 10.1146/annurev.biophys.093008.131348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe B, Leonhardt H, Solovei I. Differentiation and large scale spatial organization of the genome. Curr Opin Genet Dev. 2010;20:562–9. doi: 10.1016/j.gde.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Jost KL, Haase S, Smeets D, Schrode N, Schmiedel JM, Bertulat B, et al. 3D-Image analysis platform monitoring relocation of pluripotency genes during reprogramming. Nucleic Acids Res. 2011;39:e113. doi: 10.1093/nar/gkr486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Grant JL, Caddle LB, Atzema E, Mills KD, Arneodo A. Chromosome territories have a highly nonspherical morphology and nonrandom positioning. Chromosome Res. 2007;15:899–916. doi: 10.1007/s10577-007-1172-8. [DOI] [PubMed] [Google Scholar]
- Kind J, van Steensel B. Stochastic genome-nuclear lamina interactions: modulating roles of Lamin A and BAF. Nucleus. 2014;5:124–30. doi: 10.4161/nucl.28825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T, Kohwi Y, Takahashi K, Richards HW, Ayers SD, Han HJ, et al. SATB1-mediated functional packaging of chromatin into loops. Methods. 2012;58:243–54. doi: 10.1016/j.ymeth.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T, Poterlowicz K, Ordinario E, Han HJ, Botchkarev VA, Kohwi Y. Genome organizing function of SATB1 in tumor progression. Semin Cancer Biol. 2013;23:72–9. doi: 10.1016/j.semcancer.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;9:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven EN, Oti M, Niehues H, van Heeringen SJ, Schalkwijk J, Stunnenberg HG, et al. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015a;16:863–78. doi: 10.15252/embr.201439941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven EN, van Bokhoven H, Zhou H. Gene regulatory mechanisms orchestrated by p63 in epithelial development and related disorders. Biochim Biophys Acta. 2015b;1849:590–600. doi: 10.1016/j.bbagrm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Lajoie BR, Dekker J, Kaplan N. The Hitchhiker’s guide to Hi-C analysis: practical guidelines. Methods. 2015;72:65–75. doi: 10.1016/j.ymeth.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8:104–15. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–93. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YS, Zhang B, Spector DL. Biogenesis and function of nuclear bodies. Trends Genet. 2011;27:295–306. doi: 10.1016/j.tig.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardaryev AN, Gdula MR, Yarker JL, Emelianov VN, Poterlowicz K, Sharov AA, et al. p63 and Brg1 control developmentally regulated higher-order chromatin remodelling at the epidermal differentiation complex locus in epidermal progenitor cells. Development. 2014;141:101–11. doi: 10.1242/dev.103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaki Y, Gunkel M, Schermelleh L, Beichmanis S, Neumann J, Heidemann M, et al. Functional Nuclear Organization of Transcription and DNA Replication: A Topographical Marriage between Chromatin Domains and the Interchromatin Compartment. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2010.75.042. [DOI] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown PC, Shaw PJ. Chromatin: linking structure and function in the nucleolus. Chromosoma. 2009;118:11–23. doi: 10.1007/s00412-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Naumova N, Imakaev M, Fudenberg G, Zhan Y, Lajoie BR, Mirny LA, et al. Organization of the mitotic chromosome. Science. 2013;342:948–53. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–5. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh IY, Albea DM, Goodwin ZA, Quiggle AM, Baker BP, Guggisberg AM, et al. Regulation of the Dynamic Chromatin Architecture of the Epidermal Differentiation Complex Is Mediated by a c-Jun/AP-1-Modulated Enhancer. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada LA, Sotiriou S, Misteli T. Spatial genome organization. Exp Cell Res. 2004;296:64–70. doi: 10.1016/j.yexcr.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Pederson T. The nucleus introduced. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto CN, Valdes VJ, Bardot ES, Ezhkova E. Epigenetic regulation of epidermal differentiation. Cold Spring Harb Perspect Med. 2014:4. doi: 10.1101/cshperspect.a015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken J, Brero A, Koehler D, Schmid VJ, Strauss A, Wuensch A, et al. Reprogramming of fibroblast nuclei in cloned bovine embryos involves major structural remodeling with both striking similarities and differences to nuclear phenotypes of in vitro fertilized embryos. Nucleus. 2014;5:555–89. doi: 10.4161/19491034.2014.979712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–80. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genet. 2003;34:287–91. doi: 10.1038/ng1177. [DOI] [PubMed] [Google Scholar]
- Roukos V, Misteli T. The biogenesis of chromosome translocations. Nat Cell Biol. 2014;16:293–300. doi: 10.1038/ncb2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol. 2010;190:165–75. doi: 10.1083/jcb.201002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S, Clay I, Fraser P. The transcriptional interactome: gene expression in 3D. Curr Opin Genet Dev. 2010;20:127–33. doi: 10.1016/j.gde.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Sequeira-Mendes J, Gutierrez C. Genome architecture: from linear organisation of chromatin to the 3D assembly in the nucleus. Chromosoma. 2015 doi: 10.1007/s00412-015-0538-5. [DOI] [PubMed] [Google Scholar]
- Sexton T, Cavalli G. The Role of Chromosome Domains in Shaping the Functional Genome. Cell. 2015;160:1049–59. doi: 10.1016/j.cell.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Solovei I, Cremer M. 3D-FISH on cultured cells combined with immunostaining. Methods Mol Biol. 2010;659:117–26. doi: 10.1007/978-1-60761-789-1_8. [DOI] [PubMed] [Google Scholar]
- Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–45. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- Szczerbal I, Bridger JM. Association of adipogenic genes with SC-35 domains during porcine adipogenesis. Chromosome Res. 2010;18:887–95. doi: 10.1007/s10577-010-9176-1. [DOI] [PubMed] [Google Scholar]
- Tough DF, Lewis HD, Rioja I, Lindon MJ, Prinjha RK. Epigenetic pathway targets for the treatment of disease: accelerating progress in the development of pharmacological tools: IUPHAR Review 11. Br J Pharmacol. 2014;171:4981–5010. doi: 10.1111/bph.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbokhoven H, Melino G, Candi E, Declercq W. p63, a story of mice and men. J Invest Dermatol. 2011;131:1196–207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- Williams RR, Broad S, Sheer D, Ragoussis J. Subchromosomal positioning of the epidermal differentiation complex (EDC) in keratinocyte and lymphoblast interphase nuclei. Exp Cell Res. 2002;272:163–75. doi: 10.1006/excr.2001.5400. [DOI] [PubMed] [Google Scholar]
- Zane L, Sharma V, Misteli T. Common features of chromatin in aging and cancer: cause or coincidence? Trends Cell Biol. 2014;24:686–94. doi: 10.1016/j.tcb.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]