Abstract
Kainate receptors (KARs) are one of the three subtypes of ionotropic glutamate receptors in the CNS. These receptors are widely expressed pre- and postsynaptically throughout the brain. Thus, kainate receptor activation mediates a large variety of pre- and postsynaptic effects on either glutamatergic or GABAergic synaptic transmission. Although ionotropic functions for KAR have been described in multiple brain regions, there is considerable evidence from various CNS regions that KARs activation modulates GABA release through either G-protein dependent metabotropic pathway or secondary activation of G-protein coupled receptors. In the present chapter, we provide further evidence supporting that these two pathways are also involved in the modulation of GABA release in specific basal ganglia nuclei. Because of their more subtle effects on neurotransmisison regulation than other ionotropic glutamate receptors, KARs represent interesting targets for the future development of pharmacotherapy for basal ganglia diseases.
Introduction
Kainate receptors (KARs) are one of the three subtypes of ionotropic glutamate receptors in the CNS, made up of a combination of GluR5, GluR6, GluR7, KA1 and KA2 subunits.1, 2 These receptors are widely expressed pre- and postsynaptically throughout the brain.3–9 Cloning technology and recent development of drugs that could discriminate between kainate and AMPA receptors have led to further characterization of the pharmacological and physiological properties of KARs during the past decade.7, 8 Kainate receptors have direct actions on intrinsic cell excitability in the hippocampus10 and modulate both glutamatergic and GABAergic synaptic transmission in a number of brain regions.7, 8 In the hippocampus and amygdala, synaptically released glutamate from nearby excitatory synapses, can activate KARs in GABAergic terminals, thereby providing an endogeneous, physiologically relevant, mechanism mimicking the effect of exogenous KA application on inhibitory synaptic transmission.11, 12 Although the hippocampal KARs surely deserved most attention, data from our laboratory and others have provided further evidence for a widespread pre and postsynaptic localization of KARs and their functions in many other CNS structures, including the cerebral cortex, hypothalamus, cerebellun, spinal cord and basal ganglia.5, 7, 9, 13 In this chapter, we will provide an overview of our current understanding of the localization and function of KARs in the basal ganglia and discuss their potential relevance as novel targets for movement disorders therapy. Each basal ganglia nucleus will be discussed in turn, followed by concluding remarks related to the potential impact KARs may have in regulating basal ganglia function under normal and pathological conditions. Additional information about the basal ganglia KARs localization can also be found in a previous review.14
Striatum
The mRNA for GluR6, GluR7 and KA2, but not GluR5 and KA1, are expressed in rat and mice striatum.15, 16 Double in situ hybridization studies dermonstrated that most striatal projection neurons labeled for either enkephalin or substance P mRNAs co-express GluR6 mRNA.17 The expression of KARs subunits in striatal interneurons remains to be determined. Consistent with these results, strong cellular and neuropil GluR6/7 immunoreactivity was found in the monkey striatum.3, 5 At the ultrastructural level, both GluR6/7 and KA2 are expressed in subsets of glutamatergic terminals, some of which in the sensorimotor putamen, originate from the motor cortex or the centromedian thalamic nucleus5 (Fig. 1). Presynaptic KARs are either extrasynaptic, away from the main release sites of neurotransmitter or associated with the presynaptic active zones of glutamatergic synapses (Fig. 1).5 At the postsynaptic level, the majority of GluR6/7 and KA2 labeling is found extrasynaptically along the plasma membrane of spines and dendrites contacted by glutamatergic inputs, though some labeling can occasionally be found in the postsynaptic density of glutamatergic synapses (Fig. 1).5
Figure 1.

Pre and postsynaptic expression of GluR6/7 and KA2 immunoreactivity in the monkey striatum. A) GluR6/7-immunoreactive elements in the body of the caudate nucleus. Note the presence of labeled terminals (Te) forming asymmetric synapses (arrowheads) and immunoreactive dendrites (Den). The asterisks indicate unlabeled boutons. B) Presynaptic and postsynaptic GluR6/7 labeling at an asymmetric axospinous synapse as reviewed with the postembedding immunogold method. C) Dense KA2 labeling (arrows) in the presynaptic gird of an asymmetric axospinous synapse. Scale bars: A: 0.3 μm; B: 0.2 μm (valid for C). (See ref. 5 for more details). From: Kieval JZ et al. J Neurosci 2001; 21:8746–8757;5 © 2001 with permission from the Society for Neuroscience.
Agonist-induced activation of KARs in slices of rat striatum modulates GABAergic transmission.17, 18 Domoate (200 nM–500 nM), an AMPA/KAR agonist, increases the frequency of spontaneous GABAergic IPSCs (SIPSCs) with low amplitude, which are likely generated by intrinsic axon collaterals of GABAergic projection neurons,20, 21 but does not have any effect on the frequency of large amplitude IPSCs most likely mediated by GABAergic afferents from fast spiking striatal interneurons19–21 or projections from the globus pallidus.22–25 Domoate also decreases the IPSCs amplitude evoked by intrastriatal stimulation.17 These effects are mediated by activation of GluR6-containing KARs because they are lost in GluR6-deficient mice.17 Thus, these differential effects of KARs agonist on sIPSC of different amplitude suggest a variable degree of expression and function of presynaptic KARs on intrinsic GABAergic projections from striatal output neurons or interneurons. The lack effect of domoate on large amplitude sIPSCs suggests that low concentrations of domoate do not trigger spike discharge in GABAergic interneurons.
As described in hippocampus and spinal cord,26, 27 the KAR-induced depression of GABAergic transmission in the striatum involves activation of secondary G-protein coupled receptors.17 Domoate-induced inhibition of evoked IPSCs (eIPSCs) is, indeed, significantly reduced by A2A receptor antagonists (Fig. 2). These observations, combined with electron microscopic evidence for the localization of A2A receptor immunoreactivity in intrastriatal GABAergic axon collaterals of striatopallidal neurons,28, 29 provide a substrate whereby KARs could regulate GABAergic transmission via indirect activation of presynaptic A2A receptors. However, KARs activation does not affect the frequency of miniature IPSCs (mIPSCs), while increasing that of evoked and spontaneous IPSCs, in the rat nucleus accumbens, suggesting the involvement of postsynaptic KARs.18 Together, these studies provide solid evidence that KARs are clearly involved in the regulation of inhibitory transmission in both the dorsal and ventral striatum, but significant work remains to be done to elucidate the exact mechanisms underlying these effects. The role of KARs on GABAergic interneurons must also be examined carefully.
Figure 2.
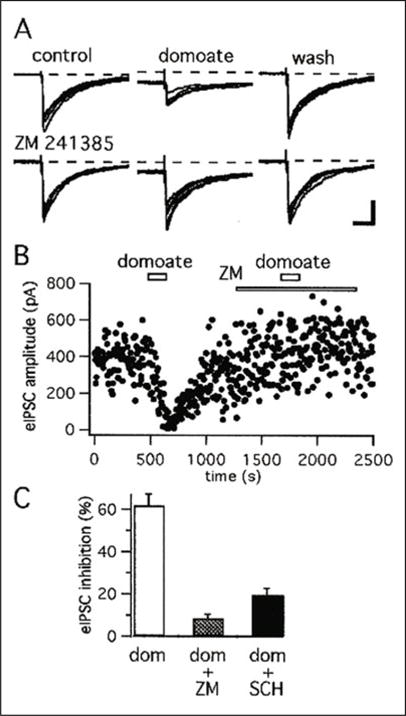
A2A receptor antagonists block the action of domoate on evoked IPSCs. A) IPSCs were evoked by intrastriatal stimulation (at a rate of 0.2 Hz) in the presence of NBQX (1 μM). Top traces, Domoate (500 nM for 2 min) reversibly decreased evoked IPSC amplitude. Bottom traces, In the same cell, perfusion of the slice with the selective A2A antagonist ZM 241385 (1 μM) prevents the action of domoate on evoked IPSC amplitude. The dotted line represents the level of the control inward current. B) For the same experiment, plot of the amplitude of evoked IPSCs as a function of the time. Domoate is applied at the time indicated by the open horizontal bars. As indicated by the bottom horizontal bar, ZM 241385 (1 μM) is perfused several minutes before the second application of domoate. C) Histogram of the average (± SEM) inhibition by domoate (500 nM for 2 min) of evoked IPSC amplitude in control condition (n = 18), in the presence of ZM 241385 (1 μM) (n = 7) and in the presence of SCH-58261 (1 μM) (n = 6). For both antagonists, the difference in inhibition versus domoate was significant with P < 0.001. Reprinted from Chergui K et al. J Neurosci 2000; 20(6):2175–2182;17 © 2000 with permission from the Society for Neuroscience.
Globus Pallidus
Globus pallidus neurons express strong mRNA for GluR6 and KA2 in rodents.16 Consistent with these findings, neurons in the external (GPe) and internal (GPi) pallidal segments in monkeys display moderate to strong GluR6/7 and KA2 immunoreactivity.30 At the electron microscopic level, GluR6/7 labeling is expressed both postsynaptically in dendrites of pallidal neurons and presynaptically in GABAergic striatal terminals and putative glutamatergic terminals.9, 30 In addition, significant GluR6/7 immunoreactivity is expressed in unmyelinated axons throughout both pallidal segments suggesting a presynaptic role for KARs in this brain region.9, 30 The pattern of KARs immunoreactivity in the monkey pallidum is very similar to that found in both adult and young rats GP (Fig. 3).
Figure 3.

Pre and postsynaptic expression of GluR6/7 immunoreactivity in rat and monkey GP. GluR6/7-labeled terminals (Te) forming symmetric (A,C) or asymmetric (B) axon-dendritic synapse in rat and monkey pallidum. C) shows a GluR6/7-Postive axon terminals (Te) enriched in GABA immunoreactivity forming a symmetric axon-dendritic synapse on a labeled dendrite. Note the low density of gold particles associated with a putative glutamatergic terminal (u.Te) that forms an asymmetric synapse (arrow) on the same dendrite. Scale bars: A: 0.5 μm; B: 0.3 μm; C: 0.3 μm. (See references 9 and 30 for more details.) Reprinted from: Kane-Jackson R, Smith Y. Neuroscience 2003; 120:285–289;30 with permission from Elsevier; and from Jin X-T et al. Eur J Neurosci 2006; 23:374–386;9 © 2006 with permission from Wiley-Blackwell.
In line with these immunocytochemical data, KA application inhibits GABAergic synaptic transmission through presynaptic mechanisms in slices of rat GP (Fig. 4). On the other hand, KA (1 μM) does not have a signifficant effect on whole cell resistance of rat GP neurons9 suggesting that the KAR-induced depression of eIPSCs is not due to postsynaptic changes of the passive membrane properties of GP cells.
Figure 4.

KAR activation increases paired pulse facilitation ratio (PPFR) and reduces the frequency, but not the amplitude, of mIPSCs at GABAergic synapses in the GP. A) Paired IPSCs were recorded before (left trace) and during (middle) 1 μM KA application. The right trace shows the KA-induced effect after scaling to the peak of the first IPSC. B) The same neuron presented in (A) shows the time course of increased paired-pulse facilitation ratio (PPFR) of IPSCs in response to 1 μM KA application (left graph) and the effect of KA on PPFR expressed as a ratio of P2/P1 (mean ± S.E.M.) (right graph). C) A summary bar graph shows that KA (0.1–0.3 μM) significantly reduces the frequency of mIPSCs, which is blocked in the presence of 50 μM CNQX. D) A summary bar graph shows that neither KA nor KA together with CNQX affects the amplitude of mIPSCs. Asterisks indicate a significant difference from control (* < 0.01), NS indicates nonsignificant differences; and n indicates the number of cells tested under each condition. (See ref. 13 for more details.) From X-T Jin, Smith Y et al. Neuroscience 2007; 149:338–34913 © 2007 with permission from Elsevier.
As discussed in other chapters, since the pioneer publication of Rodriguez-Moreno and Lerma in 1998,31 various complex mechanisms involving G-proteins have been demonstrated by which KARs modulate GABAergic synaptic transmission in the hippocampus and other brain regions (reviewed in refs. 7,8,32–34). We recently studied the effect of another G-protein inhibitor N-ethylmaleimide (NEM) on KA-induced inhibition of GABAergic transmission in slices of rat GP and found that this G-protein antagonist is capable of blocking presynaptic KAR-induced inhibition of glutamatergic transmission in this brain region.9 We also demonstrated that the KAR-mediated presynaptic modulation of GABAergic synaptic transmission in GP is abolished by NEM (Fig. 5A,B), likely though a presynaptic site of action because the KAR-mediated decrease in mIPSCs frequency is also significantly reduced by this drug (Fig. 5C–E). Although the exact mechanisms by which G-proteins contribute to the KAR-mediated presynaptic effects in GP remain to be determined, the fact that these effects are not abolished after blockade of various G protein-coupled receptors, including GABAB and A2A which are involved in mediated presynaptic effects of KARs in hippocampus and striatum,17, 26, 27, 35 is strongly indicative of a specific mechanism different from that seen at other synapses.13
Figure 5.

Application of G-protein inhibitor (NEM) blocks the KAR activation-induced inhibition of GABAergic synaptic transmission in rat GP. A) Evoked IPSCs recorded in presence of NEM (left trace) and NEM together with KA (right trace). B) A summary bar graph shows that the inhibitory effect of KA on IPSC amplitude is blocked in the presence of NEM. C) Sample traces show mIPSCs in presence of NEM (left trace) and together with KA (right trace). D, E) A summary bar graph shows that KA has no effect on either mIPSCs frequency or amplitude in the presence of NEM. (See reference 13 for more details.) From X-T Jin, Smith Y et al. Neuroscience 2007; 149:338–34913 © 2007 with permission from Elsevier.
Several studies have indicated that PKC activation downstream of G-protein activity is essential for KAR-mediated pre and postsynaptic effects (reviewed by refs. 33,34). For instance, KA-induced presynaptic inhibition of GABAergic or glutamatergic transmission and KA-mediated postsynaptic inhibition of slow afterhyperpolarization currents (IsAHP) in hippocampal neurons are blocked by PKC inhibitor (Calphostin C), but not by PKA inhibitor (H-89).9, 31, 36–40 In the rat GP, presynaptic KAR-mediated effects on evoked and mIPSCs are also blocked by Calphostin, but not by H-89 (Fig. 6), providing further evidence that KAR-induced depression of GABAergic synaptic transmission in the rat GP requires G-protein and PKC activation, but does not rely on the secondary activation of G protein-coupled receptors. Thus, together with our recent study showing KAR-mediated regulation of glutamatergic transmission,9 these findings demonstrate that KARs mediate their presynaptic effects on both GABAergic and glutamatergic transmission in the GP through a metabotropic mode of action.
Figure 6.

Pretreatment with PKC inhibitor (calphostin), but not PKA inhibitor (H-89), prevents KAR activation-induced inhibition of IPSCs in rat GP. A) The time course of 1 μM KA on IPSC amplitude in the presence of 1 μM calphostin C. Three IPSCs are averaged in each trace at the time indicated by corresponding letters in the graph. B) A bar graph shows that the KAR activation-induced inhibition of IPSCs is blocked by 0.5 μM staurosporine, a broad-spectrum inhibitor of protein kinase and calphostin C, but not by H-89. There is a significant difference from control, * < 0.01. C) mIPSCs were recorded in the presence of 1 μM calphostin C and calphostin C together with 1 μM KA. D) mIPSCs were recorded in the presence of 0.5 μM H-89 together with 1 μM KA. E) Summary bar graph shows that KA has no effect on mIPSCs frequency in the presence of 0.5 μM staurosporine and calphostin C, but not in the presence of H-89. F) Summary bar graph shows that KA has no effect on mIPSCs amplitude in the presence of staurosporine, calphostin C and H-89. *P < 0.05. The y axis of all graphs respresents percent of control. (See ref. 13 for more details.) From X-T Jin, Smith Y et al. Neuroscience 2007; 149:338–34913 © 2007 with permission from Elsevier.
Substantia Nigra Pars Compacta
Dopaminergic neurons in the substantia nigra pars compacta (SNc) express the highest level of mRNA for GluR5 and GluR7 subunits within the basal ganglia.16 In contrast, only low level of GluR6 and KA2 is found in SNc neurons, while no detectable mRNA for KA1 is observed in these cells.16 Although the expression of GluR6, GluR7 and KA2 subunits in SNc was confirmed by light microscopic immunocytochemistry,41 much remains to be known about the exact cellular and subcellular localization of these subunits in the SNc.
Kainate application increases the frequency of mIPSCs, without changing their amplitude, in SNc neurons.35 This presynaptic facilitatory effect of KA on the frequency of mIPSCs is suppressed in either Na+-free or Ca2-free external solution and in presence of voltage-dependent Ca2+ channel blockers supporting a direct presynaptic ionotropic mode of action.35 On the other hand, KAR activation inhibits eIPSCs and this inhibitory effect is reduced in the presence of GABAB receptor antagonist, but not other G-protein coupled-receptor antagonists (Fig. 7). Several hypotheses have been proposed to explain the possible mechanisms (s) underlying the presynaptic KAR-induced modulation of GABAergic transmission in various brain regions (reviewed by references 7 and 8). These include direct inhibition of GABA release from terminals,13, 31, 37, 42, 43 a modulation of axonal excitability,44, 45 or an indirect GABAB receptor-mediated effect.26, 27 Data shown in Figure 7 argue that KA-induced inhibition of eIPSCs in the SNc involve the secondary activation of GABAB autoreceptors. Thus, presynaptic KARs regulate the SNc circuitry through two functionally distinct opposing mechanisms: a direct presynaptic ionotropic mode of action that facilitates GABA release and a secondary inhibitory mechanism that involves presynaptic GABAB autoreceptors.
Figure 7.
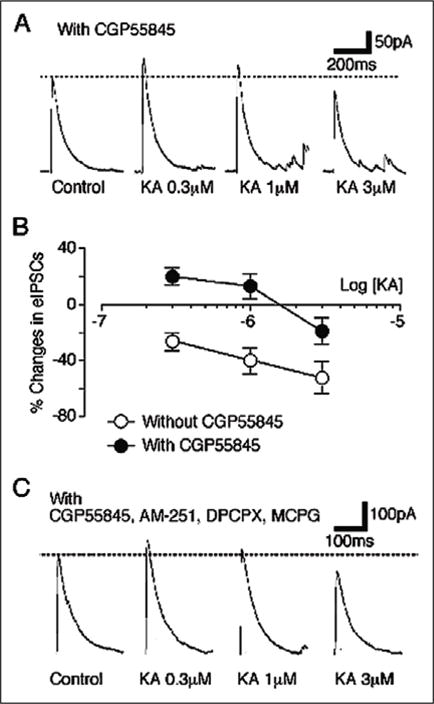
Involvement of GABAB receptors in the KA-induced inhibition of eIPSCs in dopaminergic neurons on the SNc. A) Typical traces of GABAergic eIPSCs observed during application of KA at various concentrations in the presence of 10 μM CGP55845. Dotted line represents control eIPSCs amplitude. B) Concentration-response relationship of KA action on eIPSC amplitude. Each point is normalized to respective control and represents the mean of 6 neurons. C) Typical traces of GABAergic eIPSCs observed during application of KA at various concentration in presence of CGP55845 (10 μM), AM-251 (10 μM), DPCPX (100 nM) and MCPG (1 mM). Reprinted from with permission from Nakamura M et al. J Neurophysiol 2003; 90:1662–1670.35
In contrast to SNc, neurons in the substantia nigra pars reticulata (SNr) express the highest level of mRNA for GluR5 and GluR6 subunits within the basal ganglia, while no detectable mRNA for the other kainate receptor subunits is found in these neurons.16 However, immunocytochemical study showed that protein expression of both GluR6/7 and KA2 in the rat SNr.41 The role of KARs in the SNr remains to be established.
Subthalamic Nucleus
Neurons in subthalamic nucleus (STN) express a high level of mRNA for the GluR6 subunit while they display very low level of GluR7 mRNA, or are almost completely devoid of GluR5, KA1 and KA2 subunits mRNA expression in rats.16 The high expression level of the GluR6 subunit was confirmed by immunocytochemistry in the rat STN.41 In contrast to mRNA data, a significant level of KA2 subunit immunoreactivity is also found in the rat STN.41 The physiology of KARs in STN remains unknown.
Conclusion
The past decade has witnessed significant development in our understanding of KARs-mediated regulation of GABAergic synaptic transmission in the CNS.7, 8, 32, 43 In the hippocampus, there is significant evidence that converges towards two principal mechanisms of action; a G-protein-coupled, PKC-dependent, metabotropic mechanism or the secondary activation of G-protein coupled receptors.26, 31, 36, 37 Data reviewed in this chapter provide further evidence for these two pathways in the modulation of GABA release in some basal ganglia nuclei. However, the findings presented in this chapter also highlight a significant degree of heterogeneity by which KARs mediate their effects across basal ganglia nuclei. Future studies aimed at characterizing the localization and function of KARs in the basal ganglia of animal models of Parkinson’s disease or other movement disorders may provide some insight about the potential role these receptors may play in the pathophysiology of movement disorders. Knowing that overactive glutamatergic transmission is a cardinal feature of basal ganglia pathophysiology in PD, a deeper understanding of KARs combined with the development of novel compounds that could selectively modulate activity of these receptors may pave the way for new pharmacotherapeutic approaches in PD and other movement disorders.
Acknowledgments
This research was supported by a grant from the U S Army, the Yerkes Primate Center NIH base Grant and an Award from Merck/Center for Neurodegenerative Disease at Emory University.
Footnotes
Kainate Receptors: Novel Signaling Insights, edited by Antonio Rodríguez-Moreno and Talvinder S. Sihra.
References
- 1.Hollman M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Bettler B, Mulle C. Neurotransmitter receptors II. AM PA and kainat ereceptors Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- 3.Charara A, Blankstein E, Smith Y. Presynaptic kainate receptors in the monkey striatum. Neuroscience. 1999;91(4):1195–1200. doi: 10.1016/s0306-4522(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 4.Lerma J, Paternain AV, Rodriguez-Moreno A, et al. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- 5.Kieval JZ, Hubert GW, Charara A, et al. Subcellular and subsynaptic localization of presynaptic and postsynaptic kainate receptor subunits in the monkey striatum. J Neurosci. 2001;21:8746–8757. doi: 10.1523/JNEUROSCI.21-22-08746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 7.Huettner JE. Kainate receptors and synaptic transmission. Prog in Neurobiol. 2003;70(5):387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4(6):481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- 9.Jin X-T, Paré JF, Raju DV, et al. Localization and function of pre and postsynaptic kainate receptors in the rat globus pallidus. Eur J Neurosci. 2006;23:374–386. doi: 10.1111/j.1460-9568.2005.04574.x. [DOI] [PubMed] [Google Scholar]
- 10.Melyan Z, Lancaster B, Wheal HV. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci. 2004;24:4530–4534. doi: 10.1523/JNEUROSCI.5356-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga MFM, Aroniadou-Anderjaska V, Xie J, et al. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23(2):442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cossart R, Tyzio R, Dinocourt C, et al. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29(2):497–508. doi: 10.1016/s0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 13.Jin X-T, Smith Y. Activation of presynaptic kainate receptors suppresses GABAergic synaptic transmission in the globus pallidus. Neuroscience. 2007;149:338–349. doi: 10.1016/j.neuroscience.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith Y, Charara A, Paquet M, et al. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat. 2001;22:13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 15.Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14(9):5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff S, Barhanin J, Bettler B, et al. Spatial distribution of kainate receptor subunit mRNA in the mouse basal ganglia and ventral mesencephalon. J Comp Neurol. 1997;379:541–562. doi: 10.1002/(sici)1096-9861(19970324)379:4<541::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Chergui K, Bouron A, Normand E, et al. Functional GluR6 kainate receptors in the striatum: indirect downregulation of synaptic transmission. J Neurosci. 2000;20(6):2175–2182. doi: 10.1523/JNEUROSCI.20-06-02175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowder TL, Ariwodola OJ, Weiner JL. Kainate receptor activation potentiates GABAergic synaptic transmission in the nucleus accumbens core. Brain Res. 2006:73–82. doi: 10.1016/j.brainres.2005.12.133. [DOI] [PubMed] [Google Scholar]
- 19.Koos T, Tepper J. Inhibittory control of neurostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 20.Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobio. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is week or nonexistent in the rat neostriatum. J Neurophysio. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- 22.Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res. 1991;564:296–305. doi: 10.1016/0006-8993(91)91466-e. [DOI] [PubMed] [Google Scholar]
- 23.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 24.Bevan MD, Booth PA, Eaton SA, et al. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith Y, Bevan MD, Shink E, et al. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 26.Frerking M, Petersen CC, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc Natl Acad Sci USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerchner GA, Wilding TJ, Li P, et al. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hettinger BD, Lee A, Linden J, et al. Ultrastructural localization of adenosine A2Areceptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Bogenpohl JW, Pare JF, Smith Y. Subcellular localization of adenosine A2A receptors in the striatum and globus pallidus of monkey and rat. Soc Neurosci Abstr. 2008:P-056. [Google Scholar]
- 30.Kane-Jackson R, Smith Y. Pre-synaptic kainate receptors in GABAergic and Glutamatergic axon terminals in the monkey globus pallidus. Neuroscience. 2003;120:285–289. doi: 10.1016/s0306-4522(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 32.Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Moreno A, Sihra TS. Metabotropic actions of kainate receptors in the CNS. J Neurochem. 2007;103:2121–2135. doi: 10.1111/j.1471-4159.2007.04924.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Moreno A, Sihra TS. Kainate receptors with a metabotropic modus operandi. Trends Neurosci. 2007;30(12):630–637. doi: 10.1016/j.tins.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Jang IS, Ishibashi H, et al. Possible roles of kainate receptors on GABAergic nerve terminals projecting to rat substantia nigra dopaminergic neurons. J Neurophysiol. 2003;90:1662–1670. doi: 10.1152/jn.01165.2002. [DOI] [PubMed] [Google Scholar]
- 36.Cunha RA, Malva JO, Ribeiro JA. Pertussis toxin prevents presynaptic inhibition by kainate receptors of rat hippocampal [3H] GABA release. FEBS Letters. 2000;469(2–3):159–162. doi: 10.1016/s0014-5793(00)01272-2. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Moreno A, Lopez-Garcia JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc Natl Acad Sci USA. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34(1):107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- 39.Melyan Z, Lancaster B, Wheal HV. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci. 2004;24:4530–4534. doi: 10.1523/JNEUROSCI.5356-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauri SE, Segerstrale M, Vesikansa A, et al. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci. 2005;25:4473–4484. doi: 10.1523/JNEUROSCI.4050-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainat receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 43.Maingret F, Lauri SE, Taira T, et al. Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol. 2005;567:131–142. doi: 10.1113/jphysiol.2005.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semyanov A, Kullmann DM. Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nature Neurosci. 2001;4:718–723. doi: 10.1038/89506. [DOI] [PubMed] [Google Scholar]
- 45.Kang N, Jiang L, He W, et al. Presynaptic inactivation of action potentials and postsynaptic inhibition of GABAA currents contribute to KA-induced disinhibition in CA1 pyramidal neurons. J Neurophysiol. 2004;92:873–882. doi: 10.1152/jn.01231.2003. [DOI] [PubMed] [Google Scholar]


