Abstract
BACKGROUND
Biotin-labeled red blood cells (BioRBC) are used for in vivo kinetic studies. Because BioRBC dosing occasionally induces antibodies, a sensitive and specific anti-BioRBC antibody detection assay and strategies for preventing antibody formation are needed.
DESIGN AND METHODS
Aims: 1) develop IgG gel card assay to evaluate preexisting (i.e., naturally occurring) and BioRBC-induced plasma antibodies; 2) compare gel card and tube agglutination assay results; 3) evaluate relationship of antibody induction to RBC biotin dose (#BioRBC × label per RBC). Reagent BioRBC were prepared using sulpho-NHS biotin ranging from 18 (BioRBC-18) to 1458 (BioRBC-1458)μg/mL RBC.
RESULTS
Among BioRBC-exposed subjects, gel card and tube assay results were concordant in 21 of 22 adults and in all 19 infant plasmas. Gel card sensitivity was >10-fold greater than the tube assay. For BioRBC exposed subjects, gel card detected induced anti-BioRBC antibodies at 12wk post-exposure or later in 3 of 26 adults (12%) at reagent BioRBC≤256, but in none of 41 infants. Importantly, induced antibodies were associated with higher BioRBC dose (P<0.008); no antibodies were detected in 18 subjects who received doses of BioRBC≤18. For non-induced antibodies, 6 of 1,125 naïve adults (0.3%) and 0 of 46 naïve infants demonstrated preexisting anti-BioRBC antibodies using reagent BioRBC-140/162. Preexisting anti-BioRBC antibodies were all neutralized by biotin compounds, while induced antibodies were not.
CONCLUSIONS
The gel card assay is more sensitive than the tube agglutination assay. We recommend reagent BioRBC-256 for identifying anti-BioRBC antibodies. Using a low total RBC biotin label dose (BioRBC≤18 μg/mL) may minimize antibody induction.
Keywords: biotin, RBC labeling, gel card agglutination, anti-biotin antibody
INTRODUCTION
The use of labeled red blood cells (RBC) for in vivo quantitation of red cell volume and red cell survival (RCS) has advanced understanding of anemia and its treatment. RBC labeling with 51Cr is the FDA’s regulatory standard method for measuring short-term RBC recovery.1 Unfortunately 51Cr RBC labeling has shortcomings including radiation exposure and inaccuracy in quantitation of long-term RCS. Radiation concern has eliminated 51Cr RBC kinetic studies in Europe and in vulnerable populations that include fetuses, infants, children, and pregnant women.2 The accuracy of 51Cr RCS is limited by variability in 51Cr RBC elution limiting 51Cr RCS to 30d.1
Biotin (Vitamin B8) labeling of RBC surface proteins followed by flow cytometric enumeration overcomes both 51Cr-labeling shortcomings.3 Flow cytometric analysis of biotin labeled RBC (BioRBC) permits accurate determination of RCS throughout >95% of the lifespan provided initial BioRBC enrichment is >0.5%.4–6 Other advantages of biotin RBC labeling include the ability: 1) to concurrently and independently track RCS in vivo for multiple RBC populations labeled at separate, discrete biotin densities; and 2) to isolate BioRBC in blood using magnetic beads for assessing biochemical and biophysical RBC changes.
Despite the advantages of the biotin method, a theoretical safety issue is the occasional development of anti-BioRBC antibodies following BioRBC exposure. We have detected transient BioRBC-induced anti-BioRBC antibody responses in four of 28 (14%) BioRBC-exposed adults using traditional tube agglutination.7 These antibodies have not caused clinical abnormalities,5,8 We speculate that this is analogous to penicillin-type drug-dependent antibodies with biotin covalently attached to RBC surface proteins acting as immunogenicity enhancing haptens.9 Although anti-BioRBC antibodies following initial BioRBC exposure have not reduced RBC survival, we reported an adult who experienced accelerated removal of BioRBC when re-challenged with BioRBC five years later in association with an anamnestic anti-BioRBC antibody response.10 Although this rapid BioRBC removal did not cause abnormalities in hematological or biotin nutritional laboratory tests, the antibody-induced shortening of BioRBC survival may render such subjects unsuitable for future BioRBC RCS studies.
A robust, sensitive, and specific assay for antibodies to BioRBC that has an objective endpoint and is easier to perform than the more subjective traditional tube agglutination8 could expand use of BioRBC in RBC kinetic studies. Doing so would facilitate wider adoption of the biotin RBC labeling method as an alternative to 51Cr labeling. In addition, a better understanding of the relationship between the density and dose of the BioRBC infused and antibody formation might lead to future approaches reducing the incidence of anti-BioRBC antibody induction. Thus, the primary objectives of the present study were: 1) to develop, characterize, and apply a commercial IgG gel card agglutination assay to detect BioRBC-induced and pre-existing anti-BioRBC antibodies; 2) to compare gel card reactivity and sensitivity with traditional tube agglutination;8 and 3) to compare antibody induction rates to the dose of BioRBC administered. A secondary objective was to determine the biotin label density of reagent BioRBC that can be reliably used for screening BioRBC-naïve subjects for pre-existing anti-BioRBC antibodies, and for detecting BioRBC-induced anti-BioRBC antibodies.
MATERIALS AND METHODS
This study received approval from the University of Iowa Committee on Research on Human Subjects.
Study subjects
Study subjects, groups of whom were tested in different laboratories, included healthy adults, hospitalized adults, and very low birth weight infants (VLBW; birth weight <1.5kg). Data from some study subjects have been previously reported.4,6,11–14 Individual subjects had variable volumes of plasma or serum available for anti-BioRBC antibody testing; small volumes limited the scope of testing. Importantly, all subjects were initially BioRBC naïve. The majority of naïve subjects who received BioRBC were screened pre-study by standard RBC alloantibody and DAT testing and were tested for induced anti-BioRBC antibodies approximately every four weeks for twelve or more weeks post-BioRBC infusion.
Laboratory methods
Biotin labeling of reagent BioRBC
Biotin density on RBC membrane has been usually defined as the ratio of the mass of the biotinylating reagent to the volume of packed RBC biotinylated.3 Consequently, BioRBC densities used in testing and infusion are designated as BioRBC-N, where N represents the ratio of sulfosuccinimidobiotin (sNHS-biotin; Pierce Chemical, Rockford, IL) (μg) to RBC (mL). The number and mass of biotins per RBC was determined as described below.
For the antibody detection assays reagent BioRBC were prepared from group o+ (to avoid possible confounding by anti-A, B or A,B in subjects plasma/serum) allogeneic fresh RBC incubated with increasing amounts of sNHS-biotin as previously described.13 To compare the relative sensitivity of the tube and gel card agglutination assays,8 a reagent density of BioRBC-54 was employed. In all other tube assays reagent BioRBC-32 was used. In gel card assays, the spectrum of reagent densities ranged from BioRBC-18 to BioRBC-1458 in evaluating assay sensitivity. This included low BioRBC densities having no effect on in vivo RBC lifespan to higher BioRBC densities that shortened RBC lifespan, i.e., BioRBC≥162.5 The lower biotin reagent densities caused no change in RBC mean corpuscular volume or in vitro morphology whereas the higher densities increased MCV and altered RBC morphology by causing swelling and spicule formation (data not shown).13 Aliquots of reagent BioRBC were maintained either at 4°C for use within one week or frozen in glycerol at −70°C. Frozen BioRBC were thawed and deglycerolized for same day use.15
RBC Labeling using DNP as surrogate for biotin
To prepare a BioRBC labeling control, RBCs were labeled with DNP using increasing concentrations of NHS-DNP (2,4-dintrophenyl-N-hydroxysuccinimide, Cisbio US, Inc.) in an analogous fashion to that for BioRBCs. Because of the different solubility characteristics of the NHS-DNP and sNHS-biotin, equal molar quantity of labeling reagent per volume of packed RBC for the two labeling reagents would not result in equal surface labeling densities; NHS-DNP is hydrophobic and can cross cellular membranes and thus labels both intracellular proteins and RBC surface proteins, producing a lower number of DNP labels on RBC surface. To achieve equivalent relative surface DNP and BioRBC label densities, a similar range of labeling reagent molar quantities were used to produce overlapping flow cytometry histograms. Label density was quantitated by staining biotin- and DNP-labeled RBCs with FITC avidin or FITC anti-DNP (Dinitrophenyl-KLH Rabbit IgG Antibody Fraction, Fluorescein Conjugate, #A-6423, ThermoFisher Scientific), respectively. Flow cytometry was performed as described above for biotin. A 0.8% packed cell suspension of BioRBCs and DNP-RBCs with equal surface densities were used as reagent RBC in the gel card assay. The positive plasma containing high titer anti-BioRBC antibody was used as control test plasma.
Estimation of number of PE molecules per cell on biotin labeled RBCs
To determine if anti-BioRBC antibody induction is associated with the dose of biotin labels on the BioRBC infused, we determine the total number of biotin labels infused. This was estimated as the product of the number of BioRBC infused times the density of biotin labels per RBC. For multidensity BioRBC infusions, this is equal to the sum of the biotin labels estimated for all BioRBC populations.
To estimate mean total biotin label per RBC administered for a representative BioRBC population, a flow cytometry assay utilizing streptavidin-phycoerythrin (SA-PE) and QuantiBRITE-PE (QB-PE; Becton Dickinson, San Jose, CA) was employed. QB-PE beads contain four pre-calibrated bead populations with known PE molecules per bead. These generated a calibration curve. BioRBC-6 were stained to saturation with SA-PE and fluorescence intensity measured. PE molecules per BioRBC were estimated using the QB-PE standard curve and fluorescence intensity of stained BioRBCs. Because fluorescence intensity per RBC is linearly related to biotinylation reagent per mL RBC,13 biotin label density was then calculated as simple ratios of the biotinylation reagent masses per mL used in labeling individual BioRBC populations.
Tube agglutination anti-BioRBC antibody detection method
The tube anti-globulin agglutination assay was performed as previously described.8 Briefly, unlabeled RBCs were used to assess non-specific agglutination; only plasma without detectable non-specific agglutination against unlabeled RBC were scored. K1 RBC antigen and anti-K1 antibody served as a positive control. Agglutination strength was scored as: 0 (no agglutination), ±, 1w, 1+, 2+, 3+, and 4+.16
Gel card assay
Positive control
In the absence of an established anti-BioRBC antibody standard, plasma from a subject exposed to BioRBC twice (Subject 521) that resulted in a high titer (1:512 at reagent density BioRBC-54) of anti-BioRBC antibody following the second exposure was employed. This plasma was diluted 1:100 in Diluent 2 (MTS9230, Ortho Clinical Diagnostics, Rochester, NY) as positive control.10
Assay procedure
Like the tube anti-globulin assay, the gel card assay is based on RBC agglutination. The gel cards were used according to the manufacturer’s recommendations (MTS Anti-IgG Card, MTS084024, Ortho Clinical Diagnostics, Rochester, NY). Briefly, 50μL of the reagent BioRBC-N or unlabeled RBC (at 0.8% hematocrit in Diluent 2) was prepared from a group O+ convenience donor and layered atop the buffer in gel card microtubes. Test plasma (25μL) was added to reagent BioRBC or RBC in the gel column and incubated at 37°C for 30min (ID-MTS Incubator MTS9680, Ortho Clinical Diagnostics, Rochester, NY). Gel cards were subsequently centrifuged at 895RPM (80-90RCF) for 10min (ID-MTS Centrifuge MT515060, Ortho Clinical Diagnostics, Rochester, NY). Doing so exposed the BioRBC/plasma mixture to the IgG Coombs reagent in the gel card.
Scoring of agglutination
The BioRBC agglutination was quantified using the manufacturer’s gradation-specific definitions of reactivity. Reactivity scores ranged from 0 (no agglutination) to 4+ (agglutinates are trapped on the surface of the gel bed). In this study, a score of 1+ (i.e., agglutination in lower half of matrix) was further sub-classified into the three increasingly reactive 1+ designations based on the manufacturer’s photographs as follows: 1) ± indicates a slanted pellet with a few agglutinated RBC; 2) 1w indicates a slanted pellet with more agglutinated RBC; and 3) 1+ indicates a disrupted RBC pellet with more agglutinated cells.
Characterization of structural specificity of anti-BioRBC antibodies
Hemagglutination inhibition (neutralization)
Hemagglutination inhibition was used to determine whether the antibody was specific to biotin, BioRBC, or both. 25μL of plasma was incubated for 15min at room temperature in the presence of soluble 16μM biotin, 8μM biocytin (a structurally related analog), biotinylated albumin, or biotinylated gelatin. Neutralized plasma was then tested by gel card. To remove unreacted reagent and hydrolysis products, albumin stock solution was biotinylated using a three-fold molar excess of sulfo-NHS biotin (Pierce Chemical) followed by dialysis. Biotinylated gelatin stock was prepared in phosphate buffered saline as 7mg biotinylated gelatin/mL.17 Specificity for BioRBC was assessed by abrogation of agglutination in the presence of biotin or its analogs.
BioRBC adsorption
Antibody adsorption was used to demonstrate specificity to BioRBC. To do this, 25uL of test plasma was incubated with increasing amounts of BioRBC (0.25, 2.5, and 25μL of packed BioRBC-512 or -1458) at 37°C for 30min. The reactions were then centrifuged to separate the plasma and RBC, with the adsorbed plasma retested in the gel card assay at the initial BioRBC agglutinating reagent density. If the agglutination pattern showed reduced BioRBC dose-dependent reactivity, this indicated that the antibody was specific to BioRBC, but not biotin.
Assay Sensitivity and Specificity
Comparative sensitivity of tube agglutination and the gel card agglutination assay was assessed by the titer of the positive control plasma at a fixed BioRBC reagent density, i.e., BioRBC-54. Assay sensitivity in the gel card assay was determined by the lowest level of reagent BioRBC density at which induced anti-BioRBC antibody reactivity was detected. Assay specificity of induced anti-BioRBC antibodies in the gel card assay was determined by demonstrating no plasma hemagglutination inhibition by biotin, or biotin attached to other molecules.
Statistics and data management
Central tendency and variability were expressed as the mean ± SE with 95% confidence limits provided where appropriate. The non-parametric Wilcoxon rank sum exact test was used in comparing mean values.
RESULTS
Standard RBC alloantibody and DAT testing
Of 26 study subjects tested for anti-BioRBC antibodies prior to the autologous BioRBC transfusion, 18 were screened for RBC alloantibodies and DAT by standard blood banking assays. There were no positive results.
Performance characteristics of gel card assay procedure
Sample type
There were no anticoagulant or sample type effects. Comparison of reactivity among six sets of concurrently collected aliquots of undiluted and 1:256 diluted high titer positive control serum, EDTA plasma, and heparinized plasma yielded identical results.
Consistency of reactivity evaluation
Using archived gel card photographs, agglutination scoring evaluated independently by two authors (RLS and JAW) was consistent.
Reproducibility
Reactivity reproducibility was also consistent in 24 gel card assays for positive control plasma diluted 1:100 when assayed at reagent BioRBC-54 using RBC at three study sites. In addition to these inter- and intra-assay controls, consistent titration endpoints, i.e., the last doubling dilution showing hemagglutination) differed by acceptable variation (±1 dilution) between repeat assays and between operators.
Frozen glycerolized reagent BioRBC
Thawed glycerolized BioRBC and unlabeled RBC yielded identical results compared to freshly prepared BioRBC and unlabeled RBC (data not shown).
Comparison of tube and gel card agglutination assay method results
Comparison of sensitivity
Using positive control plasma tested at reagent BioRBC-54 density, anti-BioRBC antibody reactivity was detectable at doubling dilutions up to 1:1024 in the gel card assay compared to only 1:64 in the tube assay (Table 1).
Table 1.
Comparison of sensitivities of tube and gel card assays at reagent BioRBC-54
| Dilution of reactive anti-BioRBC antibody positive control plasma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unlabeled RBC | 1:1 | 1:2 | 1:64 | 1:128 | 1:256 | 1:512 | 1:1024 | 1:2048 | |
| Tube method | 0 | 2+ | 2+ | 1w | 0 | 0 | 0 | 0 | 0 |
| Gel card method | 0 | 4+ | 3+ | 3+ | 3+ | 2+ | 2+ | 1+ | 0 |
Plasma reactivity among BioRBC-exposed subjects
Adults
Tube and gel card antibody agglutination assays were compared in a subset of eight Iowa BioRBC-exposed adults undergoing 11 autologous BioRBC survival studies.5 Testing was performed using similar reagent densities, i.e., BioRBC-32 (tube) and BioRBC-32 or 64 (gel card). Scoring agreed for the two assays in 21 of the 22 plasma samples. For the one plasma sample that did not agree, the tube assay score was ± (i.e., the lowest) while the gel card score was zero.
Infants
Nineteen pre-study and end of study plasma samples from 11 allogeneic BioRBC-exposed infants18 were performed in both assays using BioRBC-32 (tube) and BioRBC-54 (gel card). No plasma samples were reactive in either assay.
Gel card results for BioRBC-exposed subjects
Adults: Testing for induced reactivity using multiple densities of reagent BioRBC
Following initial transfusion of autologous BioRBC, 26 healthy adult subjects were tested intermittently at three sites for up to 5mo using the gel card method. Label densities, volume of BioRBC, and total mass of biotin label infused differed substantially among the three studies, and resulted in a much higher RBC biotin dose at the Iowa site (Table 2).5,6 The average total dose of biotin (as biotin label) was approximately 25- to 50-fold greater for the Iowa study compared to Cincinnati or Amsterdam. With the exception of the Iowa adults, the biotin dose was <10% of the recommended daily intake.19 Three of the eight BioRBC-exposed Iowa adults, but none of eight Cincinnati or ten Amsterdam adults—all of whom were exposed to BioRBC≤18μg/mL RBC—developed anti-BioRBC antibodies. When the 23 BioRBC-exposed adults without induced antibodies were compared to the three with induced antibodies, the BioRBC dose was higher in the group who developed anti-BioRBC antibodies (P=0.008).
Table 2.
Quantitation of BioRBC dose administered to study groups (mean ± SD).
| Number of Subjects | Number Anti-BioRBC Antibody Reactive† | Volume of BioRBC Transfused (mL/kg)¥ | Dose of BioRBC (No. biotin molecules in transfused BioRBC × 1012/kg) | Dose of BioRBC (% of RDI in transfused BioRBC)28,29 | Initial Post-Transfusion BioRBC Enrichment (% of total RBCs) | Time of Final Post-Transfusion Sample (wk) | |
|---|---|---|---|---|---|---|---|
| Adults | |||||||
| BioRBC-6, -18, -54, & -16213 (Iowa) | 8 | 3 | 1.09 ± 0.29 | 499 ± 126 | 53 ± 4 | 5.07 ± 0.76 | 18.5 ± 2.1 |
| BioRBC-66 (Amsterdam) | 10 | 0 | 0.34 ± 0.03 | 7.18 ± 0.90 | 0.75 ± 0.19 | 0.60 ± 0.09 | 12 |
| BioRBC-18§ (Cincinnati) | 8* | 0 | 0.286 | 19 | 1.9 | 0.50 | 24 |
| VLBW Infants | |||||||
| BioRBC-6, -18, -54, & -16218 | 19 | 0 | 2.87 ± 0.49 | 1,110 ± 338 | 9.9 ± 3.7 | 9.55 ± 2.05 | 10.9 ± 5.5 |
| BioRBC-6 & -18, or -9 & -3620 | 17 | 0 | 1.43 ± 0.61 | 61 ± 110 | 0.39 ± 0.73 | 5.99 ± 2.03 | 18.1 ± 4.6 |
Based on gel card reactivity and the post-BioRBC transfusion exposure time at which anti-BioRBC antibodies developed as indicated in Table 3.
Packed RBC volume was estimated to be approximately 0.45.
Unpublished.
Includes 4 subjects with Type 1 diabetes mellitus with their approximated data estimates in each column.
These adult samples were tested using increasing reagent BioRBC densities to increase assay sensitivity. Anti-BioRBC antibodies were first detectable only after 12wk in two subjects and after 16wk in the third (Table 3). Using a reagent cutoff of BioRBC-256 anti-BioRBC antibodies were detected in all three subjects who developed an immune response, i.e., 100% sensitivity.
Table 3.
Induced plasma anti-BioRBC antibodies by gel card among BioRBC-exposed adults*.
| Post-BioRBC exposure plasma reactivity scores with increasing BioRBC reagent density | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 12 | Week 16 | Week 20 | ||||||||||||||||
| Subject# | 32 | 64 | 128 | 256 | 512 | 1024 | 32 | 64 | 128 | 256 | 512 | 1024 | 32 | 64 | 128 | 256 | 512 | 1024 |
| 521 | 0 | 0 | 0 | ± | 1+ | 1+ | 0 | 2+ | 2+ | 2+ | 2+ | 2+ | 1+ | 2+ | 2+ | 2+ | 3+ | 3+ |
| 526 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1w | 1+ | 1+ | ND | ND | ND | ND | ND | ND |
| 528 | 0 | 0 | 0 | 0 | 1+ | 2+ | 0 | 0 | 1+ | 2+ | 3+ | 3+ | ND | ND | ND | ND | ND | ND |
Prior to Week 12, plasma from all three subjects was non-reactive at all BioRBC<256 reagent densities
Infants: Testing for induced reactivity using multiple densities of reagent BioRBC
The study designs for the two groups of infants who had BioRBC survival studies dictated BioRBC transfusion of either two or four densities (Table 2).18,20 Samples tested included 168 pre-study and monthly post-infusion plasma samples from 36 infants at reagent gel card BioRBC-54, with a subset of these tested at BioRBC-162 and BioRBC-1458 to increase the assay sensitivity. No infant developed induced anti-BioRBC antibodies.
Gel card results for BioRBC-naïve subjects
Adults: Reactivity tested using multiple reagent BioRBC densities
Among 161 healthy (i.e., not hospitalized), naïve adults tested at multiple reagent BioRBC densities, 131 (81.4%) were non-reactive at all of BioRBC densities, and 30 (18.6%) showed varying degrees of reactivity starting at BioRBC-162 (Table 4). The percent of samples with detectable preexisting anti-BioRBC antibodies increased as reagent BioRBC density increased. Most subjects with reactive plasma demonstrated increases in strength of reactivity with increasing density. No anti-biotin immune response was detected in any of the five laboratory workers regularly performing RBC biotinylation.
Table 4.
Gel card reactivity of among 161 BioRBC naïve, healthy adults.
| BioRBC Reagent Density Levels (μg NHS-biotin per mL RBC) | |||||
|---|---|---|---|---|---|
| 18 | 54 | 162 | 486 | 1458 | |
| Number first becoming reactive | 0 | 0 | 2 | 7 | 21 |
|
| |||||
| Percent first becoming reactive (95% confidence interval) | 0.0% (0%, 2.3%) |
0.0% (0%, 2.3%) |
1.2% (0.2%, 4.4%) |
4.4% (1.8%, 9.3%) |
13.0% (8.3%, 19.2%) |
|
| |||||
| Cumulative percent reactive (95% confidence interval) | 0.0% (0%, 2.3%) |
0.0% (0%, 2.3%) |
1.2% (0.2%, 4.4%) |
5.6% (2.6%, 10.4%) |
18.6% (12.9%, 25.5%) |
None of the pre-study naïve plasma samples for the eight Iowa adults later transfused with BioRBC were reactive using reagent BioRBC≤256.13 In contrast, six of these same eight pre-study naïve plasmas were reactive when tested at densities BioRBC>256, including two who developed induced anti-BioRBC antibodies and four who did not.
Adults: Expanded testing to evaluate prevalence of anti-BioRBC in naïve populations
In addition to characterization of reagent BioRBC density on reactivity of plasma from naïve healthy adults, an additional 964 plasma samples were screened for the presence of preexisting anti-BioRBC antibody. For this, samples were from de-identified hospitalized BioRBC-naïve adult subjects using a reagent density of BioRBC-140. Of these, four (0.4%) were reactive. Further multi-density testing demonstrated that one of these four naïve reactive subjects was reactive beginning at BioRBC-18 (2+ reactivity) and two more became first reactive at BioRBC-54 (2+ and 1+ reactivity). Combined with the 2 healthy naïve hospitalized subjects demonstrating preexisting reactivity at BioRBC-162, the pooled reactive rate for naïve samples tested at either BioRBC-140 or BioRBC-162 was 0.5% (6 of 1,125 plasma samples).
Infants: Reactivity tested using multiple densities of reagent BioRBC
In contrast to naïve adults, none of 83 naïve VLBW infant study subjects demonstrated plasma reactivity using reagent BioRBC-54. A subset of 46 of these 83 naïve neonates had sufficient plasma for additional testing at BioRBC-162 and BioRBC-1458; none were reactive.
Characterization of binding specificity of anti-BioRBC antibody
Agglutination results of RBC labeling using DNP-RBC as the reagent cells
The high titer positive control plasma caused the BioRBC to agglutinate in a dose dependent fashion, i.e., the higher density BioRBC produced greater agglutination. The DNP-RBC did not produce any agglutination at reagent densities up to a DNP density equivalent to BioRBC-486.
Hemagglutination neutralization (inhibition) by biotin, biocytin, and biotinylated proteins
Using the gel card agglutination inhibition assay, induced anti-BioRBC antibodies from Subjects 521, 526, and 528 were not neutralized by 16μM biotin in solution; additional testing of plasma from Subject 521 also showed no neutralization by 8μM biocytin or biotin attached to albumin or gelatin. In contrast, of the four hospitalized naïve subjects with preexisting anti-BioRBC antibodies who were reactive at BioRBC≤140 or below, all were neutralized with biotinylated albumin and biotinylated gelatin. Three of these were neutralized with biotin and one was not. Similarly, three were neutralized with biocytin and one was not. Of the 29 naïve, healthy, non-hospitalized subjects who exhibited plasma reactivity at reagent BioRBC≥162 and who had sufficient plasma for additional testing, reactivity sometimes depended on the donor providing the BioRBC. When the plasma reactivity could be reproduced, neutralization was observed with most of the biotin or biotin containing compounds.
Adsorption of anti-BioRBC antibody by prior exposure to BioRBC
Reactive plasma samples from naïve (n=5) and BioRBC exposed subjects (n=2) demonstrated a dose dependent reduction—or elimination—of gel card reactivity following incubation with increasing volumes of BioRBC-1458.
DISCUSSION
Using commercial gel cards, we developed, characterized, and applied an assay for detecting plasma anti-BioRBC antibodies in humans. This is important because of the limitations of RBC survival measurements using radioactive 51Cr labeling that include variable rates of 51Cr RBC elution and radiation exposure.3,7 Here we present evidence that a BioRBC-adapted commercial gel card agglutination assay provides a more simple, more rapid, more reliable, less subjective, and more sensitive means for assessing induced anti-BioRBC immune responses relative to the traditional tube agglutination method.8 This method can also detect preexisting anti-BioRBC antibodies among naïve subjects. Employment of pre-screening and follow up monitoring for their exclusion from initial and subsequent RBC kinetic studies respectively will help reduce the chance of artifactually reducing BioRBC survival, thereby enhancing the validity of RBC kinetic determinations.
Based on these findings, we speculate that BioRBC transfusion-induced anti-BioRBC antibodies are the result of the covalent modification of biotinylated RBC surface protein amino acid (primarily lysine) residues. This enhances RBC immunogenicity21,22 similar to alloimmunization of intrinsic RBC antigens.9 Our DNP experiments suggest that induced anti-BioRBC antibodies are highly specific to the biotin moieties and that modified RBC surface proteins are not in general epitopes for these antibodies. In screening potential participants for exclusion from RBC kinetic studies, we recommend that a gel card BioRBC-256 reagent density be used. This recommendation is based on achieving adequate sensitivity to identify all three subjects in whom plasma anti-BioRBC antibody was detectable and on the observation that subjects testing positive pre-BioRBC dosing at gel card reagent BioRBC >256μg/mL were not more likely to later develop induced antibodies. The recommendation of BioRBC-256 for screening may ultimately prove to be higher than necessary. This is based on too few—only three—subjects who developed anti-BioRBC antibodies, often with insufficient available plasma for confirmatory testing and with no plasma available >20wk post-BioRBC dosing in some subjects (Table 3).
An important finding of the present study was the significant association of BioRBC dose with induction of anti-BioRBC antibodies. While based only on three subjects who developed antibodies, none of the 18 subjects dosed with BioRBC-6 or BioRBC-18 developed antibodies. This dose dependence is mechanistically plausible based on other pre-clinical23 and clinical studies24,25 of RBC in which increasing density of RBC surface antigens increases the likelihood of developing alloimmunization once a threshold is exceeded. Thus, a lower total dose of biotin label, or a lower density of biotin label per RBC, may reduce the immunogenic potential.
Here we report antibody specificity and timing of sensitization post-BioRBC exposure. Both preexisting and induced anti-BioRBC antibodies were characterized both by neutralization of hemagglutination using soluble biotin and structurally related compounds and also by antibody adsorption using reagent BioRBCs. Plasma samples from BioRBC naïve subjects could be neutralized by soluble biotin, biocytin, biotinylated albumin, and biotinylated gelatin. This was in contrast to most BioRBC-induced reactive plasmas which could not, thus suggesting that there are structural difference in their typical epitopes. We speculate that pre-existing and induced anti-BioRBC antibodies bind to the biotin moieties per se, but that at least some induced BioRBC antibodies appear to recognize an additional epitope (perhaps the linker or the biotinylated amino acid residues) and thus still bind to BioRBC causing hemagglutination (i.e., gel card reactivity) despite prior incubation with biotin, biocytin, or a biotinylated protein. Yet the induced anti-BioRBC antibodies did not bind to RBC derivatized with the same linker but in which the biotin was replaced with DNP. While we speculate that the linker alone is not a strong enough epitope to bind anti-BioRBC antibody, steric hindrance from DNP cannot be ruled out. The origin of the preexisting anti-BioRBC antibody we identified is unknown. Possibly these individuals may have had prior environmental or dietary exposure to biotin or a related compound to invoke an immune response.
Notwithstanding these limitations, the present study provides indirect evidence that use of BioRBC is safe and accurate for assessing human RBC kinetics. This interpretation of safety rests primarily on lack of laboratory or clinical adverse effects evidenced by: 1) induced anti-BioRBC antibody production following initial BioRBC exposure has had no detectable effect on hematologic parameters or on biotin nutritional status;10 and 2) clinical features of hemolytic reactions have not been seen—presumably because the volumes of BioRBC transfused for RBC kinetic studies are small (~0.40mL of BioRBC/kg body weight) and unlikely to cause a clinically significant transfusion reaction, even if the entire BioRBC transfusion was acutely hemolyzed. This inference is supported by copious data, including mistaken transfusion of ABO incompatible RBC, and the presence of RBC incompatibility/mismatch in the setting of allogeneic peripheral blood stem cell transplantation.26 Because premature infants lacked naturally existing anti-BioRBC antibodies and not develop induced anti-BioRBC antibodies, safety issues in this study population appear even less concerning. Regarding the determination of RBC volume and short- and long-term RCS, induced anti-BioRBC antibody does not affect the accuracy of initial RBC kinetic studies. This is likely attributable to anti-BioRBC antibodies not appearing until 3–4mo post-dosing.5,8
We conclude that the performance of the gel card agglutination assay makes it preferable to its predecessor, the tube agglutination assay, for detecting plasma anti-BioRBC antibodies. The gel card assay is more sensitive, less operator dependent, and can be performed using readily available commercial reagents and equipment.27 In applying the gel card assay in detecting anti-BioRBC antibodies in population BioRBC kinetic studies, our current sensitivity and specificity based recommendation is to use a reagent density of BioRBC≤256. Because few adult study subjects had preexisting or induced anti-BioRBC antibodies had limited sample volume, the association of anti-BioRBC antibody induction and low biotin dose of the transfused BioRBC must be viewed as tentative. If confirmed, this putative association between BioRBC dose and anti-BioRBC antibody induction implies that dosing with low total BioRBC dose or density will reduce—or perhaps eliminate—this antibody induction. Because we predict that application of the population biotin RBC labeling method will advance transfusion medicine and enhance understanding of hematological disorders and RBC physiology, we speculate that development of the gel card assay will be particularly useful.
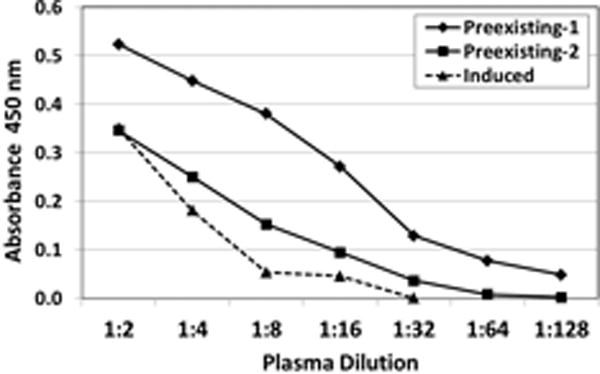
Figure 1.
Acknowledgments
We acknowledge the critiques of Sean R. Stowell, M.D., Ph.D., and Lawrence Corash, M.D., and the statistical input of M. Bridget Zimmerman, Ph.D. Melissa von Goetz and Paramjit Khera PhD provided technical help and laboratory expertise. We also acknowledge the contributions of the neonatal nurses at Iowa including Gretchen Cress, Karen Johnson, Laura Knosp, Ruthann Schrock, Sara Scott, and Jin Zhou and the Cincinnati VA Clinical Research Unit M, Colleen Rogge, Brenda Wenstrup, and Faye Hailes. Mark Hart provided editorial assistance.
Statement of Financial Support
The work was supported by NIH Program Project Grant P01 HL046925. This work also received support from the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH) (Grant 2 UL1 TR000442-06 and S10 OD016199-01A1 grant UL1TR000039), as well as VA Merit Award (1 I01 CX000121), and NIH Clinical and Translational Science Award (CTSA) program (1UL1 TR001425). The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Conflict of Interest Statements:
Robert L. Schmidt: None.
- Consultant Medday Pharmaceuticals
Robert S. Franco: None.
Robert M. Cohen: None.
- Employee Cerus Corporation, 2550 Stanwell Drive, Concord CA 94520, USA
- None for companies. Research support from NIH, US Dept. of Defense, Leukemia & Lymphoma Society of North America, William & Blanche Lawrence Hughes Foundation, Cerus Co., Terumo BCT, Zimmet Biomed., Cellphyre, Inc., and New Health Sciences, Inc.
- Unpaid advisory committee New Health Sciences, Inc.
Christof Geisen: None.
Ronald G. Strauss: None.
Alexander P. Vlaar: None.
Demet Nalbant: None.
- Consultant HemoGenix (http://hemogenix.com)
- Loan agreement Sysmex America, Inc., Hematology Analyzer
References
- 1.Recommended method for radioisotope red-cell survival studies. International Committee for Standardization in Haematology. Br J Haematol. 1980;45:659–66. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 2.Diekema DS. Conducting ethical research in pediatrics: a brief historical overview and review of pediatric regulations. J Pediatr. 2006;149:S3–11. doi: 10.1016/j.jpeds.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Mock DM, Widness JA, Veng-Pedersen P, Strauss RG, Cancelas JA, Cohen RM, Lindsell CJ, Franco RS. Measurement of posttransfusion red cell survival with the biotin label. Transfus Med Rev. 2014;28:114–25. doi: 10.1016/j.tmrv.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–91. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters AL, Beuger B, Mock DM, Widness JA, de Korte D, Juffermans NP, Vlaar AP, van Bruggen R. Clearance of stored red blood cells is not increased compared with fresh red blood cells in a human endotoxemia model. Transfusion. 2016;56:1362–9. doi: 10.1111/trf.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock DM, Widness JA, Strauss RG, Franco RS. Posttransfusion red blood cell (RBC) survival determined using biotin-labeled RBCs has distinct advantages over labeling with (51) Cr. Transfusion. 2012;52:1596–8. doi: 10.1111/j.1537-2995.2012.03588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordle DG, Strauss RG, Lankford G, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–9. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 9.Petz LD, Garratty G, Petz LD. Drug-induced immune hemolytic anemia. In: Petz LD, Garratty G, editors. Immune Hemolytic Anemias. Philadelphia, Pa: Churchill Livingstone/Elsevier Science; 2004. pp. 261–317. [Google Scholar]
- 10.Stowell SR, North AK, Franco RS, Cancelas JA, Mock DM, Strauss RG, Geisen C, Schmidt RL, Nalbant D, Widness JA. Shortened survival of biotin-labeled RBCs following a second BioRBC transfusion in an adult with a previous BioRBC antibody response. Transfusion. 2014;54:52A. [Google Scholar]
- 11.Van ’t Erve TJ, Wagner BA, Martin SM, Knudson CM, Blendowski R, Keaton M, Holt T, Hess JR, Buettner GR, Ryckman KK, Darbro BW, Murray JC, Raife TJ. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015 doi: 10.1111/trf.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freise KJ, Widness JA, Veng-Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther. 2010;332:229–37. doi: 10.1124/jpet.109.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mock DM, Matthews NI, Zhu S, Burmeister LF, Zimmerman MB, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51:148–57. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalbant D, Bhandary P, Matthews NI, Schmidt RL, Bogusiewicz A, Cress GA, Zimmerman MB, Strauss RG, Mock DM, Widness JA. Comparison of multiple red cell volume methods performed concurrently in premature infants following allogeneic transfusion. Pediatr Res. 2013;74:592–600. doi: 10.1038/pr.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid P, Huvard MJ, Lee-Stroka AH, Lee JY, Byrne KM, Flegel WA. Red blood cell preservation by droplet freezing with polyvinylpyrrolidone or sucrose-dextrose and by bulk freezing with glycerol. Transfusion. 2011;51:2703–8. doi: 10.1111/j.1537-2995.2011.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brecher ME, Leger RM, Linden JV, Roseff SD, editors. Technical Manual. Bethesda, MD: American Association of Blood Banks; 2005. Methods Section 1: General Laboratory Methods; pp. 728–9. [Google Scholar]
- 17.Suzuki T, Dale GL. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987;70:791–5. [PubMed] [Google Scholar]
- 18.Widness JA, Nalbant D, Matthews NI, Strauss RG, Schmidt RL, Cress GA, Zimmerman MB, Mock DM. Tracking donor RBC survival in premature infants: agreement of multiple populations of biotin-labeled RBCs with Kidd antigen-mismatched RBCs. Pediatr Res. 2013;74:689–97. doi: 10.1038/pr.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(U.S.). NRC. Recommended dietary allowances. Washington, D.C.: National Academy Press; 1998. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, pantothenic acid, biotin, and choline. [PubMed] [Google Scholar]
- 20.Widness JA, Kuruvilla DJ, Mock DM, Matthews NI, Nalbant D, Cress GA, Schmidt RL, Strauss RG, Zimmerman MB, Veng-Pedersen P. Autologous Infant and Allogeneic Adult Red Cells Demonstrate Similar Concurrent Post-Transfusion Survival in Very Low Birth Weight Neonates. J Pediatr. 2015;167:1001–6. doi: 10.1016/j.jpeds.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogusiewicz A, Mock NI, Mock DM. A biotin-protein bond with stability in plasma. Anal Biochem. 2005;337:98–102. doi: 10.1016/j.ab.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Zempleni J, Mock DM. Chemical synthesis of biotinylated histones and analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis/streptavidin-peroxidase. Arch Biochem Biophys. 1999;371:83–8. doi: 10.1006/abbi.1999.1431. [DOI] [PubMed] [Google Scholar]
- 23.Stowell SR, Arthur CM, Smith NH, Zimring JC, Hendrickson JE. Impact of RBC copy number and dose on murine immune responses to transfused KEL RBCs. Transfusion. 2014;54:16A. [Google Scholar]
- 24.Daniels G. Variants of RhD–current testing and clinical consequences. Br J Haematol. 2013;161:461–70. doi: 10.1111/bjh.12275. [DOI] [PubMed] [Google Scholar]
- 25.Shao CP, Wang BY, Ye SH, Zhang WL, Xu H, Zhuang NB, Wu XY, Xu HG. DEL RBC transfusion should be avoided in particular blood recipient in East Asia due to allosensitization and ineffectiveness. J Zhejiang Univ Sci B. 2012;13:913–8. doi: 10.1631/jzus.B1100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowley SD. Hematopoietic stem cell transplantation between red cell incompatible donor-recipient pairs. Bone Marrow Transplant. 2001;28:315–21. doi: 10.1038/sj.bmt.1703135. [DOI] [PubMed] [Google Scholar]
- 27.AuBuchon JP, de Wildt-Eggen J, Dumont LJ, Biomedical Excellence for Safer Transfusion C, Transfusion Medicine Resource Committee of the College of American P Reducing the variation in performance of antibody titrations. Arch Pathol Lab Med. 2008;132:1194–201. doi: 10.5858/2008-132-1194-RTVIPO. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, pantothenic acid, biotin, and choline. In: Food and Nutrition Board Institute of Medicine, editor. Recommended Dietary Allowances. Washington, D.C.: National Academy Press; 1998. pp. 374–89. [Google Scholar]
- 29.National Research Council. Dietary Reference Intakes Tables – The Complete Set (PDF) [monograph on the internet] Washington, D.C.: The National Academies; 2005. Available from: http://www.nap.edu/ [Google Scholar]


