Abstract
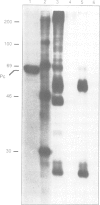
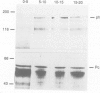
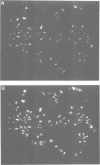
The polycomb group (Pc-G) genes are responsible for maintaining the repressed state of homeotic genes during development. It has been suggested that the Pc-G exerts its transcriptional control by regulating higher order chromatin structure. In particular, the finding of genetic and molecular similarities to components involved in heterochromatin formation, led to the proposal that homeotic genes are permanently repressed by mechanisms similar to those responsible for heterochromatin compaction. Because of synergistic effects, Pc-G gene products are thought to act in a multimeric complex. Using immunoprecipitation we show that two members of the Pc-G, Polycomb and polyhomeotic, are constituents of a soluble multimeric protein complex. Size fractionation indicates that a large portion of the two proteins are found in a distinct complex of molecular weight 2-5 x 10(6) Da. During embryogenesis the two proteins show the same spatial distribution. In addition, by double-immunofluorescence labelling we can demonstrate that Polycomb and polyhomeotic have exactly the same binding patterns on polytene chromosomes of larval salivary glands. We propose that some Pc-G proteins act in multimeric complexes to compact the chromatin of stably repressed genes like the homeotic regulators.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N., Charlton J., Brunk B. Genetic interactions of the suppressor 2 of zeste region genes. Dev Genet. 1989;10(3):249–260. doi: 10.1002/dvg.1020100314. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Blix P. M., Rubenstein A. H., Tager H. S. Iodotyrosylation of peptides using tertiary-Butyloxycarbonyl-L-[125I]iodotyrosine N-hydroxysuccinimide ester. Anal Biochem. 1980 Mar 15;103(1):70–76. doi: 10.1016/0003-2697(80)90238-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breen T. R., Duncan I. M. Maternal expression of genes that regulate the bithorax complex of Drosophila melanogaster. Dev Biol. 1986 Dec;118(2):442–456. doi: 10.1016/0012-1606(86)90015-1. [DOI] [PubMed] [Google Scholar]
- Brunk B. P., Martin E. C., Adler P. N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991 Sep 26;353(6342):351–353. doi: 10.1038/353351a0. [DOI] [PubMed] [Google Scholar]
- Busturia A., Morata G. Ectopic expression of homeotic genes caused by the elimination of the Polycomb gene in Drosophila imaginal epidermis. Development. 1988 Dec;104(4):713–720. doi: 10.1242/dev.104.4.713. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J. E., García-Bellido A. Interactions of Polycomb and trithorax with cis regulatory regions of Ultrabithorax during the development of Drosophila melanogaster. EMBO J. 1990 Dec;9(13):4267–4275. doi: 10.1002/j.1460-2075.1990.tb07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Sharma S., Keelan D. J., Lewis E. B. The molecular genetics of the bithorax complex of Drosophila: cis-regulation in the Abdominal-B domain. EMBO J. 1990 Dec;9(13):4277–4286. doi: 10.1002/j.1460-2075.1990.tb07876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- DeCamillis M., Cheng N. S., Pierre D., Brock H. W. The polyhomeotic gene of Drosophila encodes a chromatin protein that shares polytene chromosome-binding sites with Polycomb. Genes Dev. 1992 Feb;6(2):223–232. doi: 10.1101/gad.6.2.223. [DOI] [PubMed] [Google Scholar]
- Deatrick J., Daly M., Randsholt N. B., Brock H. W. The complex genetic locus polyhomeotic in Drosophila melanogaster potentially encodes two homologous zinc-finger proteins. Gene. 1991 Sep 15;105(2):185–195. doi: 10.1016/0378-1119(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Denell R. E., Frederick R. D. Homoeosis in Drosophila: a description of the Polycomb lethal syndrome. Dev Biol. 1983 May;97(1):34–47. doi: 10.1016/0012-1606(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Duncan I. M. Polycomblike: a gene that appears to be required for the normal expression of the bithorax and antennapedia gene complexes of Drosophila melanogaster. Genetics. 1982 Sep;102(1):49–70. doi: 10.1093/genetics/102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura J. M., Brock H. W., Santamaria P. Polyhomeotic: a gene of Drosophila melanogaster required for correct expression of segmental identity. Mol Gen Genet. 1985;198(2):213–220. doi: 10.1007/BF00382998. [DOI] [PubMed] [Google Scholar]
- Dura J. M., Ingham P. Tissue- and stage-specific control of homeotic and segmentation gene expression in Drosophila embryos by the polyhomeotic gene. Development. 1988 Aug;103(4):733–741. doi: 10.1242/dev.103.4.733. [DOI] [PubMed] [Google Scholar]
- Dura J. M., Randsholt N. B., Deatrick J., Erk I., Santamaria P., Freeman J. D., Freeman S. J., Weddell D., Brock H. W. A complex genetic locus, polyhomeotic, is required for segmental specification and epidermal development in D. melanogaster. Cell. 1987 Dec 4;51(5):829–839. doi: 10.1016/0092-8674(87)90106-1. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C., James T. C., Foster-Hartnett D. M., Hartnett T., Ngan V., Elgin S. C. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C. Position effect variegation in Drosophila: towards a genetics of chromatin assembly. Bioessays. 1989 Jul;11(1):14–17. doi: 10.1002/bies.950110105. [DOI] [PubMed] [Google Scholar]
- Frasch M. The maternally expressed Drosophila gene encoding the chromatin-binding protein BJ1 is a homolog of the vertebrate gene Regulator of Chromatin Condensation, RCC1. EMBO J. 1991 May;10(5):1225–1236. doi: 10.1002/j.1460-2075.1991.tb08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt S. J., Singh P. B. Homeogene expression patterns and chromosomal imprinting. Trends Genet. 1990 Jul;6(7):208–212. [PubMed] [Google Scholar]
- Gay N. J., Poole S., Kornberg T. Association of the Drosophila melanogaster engrailed protein with specific soluble nuclear protein complexes. EMBO J. 1988 Dec 20;7(13):4291–4297. doi: 10.1002/j.1460-2075.1988.tb03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. Nucleosomes: regulators of transcription. Trends Genet. 1990 Dec;6(12):395–400. doi: 10.1016/0168-9525(90)90299-l. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Position-effect variegation after 60 years. Trends Genet. 1990 Dec;6(12):422–426. doi: 10.1016/0168-9525(90)90304-o. [DOI] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. A gene that regulates the bithorax complex differentially in larval and adult cells of Drosophila. Cell. 1984 Jul;37(3):815–823. doi: 10.1016/0092-8674(84)90416-1. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Gelbart W. M. Genetic analysis of the enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics. 1990 Sep;126(1):185–199. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman T. C., Lewis R., Wakimoto B. Cytogenetic Analysis of Chromosome 3 in DROSOPHILA MELANOGASTER: The Homoeotic Gene Complex in Polytene Chromosome Interval 84a-B. Genetics. 1980 Jan;94(1):115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison J. A., Russell M. A. Dosage-Dependent Modifiers of Homoeotic Mutations in Drosophila melanogaster. Genetics. 1987 May;116(1):75–86. doi: 10.1093/genetics/116.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci E., Garzino V., Mary C., Bennani N., Pradel J. Modulo, a new maternally expressed Drosophila gene encodes a DNA-binding protein with distinct acidic and basic regions. Nucleic Acids Res. 1989 Oct 25;17(20):8101–8115. doi: 10.1093/nar/17.20.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. Different transcripts of the Drosophila Abd-B gene correlate with distinct genetic sub-functions. EMBO J. 1988 Oct;7(10):3233–3244. doi: 10.1002/j.1460-2075.1988.tb03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Locke J., Kotarski M. A., Tartof K. D. Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effect. Genetics. 1988 Sep;120(1):181–198. doi: 10.1093/genetics/120.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986 Dec 11;324(6097):537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- Müller J., Bienz M. Long range repression conferring boundaries of Ultrabithorax expression in the Drosophila embryo. EMBO J. 1991 Nov;10(11):3147–3155. doi: 10.1002/j.1460-2075.1991.tb04876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M., Yoshida T., Seino H., Nishitani H., Clark K. L., Sprague G. F., Jr, Frasch M., Nishimoto T. Mutation of the hamster cell cycle gene RCC1 is complemented by the homologous genes of Drosophila and S.cerevisiae. EMBO J. 1991 May;10(5):1265–1273. doi: 10.1002/j.1460-2075.1991.tb08068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R., Hogness D. S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990 Dec;6(12):416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- Pearce J. J., Singh P. B., Gaunt S. J. The mouse has a Polycomb-like chromobox gene. Development. 1992 Apr;114(4):921–929. doi: 10.1242/dev.114.4.921. [DOI] [PubMed] [Google Scholar]
- Reuter G., Giarre M., Farah J., Gausz J., Spierer A., Spierer P. Dependence of position-effect variegation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature. 1990 Mar 15;344(6263):219–223. doi: 10.1038/344219a0. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Sato T., Denell R. E. Homoeosis in Drosophila: anterior and posterior transformations of Polycomb lethal embryos. Dev Biol. 1985 Jul;110(1):53–64. doi: 10.1016/0012-1606(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Simcox A. A., Hersperger E., Shearn A., Whittle J. R., Cohen S. M. Establishment of imaginal discs and histoblast nests in Drosophila. Mech Dev. 1991 Mar;34(1):11–20. doi: 10.1016/0925-4773(91)90087-m. [DOI] [PubMed] [Google Scholar]
- Sinclair D. A., Campbell R. B., Nicholls F., Slade E., Brock H. W. Genetic analysis of the additional sex combs locus of Drosophila melanogaster. Genetics. 1992 Apr;130(4):817–825. doi: 10.1093/genetics/130.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. B., Miller J. R., Pearce J., Kothary R., Burton R. D., Paro R., James T. C., Gaunt S. J. A sequence motif found in a Drosophila heterochromatin protein is conserved in animals and plants. Nucleic Acids Res. 1991 Feb 25;19(4):789–794. doi: 10.1093/nar/19.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smouse D., Goodman C., Mahowald A., Perrimon N. polyhomeotic: a gene required for the embryonic development of axon pathways in the central nervous system of Drosophila. Genes Dev. 1988 Jul;2(7):830–842. doi: 10.1101/gad.2.7.830. [DOI] [PubMed] [Google Scholar]
- Smouse D., Perrimon N. Genetic dissection of a complex neurological mutant, polyhomeotic, in Drosophila. Dev Biol. 1990 May;139(1):169–185. doi: 10.1016/0012-1606(90)90286-r. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Struhl G. A gene product required for correct initiation of segmental determination in Drosophila. Nature. 1981 Sep 3;293(5827):36–41. doi: 10.1038/293036a0. [DOI] [PubMed] [Google Scholar]
- Struhl G., Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985 Dec 1;4(12):3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D., Bremer M. Mechanisms for the construction and developmental control of heterochromatin formation and imprinted chromosome domains. Dev Suppl. 1990:35–45. [PubMed] [Google Scholar]
- Tiong S. Y., Russell M. A. Clonal analysis of segmental and compartmental homoeotic transformations in polycomb mutants of Drosophila melanogaster. Dev Biol. 1990 Oct;141(2):306–318. doi: 10.1016/0012-1606(90)90387-x. [DOI] [PubMed] [Google Scholar]
- Wedeen C., Harding K., Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986 Mar 14;44(5):739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Jones R. S., Lasko P. F., Gelbart W. M. Homeosis and the interaction of zeste and white in Drosophila. Mol Gen Genet. 1989 Sep;218(3):559–564. doi: 10.1007/BF00332424. [DOI] [PubMed] [Google Scholar]
- Zink B., Engström Y., Gehring W. J., Paro R. Direct interaction of the Polycomb protein with Antennapedia regulatory sequences in polytene chromosomes of Drosophila melanogaster. EMBO J. 1991 Jan;10(1):153–162. doi: 10.1002/j.1460-2075.1991.tb07931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink B., Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989 Feb 2;337(6206):468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Frasch M., Wientjens E., Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991 Sep 26;353(6342):353–355. doi: 10.1038/353353a0. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Scheijen B., Wientjens E., van der Gulden H., Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991 May 31;65(5):737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]