Abstract
Background
Functional dyspepsia (FD) refers to a group of upper gastrointestinal syndromes, subdivided into two types: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS). The etiology of FD remains unclear; however, unhealthy dietary habit is one potential underlying cause. We aim to explore the association of poor dietary habits with FD and its subtypes.
Material/Methods
A validated epidemiological questionnaire was designed to investigate dietary habits and gastrointestinal syndromes. Citizens in the Baotun community of Dongguan were invited to complete the study questionnaire. All participants were asked to undergo a physical examination and a blinded physician interview. The study was conducted from June 2015 to June 2016. FD was diagnosed using ROME III criteria. The association between investigated dietary habits and dyspeptic symptoms were explored.
Results
There were 1,304 adult residents recruited for the study survey; 165 residents had existing organic dyspepsia (OD), 203 residents were diagnosed with FD, and the other 936 participants, who were without dyspeptic symptoms or functional gastrointestinal diseases, were regarded as the control group. Subtype diagnosis indicated 61 participants had EPS, 66 participants had PDS, and 76 participants had coexisting EPS and PDS. Unhealthy dietary habits were more prevalent in the FD group than in the control groups (75.86% versus 37.50%; p<0.001). FD was found to be associated with irregular mealtime, dining out, fatty food, sweet food, and coffee (p<0.05). The impact of each dietary factor varied with FD subtypes.
Conclusions
Certain types of dietary habits were positively correlated with the prevalence of FD. FD subtypes showed relatively different associations with dietary factors.
MeSH Keywords: Abdominal Pain, Dyspepsia, Food Habits
Background
Functional dyspepsia (FD) is one of the most common functional gastrointestinal disorders (FGID). The global prevalence of FD, based on ROME III definitions, has been reported to be 5.4–21.9% [1–6]. Based on ROME III criteria, FD is characterized by continuous upper gastrointestinal discomforts with no underlying organic or biochemical abnormalities [7]. Predominant dyspeptic symptoms subdivide FD into two types: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) [8]. In addition, previous clinical observations have found that EPS and PDS frequently coexisted in FD patients, suggesting that this overlap of types might need to be independently considered [9]. Regarding the course of disease, FD is not a life-threatening disease. Patients usually do not initially seek medical care, but their quality of life will be considerably impaired during the development of the disease. Eventually, a high proportion of patients will seek clinical consultation and the condition can negatively affect attendance and productivity in the workplace, posing a high global burden on medical and social resources [10].
It is generally assumed that FD is a heterogeneous condition with different underlying mechanisms contributing to the symptom patterns [11,12]. Numerous factors are suspected to induce FD, such as mental pressure, Helicobacter pylori (H. pylori) infection, and irritable bowel syndrome, as well as diet [8]. Previous studies observed impaired gastric accommodation in FD patients, which generated abdominal discomfort and pain by spurring tension-sensitive mechanoreceptors in the proximal stomach [13]. When compared to normal volunteers, FD patients showed enhanced visceral sensitivity to distension after a meal [14]. Furthermore, activation of low-grade immune system response and dysregulation of the gut-brain axis have also been proposed [15,16]. Still, those relationships have not been clearly validated. The heterogeneity in both etiology and pathophysiological mechanisms are likely to affect efficacy of therapeutic interventions. Thus, for the purpose of identifying an effective solution for FD, studies on the mechanism of FD development are urgently needed.
As a candidate for inducing FD, diet still lacks systematic research. Dietary factors mainly consist of different food types (meat, vegetables and soybeans) and different dietary patterns (frequency of daily meals, regular time of meals, and speed of chewing meals). FD patients frequently complain that their symptoms are related to or exacerbated by diet, and that they are able to tolerate only small amounts of food at one meal [17]. The majority of previous studies found ubiquitous food intolerance in FD patients [18–20]. Several food types and dietary patterns potentially are related to the induction of FD [18,21]. Studies suggest that diet might play role in the generation of FD. However, this association has been insufficiently verified; numerous studies that investigated dietary factors have shown conflicting epidemiology study results. FD has become an important public health problem all around the globe. Diet is a potential underlying factor, and is closely related to daily routines and thus easily accessible as a medical intervention. Therefore, comprehensive studies on the association between diet and FD are needed.
The present study was carried out in the Baotun community of the Houjie township, Dongguan city, Guangdong province, China. The aim of the study was to increase the understanding of the link between dyspeptic symptoms and dietary behaviors (consumption of certain food types and dietary patterns) in FD. The initial purpose was to systematically investigate the prevalence of FD and unhealthy dietary habits within the Baotun community. A secondary purpose was to explore the association of certain dietary factors with the prevalence of FD and its subtypes.
Material and Methods
Ethic statement
This study was approved by the Institutional Review Board Committee at Sun Yat-Sen Memorial Hospital. Written informed consent was obtained from each participant before their enrollment in the study.
FD symptoms and dietary habits survey
The survey was performed in the Baotun community, Houjie township, Dongguan city, Guangdong province, China. Our study initially investigated the prevalence of FD in the local community. We also retrospectively investigated the dietary habits of FD patients. The population data from the local administrative department affirmed that 1,809 permanent residents lived in the local community. Residential members enrolled in survey received detailed evaluation of digestive symptoms and dietary habits. Trained data collectors performed face-to-face interviews to obtain the study information. With consent from participants, detailed medical and medication history were acquired from the local community hospital, from previous medical records, and from clinical consultants. The overall data collection was performed from June 2015 to June 2016.
Questionnaire
The questionnaire was modified from a previous community study [22]. It consisted of four parts: 1) basic demographics of the participants: age, gender, native place, and occupation; 2) gastrointestinal symptoms: investigated dyspeptic symptoms were modified from ROME III criteria into the Chinese language, including postprandial fullness after regular sized meal, early satiation following ordinary diet, upper abdominal pain, and upper abdominal burning. Other common gastrointestinal symptoms, reflux and nausea, were also included; 3) dietary behaviors in the past two months: a) diet patterns (frequency standard: more than three times per week): irregular mealtime (at least one hour later than the normal meal time: 7: 00–9: 00 for breakfast; 11: 00–13: 00 for lunch; 17: 00–19: 00 for dinner), skipping breakfast (not having meal until 11: 00), having night snacks constantly (except for dinner; intake extra meal after dinner), dining out frequently (prefer to have meal in restaurant rather than cook by oneself); b) preferences of food (frequency standard: at least three times per week): having raw food or drinking unboiled water, preferring fatty food, preferring spicy food, drinking coffee regularly, drinking tea frequently, and addiction to alcohol; and 4) medical and medication history within past two months: chronic illnesses such as hypertension, diabetes, and strokes were included. Drugs with potential gastrointestinal side effects, such as anti-inflammatory drugs, acid-inhibitory drugs, or other types of drugs were included. A small scale pilot of the questionnaire survey was tested in advance to make sure the investigated sections were popular and easy to understand.
Health data evaluation
All the citizens who finished the questionnaires were subsequently invited to a series of clinical tests: 1) clinical consultant: detailed medical history collection and physical examination were initially implemented; 2) laboratory examinations: clinical tests consisted of peripheral blood tests (blood glucose, liver and renal biochemical test, serum tumor markers), routine urine test, and routine fecal examinations (including obscure bleeding tests, bacteria, and parasite antigens detection); 3) endoscopy: gastroscope and entreoscope were performed to detect underlying structural or organic diseases in gastrointestinal tract; 4) imaging tests: abdominal plain film and abdominal ultrasound inspection were used to evaluate gastrointestinal tract and urinary system; 5) electrocardiogram: electrocardiogram was used to screen cardiac diseases which might confuse the diagnosis of upper gastrointestinal diseases with panel test or Doppler echocardiography conducted if necessary; 6) noninvasive H. pylori tests: H. pylori antigen detection was introduced to diagnose H. pylori infection; stool sample was obtained from each subject for the detection of H. pylori antigen, and the detection kit (Certest, Spain) was used according to the manufacturer’s instructions.
Enrollment and exclusion criteria
Inclusion and exclusion criteria were applied. Inclusion criteria: all adult citizens (≥18 years old) who resided in the Baotun community for more than two years were recruited into our study. Exclusion criteria: 1) residents with unfinished questionnaires or who denied clinical examination; 2) female participants during pregnancy or lactation.
Diagnosis of FD and its subtypes
The diagnosis of FD and its subtypes was based on the ROME III criteria [23]. The diagnostic criteria for FD and its subtypes are displayed in Supplementary Tables 1 and Table 2.
Cases grouping
The feedback on questionnaires, collected medical histories, and clinical examinations were analyzed. For example, dyspeptic participants with organic or structural explanations were sorted into organic dyspepsia (OD). Except for the OD patients, participants were classified into two groups: the control group also referred to as the symptomless participants, in which the participants had no underlying organic diseases, systematic illnesses, or FD. The FD group consisted of participants who satisfied the diagnostic criteria for FD. Participants in the FD group were further subdivided into three subtype groups (PDS, EPS, and coexisting PDS and EPS).
Statistical analysis
To identify factors independently associated with dyspepsia, chi squared tests were used to explore the association of dietary factors or demographic variables (age and gender) with FD and the three subtypes. All dietary and demographic variables were then included in logistic regression (enter mode, without any elimination) with FD. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistics regression. Statistical analysis was conducted on SPSS version 20.0 (IBM Corporation, NY, USA). A p value <0.05 was considered as statistical significance, with all p values being two-tailed.
Results
Basic characteristics of the participants
The survey was conducted from June 2015 to June 2016. Of the 1,809 residents, 1,536 residents completed the questionnaires with a response rate of 84.91%. With the exception of the 232 respondents who did not met the study enrollment criteria or refused subsequent research, 1,304 participants were recruited and received a series of clinical tests. Serial supplementary tests showed 165 participants suffered from dyspeptic symptoms with structural or organic explanations and were diagnosed as OD. There were 936 volunteers who were free of organic diseases or FD (control group) and 203 patients were had FD on the basis of diagnosis criteria (FD group). Subtypes analysis found 61 EPS and 66 PDS cases, while 76 patients had coexisting EPS and PDS. Demographic data (age distribution and gender) were analyzed between the control group and the FD group. FD patients (38.40±11.03 years) were younger than symptomless participants (45.85±18.94 years) (p<0.01; Table 1). The gender distribution of FD and its subtypes were not distinct from the control group (p>0.05; Table 1).
Table 1.
Basic characters of FD group and control group.
| Characteristics | Total (n=1139) | Control (n=936) | FD (n=203) | EPS (n=61) | PDS (n=66) | EPS & PDS (n=76) | p Value* | p Value# | p Value@ | p Value& |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender, n (%) | 0.816 | 0.512 | 0.527 | 0.635 | ||||||
| Male | 568 (49.87) | 465 (49.68) | 103 (50.74) | 33 (54.10) | 30 (45.45) | 40 (52.63) | ||||
| Female | 571 (50.13) | 471 (50.32) | 100 (49.26) | 28 (45.90) | 36 (54.55) | 36 (47.37) | ||||
| Age years, mean (±SD) | 44.53 (18.01) | 45.85 (18.94) | 38.40 (11.03) | 42.51 (12.68) | 36.48 (9.85) | 36.76 (9.75) | <0.001 | 0.001 | <0.001 | <0.001 |
| Age (years), n (%) | ||||||||||
| <30 | 174 (13.34) | 135 (14.42) | 39 (19.08) | 8 (13.11) | 13 (19.7) | 18 (23.68) | ||||
| 30–39 | 411 (31.52) | 330 (35.26) | 81 (39.88) | 20 (32.79) | 33 (50.00) | 28 (36.84) | ||||
| 40–49 | 263 (20.17) | 203 (21.69) | 60 (29.48) | 20 (32.79) | 13 (19.7) | 27 (35.53) | ||||
| 50–59 | 80 (6.13) | 66 (7.05) | 14 (6.94) | 7 (11.48) | 5 (7.58) | 2 (2.63) | ||||
| 60–69 | 63 (4.83) | 57 (6.09) | 6 (2.89) | 4 (6.56) | 2 (3.03) | 0 (0) | ||||
| 70–79 | 25 (1.92) | 24 (2.56) | 1 (0.58) | 1 (1.64) | 0 (0) | 0 (0) | ||||
| ≥80 | 123 (9.43) | 121 (12.93) | 2 (1.16) | 1 (1.64) | 0 (0) | 1 (1.32) | ||||
FD – functional dyspepsia; PDS – postprandial distress syndrome; EPS – epigastric pain syndrome. p Value
Control vs. FD;
Control vs. EPS;
Control vs. PDS;
Control vs. EPS and PDS.
Etiology analysis for the dyspeptic patients
There were 368 participants who had confirmed dyspeptic symptoms. Based on clinical consultations and clinical examinations, 165 were diagnosed as OD and 203 were diagnosed as FD. Detailed etiologic causes for OD are summarized and displayed in Table 2. Data revealed 65 suffered from gastrointestinal structural diseases and 25 suffered from existing systematic diseases. Additionally, 75 patients regularly took drugs with underlying gastrointestinal side effects: anti-inflammatory drugs and acid-inhibitory drugs.
Table 2.
Etiology analysis for the OD patients.
| Etiology | Cases | Percentage (%) |
|---|---|---|
| Gastrointestinal structural diseases | 65 | 39.39 |
| Peptic ulcer | 45 | 27.27 |
| Duodenal ulcer | 36 | 21.82 |
| Gastric ulcer | 9 | 5.45 |
| Hepatitis | 5 | 3.03 |
| Enteritis | 15 | 9.09 |
| Systematic diseases | 25 | 15.15 |
| Cardiac diseases | 22 | 13.33 |
| Anemia | 3 | 1.82 |
| Drugs | 75 | 45.45 |
| Anti-inflammatory drugs | 60 | 36.36 |
| Acid-inhibitory drugs | 24 | 14.55 |
H. pylori prevalence in enrolled cases
The overall infection rate of H. pylori was 55.21% (720/1,304). Detailed infection rates in the control, FD, and OD groups were 51.92% (486/936), 57.64% (117/203), and 70.91% (117/165), respectively. Statistical analysis found no significant difference in infection rates between FD patients and symptomless participants (control group) (57.64% versus 51.92%, p=0.142). Conversely, the prevalence rate in OD patients was distinctly different from the control group (70.91% versus 51.92%, p<0.001) or FD group (70.91% versus 57.64%, p<0.001). Results of subgroups analysis were as following: EPS (59.02%, p=0.293 versus control group), PDS (50.00%, p=0.800 versus control group), and EPS and PDS (63.16%, p=0.073 versus control group). Hence, subgroup analysis on H. pylori infection was not conducted for the FD study.
Gastrointestinal symptoms patterns in FD subjects
The detailed occurrences of dyspeptic symptoms for each subtype are showed in Table 3. Subtype analysis showed that the vast majority of EPS patients had upper abdominal pain (91.80%, 59/61) while only a small proportion of them complained about upper abdominal burning (13.11%, 8/61). More PDS patients suffered from postprandial fullness (69.7%, 46/66) than early satiation (42.42%, 28/66). As for the patients with coexisting PDS and EPS, the most common symptoms were upper abdominal pain (92.11%, 70/76) and postprandial fullness (71.05%, 54/76).
Table 3.
Gastrointestinal symptoms in FD subgroups.
| Symptom, n (%) | EPS (n=61) | PDS (n=66) | EPS & PDS (n=76) | FD (n=203) |
|---|---|---|---|---|
| Upper abdominal pain | 56 (91.80) | 0 (0) | 70 (92.11) | 126 (62.07) |
| Upper abdominal burning | 8 (13.11) | 0 (0) | 18 (23.68) | 26 (12.81) |
| Early satiation | 0 (0) | 28 (42.42) | 39 (51.32) | 67 (33.00) |
| Postprandial fullness | 0 (0) | 46 (69.70) | 54 (71.05) | 100 (49.26) |
| Regurgitation | 11 (18.03) | 12 (18.18) | 16 (21.05) | 39 (19.21) |
| Nausea | 7 (11.48) | 3 (4.55) | 8 (10.53) | 18 (8.87) |
FD – functional dyspepsia; PDS – postprandial distress syndrome; EPS – epigastric pain syndrome.
General prevalence of poor dietary habits in FD and Control group
In total, 11 unhealthy dietary habits were investigated in the survey and found 75.86% of FD patients reported having poor dietary behaviors. By comparison, 37.50% of participants in the control group reported unhealthy behavior (p<0.001). From another perspective, the average number of targeted dietary behaviors in FD patients and symptomless volunteers were 2.05 and 0.70, respectively (p<0.001). The distribution of each factor between the control group and the FD group was analysis using the chi-square test (Table 4). All the aforementioned habits had significant differences between groups except for the preference for coffee (p=0.609). In line with the association of FD and diet, subtype analysis found similar positive associations with diet. However, night snacks and preference for tea showed no differences in the EPS subgroup (p=0.188 for night snacks; p=0.153 for tea). Alcohol addiction and tea preference had no significance difference between the PDS subgroup and the control group (p=0.112 for alcohol addiction; p=0.097 for tea). In the third subtype of FD, no statistic differences were found in skipping breakfast and preference for tea (p=0.084 for skipping breakfast; p=0.076 for tea).
Table 4.
Dietary patterns in control and FD group.
| Dietary behaviors n (%) | Control (n=936) | FD (n=203) | EPS (n=61) | PDS (n=66) | PDS & EPS (n=76) | p Value* | p Value# | p Value@ | p Value& |
|---|---|---|---|---|---|---|---|---|---|
| Irregular mealtime | 36 (3.85) | 52 (25.62) | 14 (22.95) | 19 (28.79) | 19 (25.00) | <0.001 | <0.001 | <0.001 | <0.001 |
| Night snacks | 63 (6.73) | 39 (19.21) | 7 (11.48) | 16 (24.24) | 16 (21.05) | <0.001 | 0.188 | <0.001 | <0.001 |
| Dining out | 30 (3.21) | 31 (15.27) | 11 (18.03) | 10 (15.15) | 10 (13.16) | <0.001 | <0.001 | <0.001 | <0.001 |
| Unprocessed water and food | 42 (4.49) | 28 (13.79) | 7 (11.48) | 8 (12.12) | 13 (17.11) | <0.001 | 0.025 | 0.013 | <0.001 |
| Skipping breakfast | 57 (6.09) | 40 (19.7) | 13 (21.31) | 18 (27.27) | 9 (11.84) | <0.001 | <0.001 | <0.001 | 0.084 |
| Fatty food | 51 (5.45) | 39 (19.21) | 11 (18.03) | 10 (15.15) | 18 (23.68) | <0.001 | 0.001 | 0.005 | <0.001 |
| Spicy food | 120 (12.82) | 58 (28.57) | 15 (24.59) | 16 (24.24) | 27 (35.53) | <0.001 | 0.018 | 0.014 | <0.001 |
| Alcohol addicted | 39 (4.17) | 25 (12.32) | 10 (16.39) | 6 (9.09) | 9 (11.84) | <0.001 | <0.001 | 0.112 | 0.007 |
| Tea | 51 (5.45) | 21 (10.34) | 6 (9.84) | 7 (10.61) | 8 (10.53) | 0.016 | 0.153 | 0.097 | 0.076 |
| Sweet food | 87 (9.29) | 46 (22.66) | 11 (18.03) | 19 (28.79) | 16 (21.05) | <0.001 | 0.042 | <0.001 | 0.003 |
| Coffee | 21 (2.24) | 6 (2.96) | 2 (3.28) | 2 (3.03) | 2 (2.63) | 0.609 | 0.647 | 0.660 | 0.689 |
FD – functional dyspepsia; PDS – postprandial distress syndrome; EPS – epigastric pain syndrome. p value:
Control vs. FD;
Control vs. EPS;
Control vs. PDS;
Control vs. EPS and PDS.
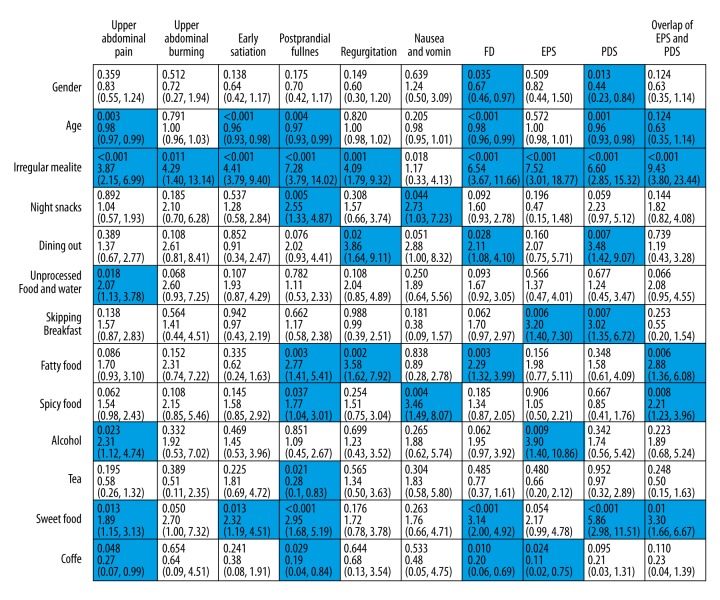
The associations between 13 variables and each digestive symptoms were independently analyzed (Figure 1). The results indicated younger age (p=0.003), irregular meal time (p<0.001), unprocessed food/water (p=0.018), alcohol addiction (p=0.023), sweet food (p=0.013), and coffee (p=0.048) were risk factors of upper abdominal pain. Dining irregularly (p=0.011) was the only factor found significant for upper abdominal burning. Age (p<0.001 for postprandial fullness; p=0.004 for early satiation), irregular mealtime (p<0.001 for postprandial fullness; p<0.001 for early satiation), and sweet food (p=0.013 for postprandial fullness; p<0.001 for early satiation) had statistical significance in postprandial fullness as well as early satiation. Moreover, night snacks (p=0.005), fatty food (p=0.003), spicy food (p=0.037), tea (p=0.021) and coffee (p=0.029) were the independent risk factors for postprandial fullness. For other dyspeptic symptoms, irregular mealtime (p=0.001), dining out (p=0.002), and fatty food (p=0.002) gained statistical meaningfulness in reflux symptom. Night snacks (p=0.044) and spicy food (p=0.004) were found as the candidates inducing nausea symptom.
Figure 1.
Logistics analysis between poor dietary habits and dyspepsia. The relationship of interested dietary habits with each dyspeptic symptom and FD subtypes were explored by binary logistics analysis. The results were noted in cells. In each cell, the first line were p value followed by OR and 95% CI. A p value <0.05 was considered as statistical significance. Those cells with positive results were stained with blue strip.
Next, the associations with multiple dietary factors and FD (and its subtypes) occurrence were analyzed (Figure 1). The analysis found females were more likely to suffer from FD (p=0.035). Residents at a younger age had more chances to develop FD (p<0.001). Certain unhealthy diet habits, dining irregularly (p<0.001), dining out (p=0.028), preference for fatty food (p=0.003), preference for sweet food (p<0.001), and preference for coffee (p=0.010) were indicated as risk factors of FD. Subtypes analysis showed pattern behavior of dining irregularly (p<0.001), skipping breakfast (p=0.006), addiction to alcohol (p=0.009), preference for coffee (p=0.024) were more likely to develop EPS. The development of PDS were gender- and age-related (p=0.013 for gender; p=0.001 for age). Moreover, irregular mealtime (p<0.001), dining out (p=0.007), skipping breakfast (p=0.007), and preference for sweet food (p<0.001) had positive association with PDS. For the overlap of EPS and PDS, younger citizens had more chances to suffer from the coexisting subtype (p=0.001). Dining irregularly (p<0.001), fatty food (p=0.006), spicy food (p=0.008), sweet food (p=0.001) were the potential candidates for the coexisting EPS and PDS subtype.
Discussion
Dyspepsia is a group of common gastrointestinal syndromes presenting upper abdominal pain, epigastric burning, early satiation, post postprandial fullness, and other upper gastroduodenal discomforts such as nausea and reflux. From an etiological viewpoint, dyspepsia can be subdivided into two categories: OD and FD. OD can be caused by benign or malignant digestive lesions, systematic syndromes, and metabolic diseases, as well as some types of drug use such as anti-inflammatory drugs and acid-inhibitory drugs. Conversely, FD is defined by recurrent or persistent symptoms originating in the gastroduodenal region with no structural or systematic abnormalities [8]. Epidemiological studies have indicated that the prevalence of FD varies from region to region and among different populations. In our survey, the occurrence of FD was estimated to be 15.57% (203/1,304) in the local community and accounted for 55.16% (203/368) of all dyspeptic patients. Our study discovered that the incidence of FD was slightly higher than that of OD (15.57% versus 12.65%) among the study enrolled residents. For the OD patients, peptic ulcer was the major cause of upper gastroduodenal symptoms, which indicated that prior exclusion of ulcerative diseases would be urgently required during dyspepsia diagnosis procedures. Moreover, chronic illnesses, such as cardiac diseases and anemia, could also be causes of dyspepsia syndromes. Additionally, medication was taken by 20.38% (75/368) of all dyspeptic patients (45.45% in the OD subgroup); medications were referred to as anti-inflammatory agents and acid-inhibitory drugs but the specific type of anti-inflammatory drug could not be verified as many people were unable to provide details. It is well recognized that nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common anti-inflammatory agents purchased over-the-counter in local pharmacies. Similarly, data on specific types of acid-inhibitory drug could not be collected. However, these results imply that medication history might have a potential association with dyspepsia. Hence, during clinic consultations with dyspepsia patients, these aforementioned aspects should be taken into consideration.
Even though several underlying explanations have been proposed, the origin and progression of FD remains unclear. Potential explanations include dysmotility and/or hypersensitivity in the upper gastrointestinal tract [24]. H. pylori infection, as a common cause of upper gastrointestinal inflammation, may also be considered. Recently, Vanhee et al. found impaired intestinal barrier and low-grade inflammation in FD cases [25]. Individuals who had a medical history of acute infectious gastroenteritis risk were more likely to develop FD [26]. Nevertheless, a population-based study concluded that H. pylori infection in FD patients did not exceed that found in healthy people [27]. In addition, H. pylori eradication only benefited a small proportion of FD patients [28,29]. Although the role of H. pylori in FD has been mired in controversy, immune mechanisms could be involved in H. pyloric infection and led to symptoms of dyspepsia [30]. Our present study showed slightly higher current infection rates of H. pylori in FD patients than in symptomless volunteers. But this difference was not statistically significant. Thus, it could not be concluded that H. pyloric infection had an adverse impact on FD prevalence. Furthermore, the intergroup analysis showed the H. pylori infection was the risk factor of OD compared to the FD group and the control group. In the future, specific investigations will be needed to probe the correlation between FD and H. pylori infection.
Dietary factors (which were the major concern of the present study) have been suspected to increase the risk of FD [31]. Previous studies have shown a potential relationship between gastrointestinal symptoms and certain types of food [32]. Reflecting from the occurrence and average amount of unhealthy dietary behaviors in our two study groups, our preliminary analysis indicated FD patients performed poorly in healthy diet behavior than symptomless volunteers. Our detailed investigation focused on certain types of dietary behavior which might influence the prevalence of FD. As aforementioned, dietary factors discussed in our survey mainly were composed of dietary patterns and types of food. The commonly reported unhealthy dietary patterns of dining irregularly, having night snacks, skipping breakfast, and dining out frequently were investigated. Numerous food types were reported as potential risk factors for FD-related symptoms. Our research concentrated on seven types of specific diets: fatty food, spicy food, sweet food, tea, alcohol, coffee, and unprocessed water/food that have been identified in the literature [19,20,31]. Spicy food, sweet snacks, and fatty food have been previously suspected as risk factors, while tea and coffee consumption have been reported to have a controversial relationship with FD [33–35]. Our study found that except for the preference for coffee, FD patients were more likely to report adverse dietary habits of interest than symptomless individuals (Table 4). Subsequent multivariate analysis supported the suggestion that irregular mealtime, dining out, intake of fatty food, sweet food, and preference for coffee were positively related to the prevalence of FD (Figure 1). Our present study concluded that unhealthy dietary habits were positively associated with FD prevalence. However, only several of the dietary factors were significantly regarded as risk factors. Moreover, two demographic factors, age and gender, were included in the multivariate analysis. FD prevalence was age-related and women were much more likely to have FD than men; which was in line with previous reports [33]. The FD prevalent tendencies based on gender differences have in general been poorly explored, and the mechanism of inter-gender differences requires more research.
Single dyspeptic symptoms unfolded different correlations with targeted dietary habits. Postprandial fullness was more likely associated with dietary factors. Up to seven of the investigated dietary behaviors had a positive association with postprandial fullness. By comparison, upper abdominal burning was only associated with irregular mealtime. The distinct differences in these results suggest that each dyspeptic symptom might be differently affected by multiple dietary factors.
Subtypes analysis of FD showed different symptom patterns between the three subgroups (Table 3). FD was diagnosed by predominant dyspeptic symptoms according to ROME III criteria. We observed that the coexistence of EPS and PDS was not rare in FD. Patients with this type of coexistence mostly complained about upper abdominal pain and postprandial fullness. Further analyses focused on certain dietary factors in FD subtypes. Comparing the FD group with its subtypes, we could find similar results in diet factors (Table 4). FD and its subtypes tended to behave unhealthier in each of the investigated diet behaviors, except for the preference for coffee. Our statistical analysis showed irregular mealtime was positively related to FD and its three subtypes. Night snacks, unprocessed food/water, and tea were not statistically meaningful in the FD group or any of the subtypes. These results suggest a similar influence of dietary factors on FD and its subtypes (Figure 1). However, FD and its subtypes showed differences in their associations with other dietary habits, suggesting the possible existence of inter-subtype differences. In order to determine different dietary effects on FD subtypes, further study of a larger sample capacity is required.
To determine the relationship between dietary habits and FD prevalence, more epidemiological research is urgently needed. Even though numerous factors may confound the correlations between diet and dyspeptic symptoms, our results suggest that some dietary behaviors are related to the prevalence of FD. This association needs more research to be validated. However, in clinical practice, dietary data may be beneficial to the investigation of dyspepsia.
Conclusions
We found FD commonly occurred in a southern China rural region. More FD patients had a history of poor dietary behaviors: dining irregularly, dining out, and preference for fatty food, sweet food, and coffee. FD subtypes were affected differently by dietary behaviors. Our research implicated unhealthy dietary habits as an independent risk factor of FD, although the actual association requires further epidemiological research. In addition, clinicians and public health advisers may want to pay attention to the influence of poor dietary habits in cases of FD.
Supplementary Tables
Supplementary Table 1.
Diagnostic criteria for FD.
| Diagnostic criteria for FD |
|
FD – functional dyspepsia.
Supplementary Table 2.
Diagnostic criteria for FD subtypes.
| A. Diagnostic criteria for PDS |
Must include one or both of the following symptoms
Supportive criteria Upper abdominal bloating or postprandial nausea or excessive belching can be present. |
| B. Diagnostic criteria for EPS |
Must include one or both of the following symptoms
|
| C. Diagnostic criteria for coexistence of EPS and PDS |
| Subjects fulfilled both PDS and EPS subtype criteria would be taken as overlap of EPS and PDS. |
FD – functional dyspepsia; PDS – postprandial distress syndrome; EPS – epigastric pain syndrome.
Acknowledgments
We would like to thank the Baotun community health station for providing health records.
Abbreviations
- FD
functional dyspepsia
- FGID
functional gastrointestinal disorder
- PDS
postprandial distress syndrome
- EPS
epigastric pain syndrome
- H. pylori
Helicobacter pylori
- OD
organic dyspepsia
- OR
odds ratio
- CI
confidence interval
- NSAIDs
nonsteroidal anti-inflammatory drugs
Footnotes
Source of support: This study was supported by National Natural Science Foundation of China (No. 81270442 and No. 81370475)
Conflict of interest
The authors declare no competing financial interests.
References
- 1.El-Serag HB, Talley NJ. Systemic review: The prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19(6):643–54. doi: 10.1111/j.1365-2036.2004.01897.x. [DOI] [PubMed] [Google Scholar]
- 2.Piessevaux H, De Winter B, Louis E, et al. Dyspeptic symptoms in the general population: A factor and cluster analysis of symptom groupings. Neurogastroenterol Motil. 2009;21(4):378–88. doi: 10.1111/j.1365-2982.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 3.Goh KL. Clinical and epidemiological perspectives of dyspepsia in a multiracial Malaysian population. J Gastroenterol Hepatol. 2011;26(Suppl 3):35–38. doi: 10.1111/j.1440-1746.2011.06648.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Jung HK, Huh KC. Current status of functional dyspepsia in Korea. Korean J Intern Med. 2014;29(2):156–65. doi: 10.3904/kjim.2014.29.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut. 2015;64(7):1049–57. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 6.Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the World. J Neurogastroenterol Motil. 2015;21(3):320–29. doi: 10.5056/jnm14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tack J, Talley NJ. Functional dyspepsia – symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10(3):134–41. doi: 10.1038/nrgastro.2013.14. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373(19):1853–63. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 9.Carbone F, Holvoet L, Tack J. Rome III functional dyspepsia subdivision in PDS and EPS: Recognizing postprandial symptoms reduces overlap. Neurogastroenterol Motil. 2015;27(8):1069–74. doi: 10.1111/nmo.12585. [DOI] [PubMed] [Google Scholar]
- 10.Sander GB, Mazzoleni LE, Francesconi CF, et al. Influence of organic and functional dyspepsia on work productivity: The HEROES-DIP study. Value Health. 2011;14(5 Suppl 1):S126–29. doi: 10.1016/j.jval.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis. 2014;32(3):222–29. doi: 10.1159/000357854. [DOI] [PubMed] [Google Scholar]
- 12.Clauwaert N, Jones MP, Holvoet L, et al. Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: Are all symptoms equal? Neurogastroenterol Motil. 2012;24(12):1088–e565. doi: 10.1111/j.1365-2982.2012.01985.x. [DOI] [PubMed] [Google Scholar]
- 13.Piessevaux H, Tack J, Wilmer A, et al. Perception of changes in wall tension of the proximal stomach in humans. Gut. 2001;49(2):203–8. doi: 10.1136/gut.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farré R, Vanheel H, Vanuytsel T, et al. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. 2013;145(3):566–73. doi: 10.1053/j.gastro.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Vanheel H, Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):142–49. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 16.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):158–67. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 17.Bisschops R, Karamanolis G, Arts J, et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. 2008;57(11):1495–503. doi: 10.1136/gut.2007.137125. [DOI] [PubMed] [Google Scholar]
- 18.Saito YA, Locke GR, 3rd, Weaver AL, et al. Diet and functional gastrointestinal disorders: A population-based case-Control study. Am J Gastroenterol. 2005;100(12):2743–48. doi: 10.1111/j.1572-0241.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 19.Filipović BF, Randjelovic T, Kovacevic N, et al. Laboratory parameters and nutritional status in patients with functional dyspepsia. Eur J Intern Med. 2011;22(3):300–4. doi: 10.1016/j.ejim.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho RV, Lorena SL, Almeida JR, et al. Food intolerance, diet composition, and eating patterns in functional dyspepsia patients. Dig Dis Sci. 2010;55(1):60–65. doi: 10.1007/s10620-008-0698-8. [DOI] [PubMed] [Google Scholar]
- 21.Karamanolis G, Tack J. Nutrition and motility disorders. Best Pract Res Clin Gastroenterol. 2006;20(3):485–505. doi: 10.1016/j.bpg.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Mak AD, Wu JC, Chan Y, et al. Dyspepsia is strongly associated with major depression and generalised anxiety disorder – a community study. Aliment Pharmacol Ther. 2012;36(8):800–10. doi: 10.1111/apt.12036. [DOI] [PubMed] [Google Scholar]
- 23.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 24.Moayyedi P. Dyspepsia. Curr Opin Gastroenterol. 2012;28(6):602–7. doi: 10.1097/MOG.0b013e328358ad9b. [DOI] [PubMed] [Google Scholar]
- 25.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63(2):262–71. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 26.Pike BL, Porter CK, Sorrell TJ, et al. Acute gastroenteritis and the risk of functional dyspepsia: A systematic review and meta-analysis. Am J Gastroenterol. 2013;108(10):1558–63. doi: 10.1038/ajg.2013.147. quiz 1564. [DOI] [PubMed] [Google Scholar]
- 27.Agréus L, Engstrand L, Svärdsudd K, et al. Helicobacter pylori seropositivity among Swedish adults with and without abdominal symptoms. A population-based epidemiologic study. Scand J Gastroenterol. 1995;30(8):752–57. doi: 10.3109/00365529509096323. [DOI] [PubMed] [Google Scholar]
- 28.Zullo A, Hassan C, De Francesco V, et al. Helicobacter pylori and functional dyspepsia: an unsolved issue? World J Gastroenterol. 2014;20(27):8957–63. doi: 10.3748/wjg.v20.i27.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sodhi JS, Javid G, Zargar SA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol. 2013;28(5):808–13. doi: 10.1111/jgh.12178. [DOI] [PubMed] [Google Scholar]
- 30.Fock KM. Functional dyspepsia, H. pylroi and post infectious FD. J Gastroenterol Hepatol. 2011;26(Suppl 3):39–41. doi: 10.1111/j.1440-1746.2011.06649.x. [DOI] [PubMed] [Google Scholar]
- 31.Keshteli AH, Feizi A, Esmaillzadeh A, et al. Patterns of dietary behaviours identified by latent class analysis are associated with chronic uninvestigated dyspepsia. Br J Nutr. 2015;113(5):803–12. doi: 10.1017/S0007114514004140. [DOI] [PubMed] [Google Scholar]
- 32.Hongo M. Epidemiology of FGID symptoms in Japanese general population with reference to life style. J Gastroenterol Hepatol. 2011;26(Suppl 3):19–22. doi: 10.1111/j.1440-1746.2011.06632.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahadeva S. Risk factors associated with dyspepsia in a rural Asian population and its impact on quality of life. Am J Gastroenterol. 2010;105(4):904–12. doi: 10.1038/ajg.2010.26. [DOI] [PubMed] [Google Scholar]
- 34.Jiang SM, Lei XG, Jia L, et al. Unhealthy dietary behavior in refractory functional dyspepsia: A multicenter prospective investigation in China. J Dig Dis. 2014;15(12):654–59. doi: 10.1111/1751-2980.12199. [DOI] [PubMed] [Google Scholar]
- 35.Saneei P, Sadeghi O, Feizi A, et al. Relationship between spicy food intake and chronic uninvestigated dyspepsia in Iranian adults. J Dig Dis. 2016;17(1):28–35. doi: 10.1111/1751-2980.12308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Diagnostic criteria for FD.
| Diagnostic criteria for FD |
|
FD – functional dyspepsia.
Supplementary Table 2.
Diagnostic criteria for FD subtypes.
| A. Diagnostic criteria for PDS |
Must include one or both of the following symptoms
Supportive criteria Upper abdominal bloating or postprandial nausea or excessive belching can be present. |
| B. Diagnostic criteria for EPS |
Must include one or both of the following symptoms
|
| C. Diagnostic criteria for coexistence of EPS and PDS |
| Subjects fulfilled both PDS and EPS subtype criteria would be taken as overlap of EPS and PDS. |
FD – functional dyspepsia; PDS – postprandial distress syndrome; EPS – epigastric pain syndrome.