Abstract
MRS 1754 [N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide] is a selective antagonist ligand of A2B adenosine receptors. This is the least well-defined adenosine receptor subtype, and A2B antagonists have potential as antiasthmatic drugs. For use as a radioligand, MRS 1754, a p-cyanoanilide xanthine derivative, was tritiated on the propyl groups in a two-step reaction using a p-carboxamido precursor, which was dehydrated to the cyano species using trifluoroacetic anhydride. [3H]MRS 1754 (150 Ci/mmol) bound to recombinant human A2B adenosine receptors in membranes of stably transfected HEK-293 cells. Specific binding was saturable, competitive, and followed a one-site model, with a KD value of 1.13 ± 0.12 nM and a Bmax value of 10.9 ± 0.6 pmol/mg protein. Specific binding utilizing 0.7 nM [3H]MRS 1754 was > 70% of total binding. The affinity calculated from association and dissociation binding constants was 1.22 nM (N = 4). Binding to membranes expressing rat and human A1 and A3 adenosine receptors was not significant, and binding in membranes of HEK-293 cells expressing human A2A receptors was of low affinity (KD > 50 nM). The effects of cations and chelators were explored. Specific binding was constant over a pH range of 4.5 to 6.5, with reduced binding at higher pH. The pharmacological profile in competition experiments with [3H]MRS 1754 was consistent with the structure–activity relationship for agonists and antagonists at A2B receptors. The Ki values of XAC (xanthine amine congener) and CPX (8-cyclopentyl-1,3-dipropylxanthine) were 16 and 55 nM, respectively. NECA (5′-N-ethylcarboxamidoadenosine) competed for [3H]MRS 1754 binding with a Ki of 570 nM, similar to its potency in functional assays. Thus, [3H]MRS 1754 is suitable as a selective, high-affinity radioligand for A2B receptors.
Keywords: G protein-coupled receptors, Tritium purines, Xanthines, Adenosine analogues
1. Introduction
Four extracellular G protein-coupled receptors for adenosine have been identified: A1, A2A, A2B, and A3 [1]. A2B receptors, which are coupled to stimulation of adenylyl cyclase [2, 3] and also lead to a rise in intracellular calcium [4], are involved in the control of vascular tone, cell growth and gene expression, mast cell degranulation, and intestinal water secretion. Activation of A2B receptors in human retinal endothelial cells may lead to neovascularization by a mechanism involving increased angiogenic growth factor expression [5]. Selective xanthine antagonists of the A2B receptor have been reported recently [6, 7]. Such antagonists are potentially useful in the treatment of asthma [8, 9] and intestinal disorders [10].
Non-selective radioligands have been used to characterize recombinant human A2B receptors overexpressed in HEK-293 cells. These include: 125I-IABOPX (125I-3-(4-amino-3-iodobenzyl)-8-(phenyl-4-oxyacetate)-1-propylxanthine) [11], [3H]CPX [9], and [3H]ZM 241385 ([3H]4-(2-[7-amino-2-}furyl{}1,2,4{triazolo}2,3-a{}1,3,5{triazin-5-ylamino]ethyl)phenol) [12]. Based on these binding assays, we have identified several new compounds with improved potency and selectivity for human A2B receptors [13, 14], culminating with aniline derivatives of the 8-phenylxanthine carboxylic congener, XCC (1, Fig. 1) [6]. A p-cyanoanilide derivative in this series, MRS 1754 (N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide, 4, Fig. 1), was 400-, 245-, and 123-fold more selective for human A2B receptors than human A1/A2A/A3 receptors, although less selective than rat A1/A2A receptors. This antagonist at a 100 nM concentration was shown to completely inhibit calcium mobilization stimulated by 1 μM NECA in HEK-293 cells expressing human A2B receptors [6]. In the present study, this selective antagonist for the A2B adenosine receptor, MRS 1754, has been prepared in tritiated form and shown to be a selective, high-affinity radioligand useful for characterizing recombinant human A2B receptors.
Fig. 1.
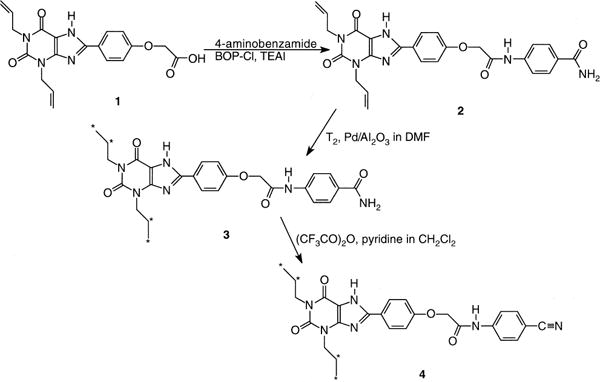
Synthesis of [3H]MRS 1754, 4, in a multi-step reaction sequence. p-Carboxamidoaniline was condensed with XCC, 1, to give the amide, 2. The 1,3-diallyl groups were tritiated to give 3. Dehydration gave the final product, 4, which was purified using TLC.
2. Materials and methods
2.1. Synthesis
2.1.1. Preparation of 3H-labelled MRS 1754
2.1.1.a N-(4-(aminocarbonyl)phenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide (2)
A solution of 1 [15] (2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-diallyl-1H-purin-8-yl)-phenoxy]acetic acid, 38 mg, 0.1 mmol), 4-aminobenzamide (27 mg, 0.2 mmol), BOP-Cl (30 mg, 0.12 mmol), and TEA (20 μL, 0.206 mmol) in 2 mL of anhydrous DMF:CH2Cl2 (1:1 mixture) was stirred at room temperature for 24 hr. The mixture was evaporated to dryness under reduced pressure, and the residue was recrystallized from a solution of CHCl3: MeOH (10:1) and washed with a solution of TEA in MeOH to afford 5 mg of 2. 1H NMR (DMSO-d6) 4.52 and 4.66 (2d, 4H, J = 3.9 Hz, 2X –NCH2–), 4.83 (s, 2H, –OCH2–), 5.04–5.17 (m, 4H, 2X =CH2), 5.83–6.03 (m, 2H, 2X –CH=), 7.14 (d, 2H, J = 8.8 Hz, Ar), 7.26 (bs, 1H, –NH2), 7.71 (d, 2H, J = 8.8 Hz, Ar), 7.85 (m, 3H, Ar and –NH2), 8.09 (d, 2H, J = 8.8 Hz, Ar), 10.35 (s, 1H, –NH–). HRMS (EI, M+) for C26H24N6O5: Calc. 500.1808. Found 500.0825.
2.1.1b [3H]N-(4-(Aminocarbonyl)phenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy-]acetamide (3)
1.7 mg (3.4 μM) of 2 was dissolved in 2 mL DMF, and 1 mL ethanol was added. The compound was reduced in an atmosphere of tritium gas utilizing 10 mg of 5% Pd/Al2O3 for 4 hr at room temperature. After removal of labile tritium by evaporation several times with DMF-EtOH, an assay indicated ~400 mCi of crude product. TLC on LKC5F plates using CHCl3:MeOH (50:5, v/v) followed by scanning showed a radiochemical purity of ~80%. The crude product was purified by preparative TLC using CHCl3:MeOH (50:3, v/v) as the developing solvent. The purified product was dissolved in ethanol and submitted for mass spectral analysis for specific activity determination (150 Ci/mmol). The purified material (125 mCi) was stored in 10 mL ethanol at −20° and had a radiochemical purity of > 98%. The product was compared with the corresponding unlabeled form of 3, for which spectroscopic data were reported [6].
2.1.1c [3H]N-(4-Cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide (4, [3H]MRS 1754)
10 mCi of purified 3 was dissolved in 200 μL of methylene chloride, and 20 μL of pyridine was added. The reaction mixture was cooled to −76°, and 6 μL of trifluoroacetic anhydride was added. The reaction was warmed to room temperature, and an aliquot was removed for TLC analysis [CHCl3:MeOH (50:2, v/v)]. To the crude reaction mixture (~60% product) was added 200 μL methanol and 200 μL TEA, followed by rotary evaporation to dryness. The product was purified by TLC using CHCl3: MeOH (50:1, v/v) as solvent. The product was eluted from the plate using ethanol and stored at ~1 mCi/mL in ethanol at −20°. TLC [CHCl3:MeOH (50:1, v/v)] indicated a radiochemical purity of > 97% (yield: 5.2 mCi). The product was compared with a corresponding unlabeled form of 4, for which spectroscopic data have been reported [6].
2.2. Pharmacological methods
A 20 nM stock solution of [3H]MRS 1754 was prepared in an equivolume mixture of DMSO and assay medium, which consisted of 50 mM Tris buffer containing 5 mM Mg2+ and 1 mM EDTA, at pH 6.5. Membranes from HEK-293 cells stably expressing the human A2B receptor, prepared as reported [6] or obtained from a commercial source (Batch 1365, Receptor Biology, Inc., Beltsville, MD), were studied. Glass incubation tubes contained a total volume of 100 μL, consisting of a suspension in Tris buffer (as above) containing membranes (30 μg protein, stored at −80°) and [3H]MRS 1754 (final concentration 0.7 nM), and a solution of the competing compound, where applicable. Nonspecific binding was determined in the presence of 100 μM NECA (RBI-Sigma). SCH 58261 (5-amino-7-(2-phenylethyl)-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine) was the gift of Dr. Ennio Ongini, Schering-Plough, S.p.a. All non-radioactive compounds were initially dissolved in DMSO, and diluted with buffer to the final concentration, with the amount of DMSO in the final assay tubes being consistently 4.5%.
Incubations were terminated by rapid filtration over Whatman GF/B filters, which had been presoaked in 0.5% polyethyleneimine, using a Brandell cell harvester. The tubes were rinsed three times with 2 mL of ice-cold Tris buffer (pH 6.5).
For saturation studies, the concentration of [3H]MRS 1754 ranged from 0.1 to 20 nM. For competition experiments, at least six different concentrations of competitor, spanning three orders of magnitude adjusted appropriately for the ic50 of each compound, were used. The ic50 values, calculated with the nonlinear regression method implemented in the Prism program (GraphPAD), were converted to apparent Ki values using the Cheng–Prusoff equation [16].
3. Results
The A2B receptor-selective xanthine antagonist MRS 1754 [6] was synthesized in tritiated form (Fig. 1) for use as a radioligand in a two-step tritiation sequence. The precursor 1,3-diallyl amide, 2, was prepared from the corresponding carboxylic acid, 1, reported previously [15]. An intermediate tritiated carboxamide derivative, 3, was dehydrated using trifluoroacetic anhydride, and the final product, 4, was purified using TLC. [3H]MRS 1754 was found to bind specifically to the human A2B receptor expressed in HEK-293 cells, using 100 μM NECA to define nonspecific binding. Since MRS 1754 is a relatively hydrophobic molecule and has low aqueous solubility, a stock solution of the radioligand in 50% aqueous DMSO was prepared, which allowed the concentration of the dissolved xanthine to remain constant.
To optimize specific binding, the pH dependence (Fig. 2), temperature dependence, and optimal amount of protein (Fig. 3) were determined. In the range of pH 4.5 to 6.5, the level of specific binding of 0.7 nM [3H]MRS 1754 was constant, whereas raising the pH (6.5 to 8.0) decreased the level by approximately 10–15% for each 0.5 pH unit. The specific binding increased linearly with increasing amounts of protein (2–30 μg) present in each tube (Fig. 3). At 25°, the level of specific binding of 0.7 nM [3H]MRS 1754 was nearly identical to that at 37°; thus, subsequent binding was carried out at 25°. Using optimal ligand binding conditions, the association and dissociation binding kinetics were determined (Fig. 4), using 100 μM NECA to induce dissociation. Binding reached equilibrium at 40 min and remained constant for the following 80 min. The standard time of incubation selected for subsequent experiments was 60 min. The kinetics of the association appeared monophasic with a T1/2 value of 7.65 ± 0.28 min. At equilibrium, the nonspecific binding did not exceed 30% of the total [3H]MRS 1754 bound. The association and dissociation rate constants were 0.022 ± 0.003 min−1 nM−1 and 0.027 ± 0.001 min−1, respectively, resulting in a kinetic KD (k−1/k1) value of 1.22 nM (N = 4), in good agreement with the equilibrium determination.
Fig. 2.
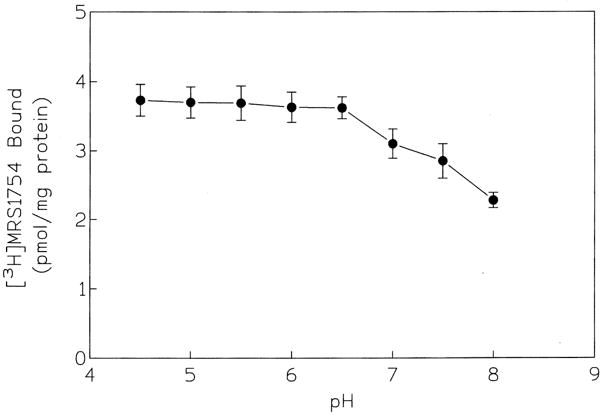
pH dependence of specific binding of [3H]MRS 1754 to human A2B receptors. [3H]MRS 1754 (0.7 nM) was incubated with membranes for 60 min at 25°. The curve represents the mean ± SEM of four determinations.
Fig. 3.
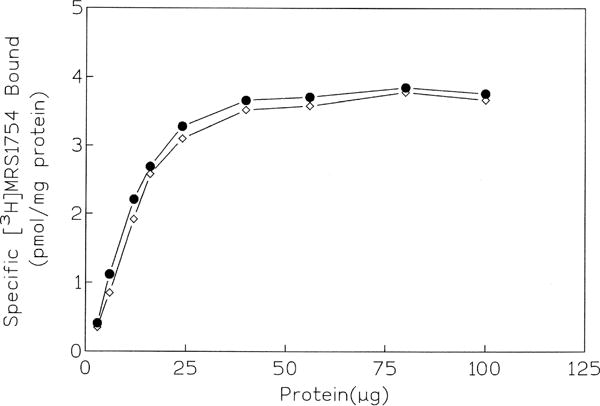
Dependence of the specific binding of [3H]MRS 1754 to the human A2B receptor on the amount of protein present in each assay tube at 25° (●) or 37° (◊). [3H]MRS 1754 (0.7 nM) was incubated with membranes for 60 min (N = 2).
Fig. 4.
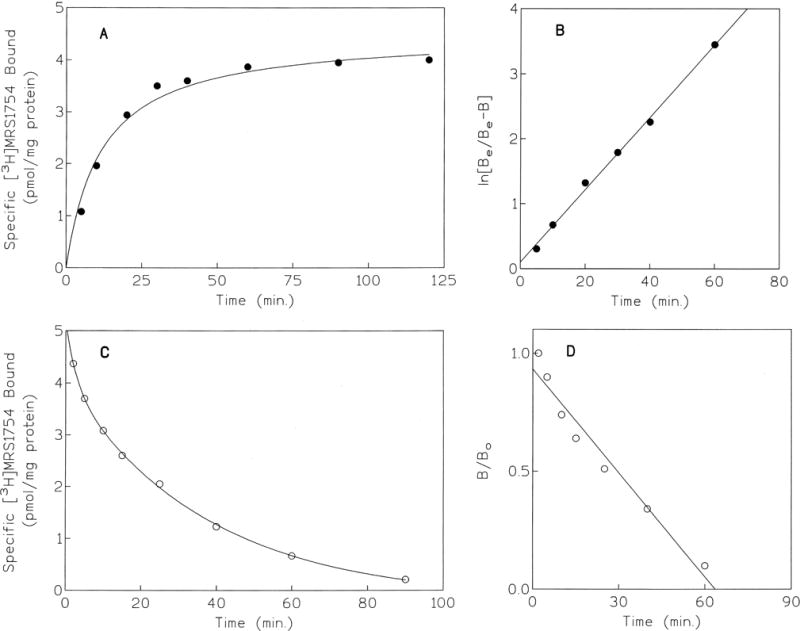
Association (A, B) and dissociation (C, D) kinetics of [3H]MRS 1754 binding to HEK-293 cell membranes expressing the human A2B receptor. [3H]MRS 1754 (0.7 nM) was incubated with membranes (30 μg protein) at 25°. Dissociation was initiated by the addition of 100 μM NECA. The results shown are representative of three separate determinations.
The effects of cations and chelators on specific [3H]MRS 1754 binding to human A2B receptors expressed in HEK-293 cell membranes were studied (Table 1). The presence of the divalent cations Zn2+ (≥1 mM) and Mn2+ (1 mM) significantly decreased the amount of specific binding of 0.7 nM [3H]MRS 1754, whereas Na+ had no effect. The effect of Zn2+ to inhibit binding appeared to be concentration-dependent, with an IC50 of ~1 mM. The cations Ca2+ or Mg2+ (10 mM) caused a 20% reduction in the amount of specific binding of 0.7 nM [3H]MRS 1754, and the addition of chelating agents in the presence of Ca2+ (EGTA or EDTA) or Mg2+ (EDTA) restored the full degree of binding.
Table 1.
Effect of cations and chelators on [3H]MRS 1754 binding
| Reagent | Concn (mM) |
Bound (% of control) |
|---|---|---|
| EDTA | 1 | 97 ± 3 |
| Mg2+ | 1 | 90 ± 7 |
| 5 | 79 ± 5 | |
| 10 | 79 ± 3 | |
| Mg2+(+ EDTA) | 1 | 116 ± 8 |
| Ca2+ | 1 | 93 ± 5 |
| 10 | 78 ± 7 | |
| Ca2+(+ EDTA) | 1 | 105 ± 4 |
| Ca2+(+ EGTA) | 1 | 110 ± 1 |
| Zn2+ | 1 | 52 ± 2 |
| 5 | 36 ± 9 | |
| 10 | 23 (N = 1) | |
| Mn2+ | 1 | 75 ± 7 |
| Na+ | 10 | 101 ± 0.5 |
| 100 | 101 ± 1 |
Data are means ±SD from three separate determinations measured in duplicate. Control value was 100% (coresponding to 3600 cpm). Nonspecific binding was determined in the presence of 100 μM NECA. [3H]MRS 1754 was present at a final concentration of 0.7 nM. The concentration of EDTA or EGTA was 1 mM.
Under optimized equilibrium conditions (60-min incubation at 25° with 30–35 μg protein/tube, in Mg2+-containing medium), binding of [3H]MRS 1754 to membranes of HEK-293 cells expressing the human A2B receptor was saturable and was best described by a one-site model (GraphPad, Prism). The percent of specific binding using a 0.7 nM concentration of the radioligand was > 70% of total binding. A representative saturation isotherm and a Scatchard transformation of the same data are shown in panels A and B of Fig. 5. The Bmax value was 10.9 ± 0.6 pmol/mg protein (N = 4). The KD value obtained from the saturation experiments was 1.13 ± 0.12 nM, which was in good agreement with the KD value of 1.22 ± 0.22 nM determined in kinetic studies.
Fig. 5.
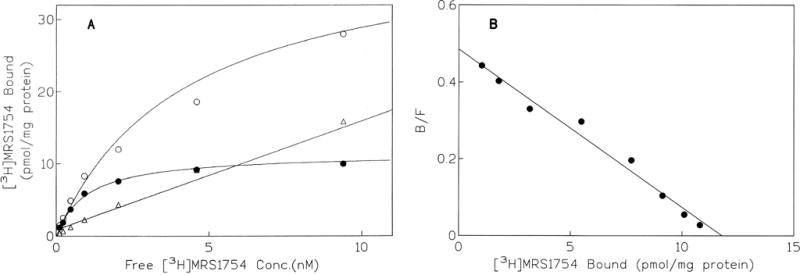
(A) Saturation isotherm of [3H]MRS 1754 binding to human A2B receptors expressed in HEK-293 cell membranes, and (B) Scatchard analysis of the same data. [3H]MRS 1754 (0.1 to 20 nM) was incubated with 30 μg of membranes for 60 min at 25°. Total binding (○), specific binding (●), and nonspecific binding (Δ), determined using 100 μM NECA, are shown in (A). Four experiments were carried out; data from one representative experiment are shown.
Levels of binding of [3H]MRS 1754 that was displaceable by 100 μM NECA in membranes expressing other adenosine receptor subtypes, e.g. rat and human A1 and A3 adenosine receptors (Table 2), were not significant. The only significant binding was to membranes expressing A2B receptors, and this binding displayed characteristics of the A2B subtype. Radioligand binding in membranes of HEK-293 cells expressing human A2A receptors was, in all experiments, either undetectable or not greater than 8% of the total binding and, when observed, was not displaceable using the A2A selective antagonist SCH 58261.
Table 2.
Binding of a single concentration of [3H]MRS 1754 in membranes of cells expressing four adenosine receptor subtypes
| Cell-Subtype | Bound (pmol/mg protein) | Referencea |
|---|---|---|
| CHO-hA1 | 0,22 ± 0.09 | [17] |
| HEK-hA2A | b | [9] |
| HEK-hA2B | 6.89 ± 0.63 | [9,12,14] |
| CHO-hA3 | 0.02 ± 0.02 | [26] |
| Rat brain-rA1 | 0.17 ± 0.04 | [6,15] |
| CHO-rA3 | 0.06 ± 0.07 | [15] |
Data are means ± SD from 3–6 separate determinations measured in duplicate. Nonspecific binding was determined in the presence of 100 μM NECA. [3H]MRS 1754 was present at a concentration of 0.7 nM, unless indicated.
References describe the source of each preparation and the pharmacological characteristics of each receptor. These citations also demonstrate that substantial binding to the indicated receptors was observed using radioligands having high affinity for each receptor. Thus, the expression levels of A1, A2A, and A3 receptors were much higher than indicated by the level of binding of [3H]MRS 1754.
Binding was either undetectable or ≤ 8% of total binding, using 5–10 μg protein/tube (N = 10). This did not represent specific binding to A2A receptors, since binding was not displaceable by the A2A selective antagonist SCH 58261. The concentration of [3H]MRS 1754 was 1.0 nM.
The pharmacological profile for known adenosine receptor ligands in competition for [3H]MRS 1754 binding was consistent with the SAR for antagonists (Fig. 6A) and agonists (Fig. 6B) noted previously at A2B receptors [6, 11, 12]. The most potent displacer of [3H]MRS 1754 binding (Table 3) was MRS 1754, itself, with a Ki value of 1.45 nM. This value was consistent with the observed KD value and with a Ki value of 1.97 nM [6], determined by competition for binding of 125I-IABOPX or [3H]ZM 241385. The potent xanthine derivatives XAC and CPX, and the triazoloquinazoline CGS 15943 [18], had Ki values of 16, 55, and 34 nM, respectively. The triazolotriazine ZM 241385 [19] had a Ki value of 145 nM, somewhat less potent than previously determined [7, 12]. Another potent xanthine derivative, XCC [14], which is a precursor of MRS 1754, had a Ki value of 54 nM in binding to human A2B receptors, similar to the value reported previously [12]. Enprofylline (3-propylxanthine) and theophylline were roughly equipotent in displacing binding of [3H]MRS 1754. Alloxazine, which has been reported to be moderately selective (10-fold) for A2B versus A1 and A2A receptors [20], had a Ki value of 2.04 μM. DAX, a xanthine derivative of interest as a treatment for cystic fibrosis [21], displaced binding with a Ki value of 408 nM. The Hill coefficients (nH) in the competition experiments were in the range of 0.8 to 1.0 for antagonists and agonists.
Fig. 6.
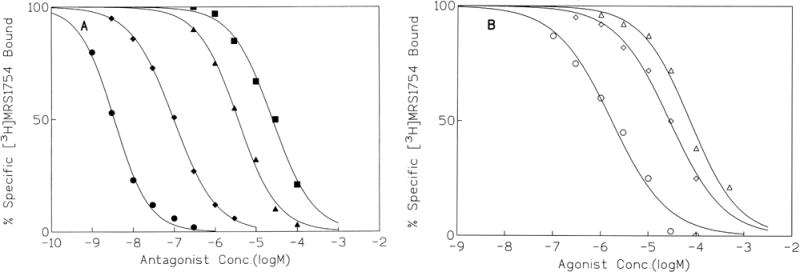
Effects of (A) the antagonists MRS 1754 (●), CPX (◆), alloxazine (▲), and enprofylline (■) and (B) the agonists NECA (○), R-PIA (◊), and SPA (Δ) on radioligand binding to human A2B receptors expressed in HEK-293 cell membranes. Membranes (30 μg) were incubated for 60 min at 25° with 0.7 nM [3H]MRS 1754. Nonspecific binding was determined with 100 μM NECA. The data are representative of four experiments.
Table 3.
Ki values for displacement of [3H]MRS 1754 binding to human A2B receptors expressed in HEK-293 cell membranes
| Compound | Ki(nM) |
|---|---|
| Antagonists | |
| MRS 1754 | 1.45 ± 0.21 |
| XAC | 16.0 ± 0.7 |
| CGS 15943 | 34.2 ± 1.0 |
| XCC | 53.6 ± 3.8 |
| CPX | 54.6 ± 12.1 |
| ZM 241385 | 145 ± 15 |
| DAX | 408 ± 54 |
| Alloxazine | 2,040 ± 570 |
| Enprofylline | 19,800 ± 6,120 |
| Theophylline | 15,200 ± 4,100 |
| Agonists | |
| NECA | 570 ± 170 |
| R-PIA | 13,900 ± 3,400 |
| SPA | 24,700 ± 7,500 |
| CPA | 20,600 ± 7,500 |
| CGS 21680 | 14 ± 2% displacement at 100 μM |
Specific binding was approximately 75% of total binding. Values are means ±SEM of 3–7 separate experiments.
Among agonists (Fig. 6B), as in functional assays [20, 22], NECA was more potent than N6-substituted analogues in binding competition. The A2A-selective agonist CGS 21680 (2-[4-[(2-carboxyethyl)phenyl]ethyl-amino]-5′-N-ethylcarbamoyladenosine) did not displace [3H]MRS 1754 binding significantly, even at a concentration of 100 μM, which is consistent with functional studies showing this agonist to be inactive at A2B receptors and selective for the A2A-receptor subtype [3].
4. Discussion
Following synthesis by an efficient multi-step method, [3H]MRS 1754 was shown to bind with high affinity to a single class of binding sites in membranes of HEK-293 cells expressing the human A2B receptor. The pharmacological characteristics of this binding site resemble the functional characteristics of A2B receptors [7, 11, 19, 20, 22]. [3H]MRS 1754 is selective for the A2B receptor, with very low affinity for A1 and A3 receptors of both humans and rats. In cells expressing human A2A receptors, the low levels of binding of [3H]MRS 1754 were demonstrated not to represent binding to this receptor subtype. Thus, due to its high affinity and selectivity, [3H]MRS 1754 has advantages over [3H]CPX, [3H]ZM 241385, and 125I-IABOPX as a radioligand for A2B receptors.
Theophylline is widely used as an antiasthmatic drug, although its mechanism of action is uncertain. The related xanthine enprofylline (3-propylxanthine) [9, 13], which is also therapeutically efficacious in the treatment of asthma, was earlier thought to act through a non-adenosine receptor-mediated mechanism due to its low affinity at A1 and A2A receptors. However, the discovery that enprofylline has greater than anticipated affinity and slight selectivity at the A2B subtype [9] supports the hypothesis that A2B receptor antagonism may contribute to the antiasthmatic activity of xanthines [8, 23, 24]. This hypothesis was strengthened by functional effects of A2B receptor activation observed in mast cells of dogs, mice, and humans [8, 25]. Thus, potent and/or selective A2B receptor antagonists may provide new therapeutic agents.
In conclusion, [3H]MRS 1754 binding to recombinant human A2B receptors in membranes is a practical method for characterizing these receptors and their ligands in recombinant systems. This radioligand is yet to be characterized in cells and tissues endogenously expressing A2B receptors and in the presence of other subtypes of adenosine receptors. Also, the affinity of MRS 1754 at A2B receptors in other species is yet to be determined. The development of binding assays for this subtype of adenosine receptors that are useful with cell membranes will aid in the elucidation of the SAR of A2B receptor agonists and antagonists, which are currently being synthesized [6,16,22,26].
Abbreviations
- BOP-Cl
bis(2-oxo-3-oxazolidinyl)phosphinic chloride
- CGS 15943
9-chloro-2-(2-furanyl) [1, 2, 4]triazolo[1,5-c]quinazolin-5-amine
- CGS 21680
2- [4-[(2-carboxyethyl]phenyl]ethyl-amino]-5′-N-ethylcarbam-oyladenosine
- CHO cells
Chinese hamster ovary cells
- CPA
N6-cyclopentyladenosine
- CPX
8-cyclopentyl-1,3-dipropylxanthine
- DAX
1,3-diallyl-8-cyclohexylxanthine
- DMF
dimethylformamide
- HEK cells
human embryonic kidney cells
- IABOPX
3-(4-amino-3-iodobenzyl)-8-(phenyl-4-oxyacetate)-1-propylxanthine
- KD
dissociation constant
- Ki
equilibrium inhibition constant
- MRS 1754
N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide
- NECA
5′-N-ethylcarboxamidoadenosine
- R-PIA
R-N6-phenylisopropyladenosine
- SAR
structure–activity relationship
- SCH 58261
5-amino-7-(2-phenylethyl)-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
- SPA
N6-p-sulfophenyladenosine
- TEA
triethylamine
- XAC
8- [4-[[[[(2-aminoethyl]amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine
- XCC
8- [4-[(carboxymethyl]oxy]phenyl]-1,3-dipropylxanthine
- ZM 241385
4-(2-[7-amino-2-}furyl{}1,2,4{triazolo}2,3-a{}1,3,5{triazin-5-ylamino]ethyl)phenol
References
- 1.Linden J, Jacobson KA. Molecular biology of recombinant adenosine receptors. In: Burnstock G, Dobson JG, Liang BT, Linden J, editors. Cardiovascular biology of purines. Boston: Kluwer; 1998. pp. 1–20. [Google Scholar]
- 2.Daly JW, Butts-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hide I, Padgett WL, Jacobson KA, Daly JW. A2A-adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: characterization with radioligand binding and by activation of adenylate cyclase. Mol Pharmacol. 1992;41:352–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Feoktistov I, Murray JJ, Biaggioni I. Positive modulation of intracellular Ca2+ levels by adenosine A2b receptors, prostacyclin, and prostaglandin E1 via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol Pharmacol. 1994;45:1160–7. [PubMed] [Google Scholar]
- 5.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85:699–706. doi: 10.1161/01.res.85.8.699. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y-C, Ji X-d, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–72. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Zwart M, Vollinga RC, von Frijtag Drabbe Künzel JK, Beukers M, Sleegers D, IJzerman AP. Potent antagonists for the human adenosine A2B receptor. Derivatives of the triazolotriazine adenosine receptor antagonist ZM241385 with high affinity. Drug Dev Res. 1999;48:95–103. [Google Scholar]
- 8.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 9.Robeva AS, Woodard R, Jin X, Gao Z, Bhattacharya S, Taylor HE, Rosin DL, Linden J. Molecular characterization of recombinant human adenosine receptors. Drug Dev Res. 1996;39:243–52. [Google Scholar]
- 10.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–94. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 11.Linden J. Molecular characterization of A2A and A2B receptors. Drug Dev Res. 1998;43:2. [Google Scholar]
- 12.Ji X-d, Jacobson KA. Use of the triazolotriazine [3H]ZM241385 as a radioligand at recombinant human A2B adenosine receptors. Drug Des Discov. 1999;16:217–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson KA, IJzerman AP, Linden J. 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev Res. 1999;47:45–53. doi: 10.1002/(sici)1098-2299(199905)47:1<45::aid-ddr6>3.0.co;2-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y-C, Karton Y, Ji X-d, Melman N, Linden J, Jacobson KA. Acyl-hydrazide derivatives of a xanthine carboxylic congener (XCC) as selective antagonists at human A2B adenosine receptors. Drug Dev Res. 1999;47:178–88. doi: 10.1002/(sici)1098-2299(199908)47:4<178::aid-ddr4>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HO, Ji X-d, Melman N, Olah ME, Stiles GL, Jacobson KA. Structure–activity relationships of 1,3-dialkylxanthine derivatives at rat A3-adenosine receptors. J Med Chem. 1994;37:3373–82. doi: 10.1021/jm00046a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson KA, Park KS, Jiang J-l, Kim Y-C, Olah ME, Stiles GL, Ji X-d. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–65. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y-C, de Zwart M, Chang L, Moro S, von Frijtag Drabbe Künzel JK, Melman N, IJzerman AP, Jacobson KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem. 1998;41:2835–45. doi: 10.1021/jm980094b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, Coll MG. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995;115:1096–102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–14. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson KA, Guay-Broder C, van Galen PJM, Gallo-Rodriguez C, Melman N, Jacobson MA, Eidelman O, Pollard HB. Stimulation by alkylxanthines of chloride efflux in CFPAC-1 cells does not involve A1 adenosine receptors. Biochemistry. 1995;34:9088–94. doi: 10.1021/bi00028a018. [DOI] [PubMed] [Google Scholar]
- 22.de Zwart M, Link R, von Frijtag Drabbe Künzel JK, Cristalli G, Jacobson KA, Townsend-Nicholson A, IJzerman AP. A screening of adenosine analogues on the human adenosine A2B receptor as part of a search for potent and selective agonists. Nucleosides Nucleotides. 1998;17:969–86. doi: 10.1080/07328319808004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feoktistov I, Polosa R, Holgate ST, Biaggioni I. Adenosine A2B receptors: a novel therapeutic target in asthma? Trends Pharmacol Sci. 1998;19:148–53. doi: 10.1016/s0165-6147(98)01179-1. [DOI] [PubMed] [Google Scholar]
- 24.Fozard JR, Hannon JP. Adenosine receptor ligands: potential as therapeutic agents in asthma and COPD. Pulm Pharmacol Ther. 1999;12:111–4. doi: 10.1006/pupt.1999.0191. [DOI] [PubMed] [Google Scholar]
- 25.Auchampach JA, Jin J, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptors and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1998;52:846–60. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 26.Cristalli G. Synthesis and characterization of potent ligands at human recombinant adenosine receptors. Drug Dev Res. 1998;43:23. [Google Scholar]


