Abstract
Objectives
Hospital case volume has been shown to be a predictor of patient mortality for treatment for various cancers. The influence of hospital case volume on malignant melanoma survival and treatment utilization is unknown.
Methods
We used the Surveillance, Epidemiology, and End Results-Medicare linked databases to identify patients aged 65 years or older diagnosed with metastatic melanoma between 2000 and 2009. We analyzed claims data to ascertain cancer treatment variation by hospital case volume. Overall survival was evaluated using propensity score methods.
Results
Among 1438 patients, 612 (42.6%) were treated in low-volume hospitals (≤5 patients) after receiving their diagnosis, 479 (33.3%) were treated in intermediate-volume hospitals (6 to 10 patients), and 347 (24.1%) were treated in high-volume hospitals (> 10 patients). In Cox proportional hazards models, treatment in a high-volume hospital after propensity score adjustment was associated with a significant improvement in survival when adjusting for other characteristics (intermediate volume: hazard ratio [HR] = 0.70, P = 0.0007; high volume: HR = 0.63, P < 0.0001). Patients treated in high-volume hospitals were less likely to receive chemotherapy, surgery, and/or radiation therapy after a metastatic melanoma diagnosis.
Conclusions
For patients diagnosed with metastatic melanoma, being treated in a high-volume hospital was associated with an improvement in survival and lower utilization of chemotherapy, immunotherapy, surgery, and radiation therapy.
Keywords: advanced melanoma, end-of-life care, survival, hospital case volume, chemotherapy, immunotherapy, surgery, radiation therapy
There have been several studies that demonstrated associations between certain hospital procedure volumes and outcomes for cancer patients. For example, a decade ago, investigators reported a positive association between the volume of cancer resection procedures performed and the survival of patients with lung cancer and colorectal cancer as well as others.1–4 A recent meta-analysis of 101 published reports found a significant positive effect of high hospital case volume on short-term mortality for patients with gastrointestinal cancers.5 However, the influence of hospital case volume on outcomes for patients with malignant melanoma has not been investigated.
Although melanoma comprises a small proportion of skin cancer cases, 75% of skin cancer deaths are attributable to melanoma.6,7 With annual incidence increasing by 3.1%, a rate faster than for any other cancers,8,9 melanoma represents an increasing health care burden to patients, their families, and the health care system. This study sought to examine the relationship between hospital case volume, that is, the number of metastatic melanoma cases treated in a hospital, and survival for patients with metastatic melanoma. Survival outcomes likely hinge on the integration of multimodality disease-control treatments such as surgery, radiation therapy, and chemotherapy as indicated along with timely initiation of palliative therapies or hospice care. There is no single standard procedure or treatment algorithm for patients with disseminated melanoma. Therefore, we focused our investigation on outcomes for patients with a diagnosis of metastatic melanoma. We also sought to analyze use of chemotherapy, surgery, and radiation therapy according to hospital volume.
METHODS
Data Source and Patient Cohort
We used the National Cancer Institute–supported SEER-Medicare data set, which covers 17 geographic areas in the United States, representing an estimated 28% of the US population.10 The linked Medicare database includes medical claims for 97% of the US citizens aged 65 years and older.11 To obtain treatment information, we used the encrypted identifier in the Patients Entitlement and Diagnosis Summary File that links to all available Medicare claim files for inpatient care, skilled nursing facility care, home health care, hospice care, physician visits, outpatient care, and durable medical equipment. Hospital characteristics were obtained from SEER hospital files. All data were deidentified so that no protected health information could be linked to individual patients. The institutional review board from The University of Texas M.D. Anderson Cancer Center exempted this study.
We identified 1438 patients aged 65 years and older with pathologically confirmed distantly metastatic malignant melanoma (melanoma, SITE1-10 = “44”; stage IV, SEER Variable HSTST = “4”) diagnosed between January 1, 2000 and December 31, 2009. Patients were excluded if their death year and month in the SEER and Medicare data sets did not match or if their cancer diagnosis was determined after death according to autopsy or cause of death mentioned in death certificate. Patients were excluded if they did not have continuous health care coverage through enrollment in Medicare parts A and B from the date their stage IV melanoma diagnosis was made until the time of death or if they had HMO coverage during this time.
Variables
Overall survival was defined as the period beginning at the time of metastatic melanoma diagnosis until death. Age at diagnosis, sex, marital status, neighborhood income and education levels, geographic region, comorbidity score, hospital volume, and days of hospice use (categorized as no hospice, hospice 1 to 3 days, and hospice 4 + days) were the covariates used in the survival model. Age was categorized as 65 to 69, 70 to 74, 75 to 79, and 80 years or older. Marital status was categorized as married, unmarried, or other. The Charlson Comorbidity score, which is used to predict mortality, was calculated from an algorithm developed by Klabunde and colleagues and SEER-Medicare data using Medicare inpatient and outpatient claims within the 12-month window before the 30-day period before a patient’s metastatic melanoma diagnosis.12,13 The use of hospice care was identified based on any hospice admission and/or service date occurring after the metastatic melanoma diagnosis date in the hospice claims file.
Hospital volume was based on the number of patients with metastatic melanoma treated within each hospital over the entire study period. Only the claims data from the MEDPAR file were included in the estimation of hospital case volume. The available provider numbers within each claim record were used to link the SEER-Medicare provider file where detailed information about hospitals was collected by the NCI using various reports and surveys. The hospital volume variable was categorized as low-volume (≤5 patients), intermediate-volume (6 to 10 patients), or high-volume (> 10 patients). To derive best cut points for categorization of hospital volume, we divided the cohort into 10 groups and then merged groups in which patients had similar survival time. Some patients were treated at multiple hospitals after diagnosis of metastatic melanoma. Given the assumption that the access to more experienced hospital at any point of treatment process could be translated to potential outcome implication, we therefore assume patients who had any contact with a higher volume hospital were counted as a recipient of that higher volume hospital.
We used International Classification of Diseases, 9th Edition procedure codes and Current Procedural Terminology codes to identify chemotherapy, surgery, and radiation therapy services from patients’ claims file (Supplemental Digital Content I, http://links.lww.com/AJCO/A45). As most of the radiation therapy and chemotherapy were given in outpatient setting, we used all claims file to estimate the therapies received. The utilization rate was defined as the proportion of patients in each volume category who had ever received chemotherapy, surgery, and radiation therapy after their metastatic melanoma diagnosis.
Statistical Analysis
A Cox proportional hazards model controlling for demographic and clinical explanatory variables was used to assess the relationship between hospital volume and overall survival at 5 years. All hazard ratios (HRs) were calculated with 2-sided P-values and 95% confidence intervals (CIs). The potential interaction terms were also evaluated in the model. The median survival was calculated using the Kaplan-Meier estimations.
To minimize selection bias in evaluating outcomes, a propensity score-based match was used to generate a new cohort for the survival model by applying an 8- to 1-digit algorithm developed by Parsons.14 In this algorithm, all demographic variables were included in the propensity score logistic model to generate the predicted probability that is used to match the pairs. Because 3 groups existed (low-volume, intermediate-volume, and high-volume), 2-step matching was employed to generate the final propensity score–adjusted cohort. In the first step, we matched patients in low-volume hospitals with those in intermediate-volume hospitals by the predicted probability from the logistic model with patients in the low-volume and intermediate-volume-only cohort; thereafter, we matched patients in low-volume hospitals with those in high-volume hospitals by the predicted probability from the model with patients in the low-volume and high-volume-only cohort. In the second stage, patients in low-volume hospitals who were selected in both low-intermediate and low-high matched cohorts served as an index from which the paired patients from intermediate-volume and high-volume hospitals were extracted and were then merged into the final propensity score–adjusted cohort. After propensity score matching we tested the characteristics of the full sample and of the propensity score–matched sample. Compared with the original full sample, the propensity score–matched sample was more balanced. In the matched cohort, a Cox proportional hazards model evaluated the associations between 3 levels of hospital volume and overall survival in months. Statistical analysis was conducted using the SAS version 9.3 software (SAS Institute Inc., Cary, NC).
RESULTS
Patient and Hospital Characteristics
Characteristics of the entire cohort and univariate analysis of the associations of hospital volume and patient characteristics are shown in Table 1. Of the 1438 patients, 612 (42.6%) were treated in low-volume hospitals after their diagnosis, 479 (33.3%) were treated in intermediate-volume hospitals, and 347 (24.1%) were treated in high-volume hospitals. In the propensity score–matched cohort, 558 patients were evenly distributed into 3 groups with 186 patients in each.
TABLE 1.
The Characteristics of the Entire Cohort and Univariate Analysis of the Associations of Hospital Volume and Patient Characteristics
| Original Cohort (N: 1438) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total | Low Volume | % | Intermediate Volume | % | High Volume | % | P | |
| Year of death | 0.05 | |||||||
| 2000–2001 | 140 | 78 | 12.8 | 33 | 6.9 | 29 | 8.4 | |
| 2002–2003 | 260 | 118 | 19.3 | 85 | 17.8 | 57 | 16.4 | |
| 2004–2005 | 315 | 124 | 20.3 | 115 | 24.0 | 76 | 21.9 | |
| 2006–2007 | 332 | 137 | 22.4 | 108 | 22.6 | 87 | 25.1 | |
| 2008–2009 | 391 | 155 | 25.3 | 138 | 28.8 | 98 | 28.2 | |
| Age (y) | < 0.001 | |||||||
| 65–69 | 145 | 49 | 8.0 | 47 | 9.8 | 49 | 14.1 | |
| 70–74 | 290 | 141 | 23.0 | 78 | 16.3 | 71 | 20.5 | |
| 75–79 | 362 | 132 | 21.6 | 136 | 28.4 | 94 | 27.1 | |
| ≥80 | 641 | 290 | 47.4 | 218 | 45.5 | 133 | 38.3 | |
| Sex | 0.67 | |||||||
| Male | 960 | 402 | 65.7 | 327 | 68.3 | 231 | 66.6 | |
| Female | 478 | 210 | 34.3 | 152 | 31.7 | 116 | 33.4 | |
| Marital status | 0.97 | |||||||
| Unmarried | 181 | 75 | 12.3 | 63 | 13.2 | 43 | 12.4 | |
| Married | 824 | 351 | 57.4 | 270 | 56.4 | 203 | 58.5 | |
| Other | 433 | 186 | 30.4 | 146 | 30.5 | 101 | 29.1 | |
| Median household income | < 0.0001 | |||||||
| Lowest quartile | 332 | 179 | 31.0 | 103 | 22.1 | 50 | 15.1 | |
| 2nd quartile | 343 | 171 | 29.6 | 119 | 25.5 | 53 | 16.0 | |
| 3rd quartile | 344 | 129 | 22.4 | 113 | 24.2 | 102 | 30.8 | |
| Highest quartile | 356 | 98 | 17.0 | 132 | 28.3 | 126 | 38.1 | |
| Unknown | 63 | 35 | 5.7 | 12 | 2.5 | 16 | 4.6 | |
| Education <12 y | < 0.0001 | |||||||
| Lowest quartile | 349 | 112 | 19.3 | 121 | 26.5 | 116 | 34.9 | |
| 2nd quartile | 339 | 121 | 20.9 | 125 | 27.4 | 93 | 28.0 | |
| 3rd quartile | 349 | 163 | 28.1 | 114 | 25.0 | 72 | 21.7 | |
| Highest quartile | 332 | 184 | 31.7 | 97 | 21.2 | 51 | 15.4 | |
| Unknown | 69 | 32 | 5.2 | 22 | 4.6 | 15 | 4.3 | |
| Hospice care | 0.01 | |||||||
| 0–3 d | 1286 | 531 | 86.8 | 442 | 92.3 | 313 | 90.2 | |
| 4 + d | 152 | 81 | 13.2 | 37 | 7.7 | 34 | 9.8 | |
| Comorbidity scores | < 0.01 | |||||||
| 0 | 788 | 355 | 58.0 | 238 | 49.7 | 195 | 56.2 | |
| 1 | 355 | 148 | 24.2 | 140 | 29.2 | 67 | 19.3 | |
| ≥2 | 295 | 109 | 17.8 | 101 | 21.1 | 85 | 24.5 | |
| Geographic region | < 0.0001 | |||||||
| West | 636 | 294 | 48.0 | 200 | 41.8 | 142 | 40.9 | |
| Northeast | 360 | 93 | 15.2 | 115 | 24.0 | 152 | 43.8 | |
| Midwest | 152 | 73 | 11.9 | 51 | 10.7 | 28 | 8.1 | |
| South | 290 | 152 | 24.8 | 113 | 23.6 | 25 | 7.2 | |
Patients in the study cohort were treated in 838 hospitals (Table 2). Of those, 327 hospitals were teaching hospitals and 62 hospitals were referral centers. Among these hospitals, 64% had radiation oncology services available onsite and 41% had hospice services available within the hospital.
TABLE 2.
The Characteristics of Hospitals Received Patients With Metastatic Melanoma
| Total (n = 838) | N (%) |
|---|---|
| Teaching hospital | |
| Yes | 327 (39.0) |
| No | 503 (60.0) |
| Unknown | 8 (1.0) |
| Referral center | |
| Yes | 62 (7.4) |
| No | 767 (91.5) |
| Unknown | 9 (1.1) |
| Hospital urban/rural | |
| Urban | 40 (4.8) |
| Rural | 16 (1.9) |
| Unknown | 782 (93.3) |
| Radiation oncology availability | |
| Not provided | 303 (36.2) |
| Provided by staff | 378 (45.2) |
| Provided under arrangement | 157 (18.6) |
| Hospice bed | |
| Yes | 23 (2.7) |
| No | 815 (97.3) |
| Hospice services | |
| Not provided | 494 (59.0) |
| Provided by staff | 155 (18.5) |
| Provided under arrangement | 152 (22.5) |
Overall Survival
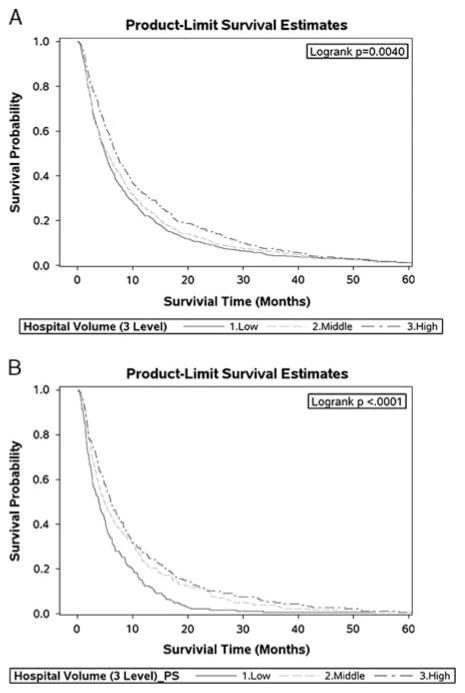
The unadjusted Kaplan-Meier survival curves for the entire cohort categorized by hospital volume are shown in Figure 1A. The median survival was 5.0 months for patients treated in low-volume hospitals, 5.5 months for patients treated in intermediate-volume hospitals, and 7.0 months for patients treated in high-volume hospitals. The survival probability curves for the propensity score–matched cohort is shown in Figure 1B. Patients in the high-volume hospital group experienced statistically significant improved survival than patients in the other 2 groups, and the survival probability for patients in intermediate-volume hospitals was better than for those in low-volume hospitals (log-rank test, P < 0.0001; Fig. 1B). The propensity score–adjusted median survivals for patients in these 3 groups were 3.9 months (low-volume), 4.9 months (intermediate-volume), and 6.0 months (high-volume).
FIGURE 1.
Comparison of survival time for metastatic melanoma patient in hospitals with different volume level: (A) is unadjusted; (B) uses propensity score–matched groups.
In the Cox proportional hazards models, treatment in high-volume hospitals was associated with a significant improvement in survival when adjusting for other characteristics (Table 3). The estimated improvements in survival for patients in high-volume hospitals were similar within the original cohort’s Cox proportional hazards model (intermediate volume: HR = 0.91; 95% CI, 0.80–1.02; high volume: HR = 0.79; 95% CI, 0.69–0.90), and propensity score–matched model (intermediate volume: HR = 0.70; 95% CI, 0.57–0.86; high volume: HR = 0.63; 95% CI, 0.51–0.77).
TABLE 3.
Hazard Ratios of Death (HRs) (95% CI) for Metastatic Melanoma Patients
| HR | 95% CI | P | |
|---|---|---|---|
| Hospital volume | |||
| Lowest | 1.00 | Reference | |
| Middle | 0.91 | 0.80–1.02 | 0.11 |
| Highest | 0.79 | 0.69–0.90 | < 0.0001 |
| Year of death | |||
| 2000–2001 | 1.00 | Reference | |
| 2002–2003 | 0.71 | 0.58–0.87 | < 0.01 |
| 2004–2005 | 0.60 | 0.49–0.74 | < 0.0001 |
| 2006–2007 | 0.57 | 0.47–0.70 | < 0.0001 |
| 2008–2009 | 0.45 | 0.37–0.56 | < 0.0001 |
| Hospice care | |||
| 0–3 days | 1.00 | Reference | |
| 4 + days | 0.55 | 0.46–0.65 | < 0.0001 |
| Comorbidity scores | |||
| 0 | 1.00 | Reference | |
| 1 | 1.14 | 1.00–1.30 | 0.04 |
| ≥2 | 1.23 | 1.08–1.41 | < 0.01 |
The other demographic variables and hospital variables such as year of death, age, sex, median household income, education, geographic region, referral center status, teaching hospital, urban/rural hospitals were all nonsignificant in the full model and were, therefore, excluded in the reduced model.
CI indicates confidence interval.
In addition to hospital case volume, other patient-related and hospital-related characteristics were significantly predicative of survival among patients diagnosed with metastatic melanoma (Table 3). Patients enrolled in ≥4 days of hospice care experienced longer survival (HR, 0.55; 95% CI, 0.46–0.65) than those who used hospice for ≤3 days. Patients who had higher comorbidity scores were likely to have shorter survival.
Hospital Volume and Use of Chemotherapy, Surgery, and Radiation Therapy
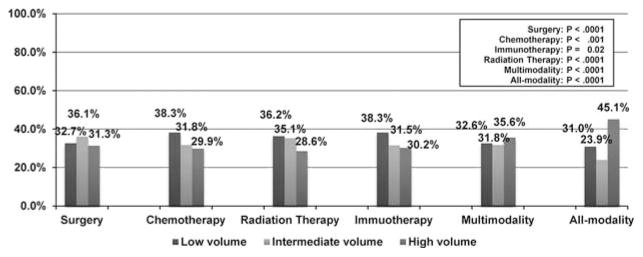
Overall rates of chemotherapy use for patients with metastatic melanoma were lower at high-volume hospitals compared with intermediate-volume and low-volume hospitals, at 29.9%, 31.8%, and 38.3%, respectively (Fig. 2). The rates of use of immunotherapy were similar (P = 0.02). Patients in high-volume hospitals experienced the lowest overall use of surgery: 31.3%, compared with 36.1% in intermediate-volume hospitals, and 32.7% in low-volume hospitals. Patients in high-volume hospitals also had a lower rate of radiation therapy use compared with intermediate-volume hospitals and low-volume hospitals. Patients in the high-volume hospitals were more likely to receive multiple modalities after diagnosis.
FIGURE 2.
Percent of patients receiving chemotherapy, immunotherapy, surgery, and radiation therapy after a metastatic melanoma diagnosis from 2000 to 2009.
DISCUSSION
We observed that higher case volume of patients diagnosed with metastatic melanoma was associated with longer survival for patients with metastatic melanoma after adjusting for age, sex, comorbidities, and other potential explanatory variables. Several studies have demonstrated that higher hospital procedure volumes for various specific procedures are associated with improved survival or complication rates.1–4,15–17 Bach et al1 found that patients with lung cancer had better survival rates in hospitals which performed a higher number of lung cancer surgeries and a 24% lower postoperative complication rate in hospitals with the highest volume of lung cancer surgeries. For patients with colon cancer, Schrag et al2 found that the difference in the 30-day postoperative mortality between hospitals performing highest and lowest volumes of surgeries was statistically significant. A study conducted by Birkmeyer et al4 examined 2.5 million patients from a nationwide sample to study the volume impact of 14 types of procedures including 6 cardiovascular procedures and 8 cancer resection procedures. The study results vary with regard to the magnitude of the associations between the procedure volumes and mortality, from 12% difference for pancreatic cancer resection to only 0.2% for carotid endarterectomy. Two recent systematic reviews evaluated the associations between hospital surgical volume and outcomes for patients with prostate and gastrointestinal cancers and revealed a significant negative relationship between hospital surgical volume and short-term mortality.5,18
Not all studies have demonstrated that hospital procedure volumes are associated with better survival outcomes. Porter et al19 analyzed the State of Washington Comprehensive Hospital Abstract Reporting System database for patients with bladder cancer who underwent cystectomy. Cumulative 90-day mortality after cystectomy for high-cystectomy, intermediate-cystectomy, and low-cystectomy volume hospitals was 5.4%, 6.9%, and 8.4%, respectively.19 However, the result was not statistically significant. Salz and Sandler20 reviewed studies on the influence of hospital surgical volume on outcomes for patients with rectal cancer. Only 2 of 8 studies that evaluated 30-day postoperative mortality revealed lower mortality in high-volume hospitals, and among 10 studies in his review 6 revealed no association between high hospital surgical volume and overall survival.
Most of the reported literature regarding hospital case volume and outcomes relates to a specific procedure for cancer treatment rather than a cancer diagnosis itself. Our study investigated case volume of diagnoses of metastatic melanoma rather than a specific procedure associated with that diagnosis. Treatment of metastatic melanoma, like head and neck cancer, involves coordinated use of multiple modalities (eg, surgery, radiation therapy, chemotherapy, immunotherapy) as well as careful consideration of patient preferences regarding quality of life for a noncurable disease (ie, when to consider hospice care). It is beyond the scope of these data to ascertain what about the higher metastatic melanoma case volume that resulted in better survival outcomes. We also found that patients who had ≥4 days of hospice enrollment experienced longer survival, a similar finding to those of other investigators who have shown that more days of hospice for patients with advanced cancer is associated with longer survival times.21,22
Our study found that patients in high-volume hospitals were less likely to receive chemotherapy, surgery, and radiation therapy. Few studies have investigated the use of chemotherapy, surgery, and radiation therapy with respect to hospital case volume. In the study by Baek et al17 on patients with rectal cancer, the authors found that sphincter-preserving procedures were more likely to be performed in high-volume hospitals. Bristow et al16 found that the high-volume hospitals were more likely to perform treatment guideline-recommended cytoreductive surgery for patients with ovarian cancer.
Our study has several limitations inherent to the use of retrospective claims data. The main limitation was that it was not possible to capture the patients whose initial diagnosis and treatment occurred outside a SEER geographic region. Therefore, the relationship of survival and hospital volume we observed may not be broadly generalizable.23 The study is also limited by the elderly population represented in the SEER-Medicare database (those aged 65 y and older), so it should be noted that these data do not reflect care for younger patients with metastatic melanoma.24–26 The SEER code used to identify stage IV disease in this cohort is assigned if metastasis develops within 6 months of diagnosis or at the end of initial treatment; therefore, some patients might have received treatments early on for curative intent before practitioners were aware that patients had disseminated disease. Moreover, exclusion of claims data for those enrolled in managed care organizations limited the sample size and generalizability of the study findings. Finally, our data do not capture any information regarding quality of life for this cohort of patients with advanced cancer.
Our study showed a significantly improved survival for patients with metastatic melanoma who received care in a hospital that had treated a relatively high volume of patients with metastatic melanoma. The implications of our findings raise several important considerations. Not every patient with metastatic melanoma has access to high-volume hospitals. The long traveling distances that may be required to access high-volume hospitals imposes physical and economic burdens on vulnerable patients. Furthermore, we are unable to determine from these data what specific factors in high-volume hospitals contributed to improved survival. However, it is possible that coordination among oncologists and other providers more experienced in care of patients with melanoma improves quality of care in such a way that could be an important factor in determining patients’ survival. Our study emphasizes the need for more research into whether or how high-volume hospitals might integrate treatment approaches in such a way that results in longer survival for patients with metastatic melanoma compared with patients who are treated at hospitals that see fewer patients with this disease.
Supplementary Material
Acknowledgments
The authors thank Brenda Moss Feinberg, ELS, from the Department of Scientific Publications at MD Anderson for her professional editing for the manuscript. The authors also thank the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS) Inc.; and the SEER Program registries in the creation of the SEER-Medicare database. This manuscript has been approved by IMS as compliant with the database user agreement.
Supported in part by a grant from the Agency for Healthcare Research and Quality (grant # R01-HS018956) and in part by a grant from the Cancer Prevention and Research Institute of Texas (Multi-Investigator Award grant # RP101207).
Footnotes
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.amjclinicaloncology.com.
References
- 1.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 3.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003;83:68–78. doi: 10.1002/jso.10244. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 5.Gruen RL, Pitt V, Green S, et al. The Effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin. 2009;59:192–211. doi: 10.3322/caac.20018. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. [Accessed May 15, 2013];Cancer Facts and Figures. 2011 Available at: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2011.
- 7. [Accessed May 15, 2013];Melanoma Skin Cancer Overview. 2012 Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003063-pdf.pdf.
- 8.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Accessed May 15, 2013];SEER Cancer Statistics Review, 1975–2005. 2009 Available at: http://seer.cancer.gov/csr/1975_2005/results_merged/topic_topfifteen.pdf.
- 10.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites1969–1999. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 doi: 10.1097/01.MLR.0000020942.47004.03. IV–3–18–IV–. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed March 5, 2013];SEER-Medicare: calculation of comorbidity weights. 2010 Available at: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 13.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 14.Parsons L. Performing a 1:N case-control match on propensity score. Proceedings of the Twenty-ninth Annual SAS Users Group International (SUGI) Conference; May 9–12, 2004.SAS Institute; [Google Scholar]
- 15.Bristow RE, Palis BE, Chi DS, et al. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Bristow RE, Zahurak ML, Diaz-Montes TP, et al. Impact of surgeon and hospital ovarian cancer surgical case volume on inhospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334–338. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Baek J-H, Alrubaie A, Guzman E, et al. The association of hospital volume with rectal cancer surgery outcomes. Int J Colorectal Dis. :1–6. doi: 10.1007/s00384-012-1536-1. [DOI] [PubMed] [Google Scholar]
- 18.Barocas DA, Mitchell R, Chang SS, et al. Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol Onco. 2010;28:243–250. doi: 10.1016/j.urolonc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Porter M, Gore J, Wright J. Hospital volume and 90-day mortality risk after radical cystectomy: a population-based cohort study. World J Urol. 2011;29:73–77. doi: 10.1007/s00345-010-0626-3. [DOI] [PubMed] [Google Scholar]
- 20.Salz T, Sandler RS. The effect of hospital and surgeon volume on outcomes for rectal cancer surgery. Clin Gastroenterol Hepatol. 2008;6:1185–1193. doi: 10.1016/j.cgh.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor SR, Pyenson B, Fitch K, et al. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33:238–246. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Saito A, Landrum M, Neville B, et al. Hospice care and survival among elderly patients with lung cancer. J Palliat Med. 2011;14:929–939. doi: 10.1089/jpm.2010.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrag D, Bach PB, Dahlman C, et al. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(suppl) doi: 10.1097/00005650-200208001-00013. IV–96–103. [DOI] [PubMed] [Google Scholar]
- 24.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40:IV62–IV68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database: applications and limitations. Med Care. 2002;40:IV19–IV25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 26.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV104–IV117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.