Abstract
Cerebral small vessel diseases (SVD) range broadly in etiology but share a remarkably overlapping pathology. Features of SVD including enlarged perivascular spaces and formation of abluminal protein deposits cannot be completely explained by the putative pathophysiology. The recently discovered glymphatic system provides a new perspective to potentially address these gaps. This work provides a comprehensive review of the known factors that regulate glymphatic function and the disease mechanisms underlying glymphatic impairment emphasizing the role that aquaporin-4 (AQP4)-lined perivascular spaces, cerebrovascular pulsatility, and metabolite clearance play in normal CNS physiology. This review also discusses the implications that glymphatic impairment may have on SVD inception and progression with the aim of exploring novel therapeutic targets and highlighting the key questions that remain to be answered.
Introduction
There are 46.8 million people worldwide suffering from dementia, and while there are several distinct neurodegenerative causes, cerebral small vessel disease (SVD) can be found in all forms(1). It is considered to be the most common pathology found in the elderly and it doubles the chances that neurodegenerative pathology will lead to dementia(1, 2). Despite its importance and rising incidence, there are no available treatments. The clinical hallmarks of SVD have been well defined, yet little is known of the mechanisms that drive them(3). SVD can be diagnosed using MRI before clinical signs and symptoms arise, and subclinical progression evolves over years to decades, providing a wide therapeutic window for intervention. Understanding the mechanisms behind SVD would undoubtedly bring us closer to controlling one of the most important risk factors of potentially preventable dementia.
Cerebral Small Vessel Disease
SVD is a group of age-related neuropathological processes affecting the small perforating arteries, arterioles, capillaries, and venules resulting in damage to the cerebral white and deep grey matter (4). The term SVD encompasses six subtypes of varied etiologies that have recently been reclassified due to their remarkably similar features on MRI (5). Neuroimaging in SVD shows white matter lesions (WML), enlarged perivascular spaces (EPVS), lacunes, and cerebral microbleeds (CMB); these pathological findings manifest as cognitive decline, increased risk of stroke, and other neurological and psychiatric disorders(2). The most common subtypes of SVD are types 1–3 and will be highlighted over the course of this review.
Type 1 consists of sporadic arteriolosclerosis secondary to aging and other vascular risk factors including systemic arterial hypertension (AHT) and type 2 diabetes mellitus (DM2). Type 2 is sporadic or hereditary cerebral amyloid angiopathy (CAA). Type 3 includes all inherited or genetic SVD subtypes excluding CAA, the most common being cerebral autosomal dominant arteriopathy with subcortical ischemic strokes and leukoencephalopathy (CADASIL) (5). For in-depth reviews of the different SVD subtypes, please refer to the following excellent references(3, 6–8).
The clinical, cognitive, neuroimaging and neuropathological spectra of SVD have been well described, but our mechanistic understanding of the neuropathology is lacking. Most definitions of SVD implicate the ischemic effects of vascular pathology as the central driver of the pathophysiology. However, recent studies demonstrate that SVD pathogenesis is much more complex than initally appreciated, and revealed several inconsistencies with the previously hypothesized mechanism(5). First, the arterioles supplying tissues displaying severe pathology typically have thickened vessel walls, but are rarely occluded(9). Second, a meta-analysis devoted to evaluating cerebral blood flow alterations in patients with SVD found no evidence that hypoperfusion precedes WML(10). Third, epidemiological studies have shown that atherothromboembolic disease burden does not predict SVD severity(3, 6) and fourth, hypoperfusion is not observed in all SVD subtypes, yet they still exhibit a common pattern of injury. Fabry’s disease is an example of a type 3 genetic SVD that, contradictory to the hypoperfusion/ischemia-centered hypothesis, has marked hyperperfusion of the brain parenchyma(9). These inconsistencies suggest that there may be alternative pathomechanisms driving this overlapping pathology. Adding futher credence to this notion, SVD pathology such as EPVS, perivascular aggregation of endogenous amyloid-β (Aβ) in CAA, and granular osmiophilic material (GOM) in CADASIL are not completely explained by the classic mechanisms. The recent discovery of the glymphatic system, a brain-wide pathway for metabolic waste drainage and fluid homeostasis, provides a new perspective on SVD pathophysiology. In this article, we will review the current glymphatic literature and highlight the known mechanisms underlying glymphatic system impairment. Using the available evidence from several disease models relevant to SVD, we hope to shed light on the role that glymphatic failure may play in SVD pathogenesis and what questions must still be addressed to do so.
Glymphatic System
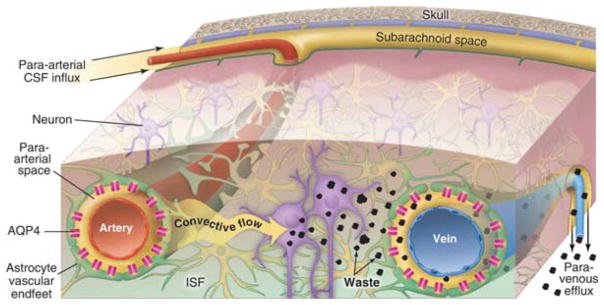
The glymphatic system is a brain-wide pathway along a system of connecting perivascular spaces (PVS) over which cerebrospinal fluid (CSF) surrounding the brain exchanges with the interstitial fluid (ISF) within the parenchyma (Figure 1). This system has been previously reviewed in detail(11). Bulk flow entry of CSF into the periarterial spaces (glymphatic influx) has been shown to be important for the brain-wide delivery of glucose(12) in energy metabolism, transport of lipids and signaling molecules(13), and apolipoprotein E (apoE)-derived from the choroid plexus(14). While glymphatic efflux of ISF has shown to play a vital role in the clearance of several metabolic waste products and other solutes such as Aβ and tau(15). Although this system has been predominately studied in rodents, the glymphatic system has also been demonstrated to exist in humans (16–19).
Figure 1. Overview of the Glymphatic System.
Convective flow of cerebrospinal fluid (CSF) enters the PVS of pial arteries and dives down into the brain through penetrating artery PVS. These PVS are surrounded on the outside by a sheath of astroglial endfeet lined with aquaporin-4 (AQP4) water channels. AQP4 facilitates the bulk flow movement of CSF into the parenchyma where it mixes with interstitial fluid (ISF). Convective ISF flow propels waste products, such as amyloid-β and tau, towards veins where they enter PVS for efflux out of the CNS. This process is regulated by changes in the extracellular space volume of the parenchyma as is seen during sleep-wake state transitions. Adapted with permission from the AAAS(126).
The mechanisms driving glymphatic fluxes have only recently begun to be pieced together (17). Glymphatic system function requires adequate CSF production by the choroid plexus to provide a pressure gradient for fluid to move from the ventricles to the subarachnoid space (SAS) and subsequently into PVS (11). The PVS is lined, almost in its entirety, by astrocytic endfeet lined with aquaporin-4 (AQP4) water channels abutting the abluminal vessel wall. Previous literature has established that AQP4 is necessary for glymphatic fluid fluxes and CSF-ISF exchange is modulated by both sleep-wake state changes and body posture(20–22).
While working to identify key factors that govern the glymphatic system, our lab and others have begun to uncover several processes that become deranged in pathological states. These discoveries have allowed us to further understand the processes underlying glymphatic dysfunction and identify overlapping phenomena across a broad range of disease mechanisms. In speculating on glymphatic failure in SVD, three sections of the pathway stand out and will be elaborated upon in the following three sections. (1) Perivascular spaces, an their structural and functional integrity, have shown to be critical to homeostatic glymphatic function, specifically the role of perivascular AQP4 polarization (20). We propose that vascular remodeling, EPVS, and the perivascular aggregation and deposition of Aβ in CAA and GOM in CADASIL could potentially influence PVS bulk flow. (2) Cerebrovascular pulsatility has been demonstrated to be a driving force for CSF flow within the PVS. Extensive experimental evidence and mathematical modeling have shown tracer movements compatible with bulk flow within these spaces, although tracer entry is size-dependent and PVS blockage (i.e. protein aggregates) has shown to affect these flows (20, 23–26). A recent theoretical model contradicts the existence of bulk flow in the PVS, but provides no supporting experimental evidence(27). Features of SVD like changes in vascular wall compliance and reactivity are likely to alter these flows significantly. (3) CNS clearance of toxic solutes such as Aβ, has been shown to be drastically reduced in several age-related and neurodegenerative disease models. This is relevant to some SVD types 2 and 3 (e.g. CAA and CADASIL) characterized by protein aggregation and deposition. Glymphatic function has been assessed in several highly relevant processes to SVD including aging(28) and diseases such as microinfarction (29, 30), stroke (31), Alzheimer’s disease (AD)(32), migraine(33), and diabetes(34). Extrapolating from the current literature, we will focus on the effects that altered PVS structure and function, arterial pulsatility, and clearance have on glymphatic function. We posit that glymphatic impairment plays an important role in SVD.
1. Perivascular Spaces
Glymphatic pathways start at PVS surrounding small penetrating arteries, also referred to as Virchow–Robin spaces (VRS), these separate the vessels from the brain parenchyma (35). VRS are cavities that contain CSF-like fluid and are restricted by vessel wall at the inner boundary and by astrocytic end-feet and pia matter on the exterior. They are functional extensions of the SAS, but are indirectly connected to the SAS due to pial separation of the two compartments(36). As vessels dive into the parenchyma, their pial covering gradually disappears giving way to an astroglial sheath that becomes the outer wall of the PVS and covers a large portion of the the brain’s microvasculature. Previous studies, using chemical fixation methods, report that astrocyte endfeet cover between 95–99% of the surface area of the capillary cross-section; however, recent estimates, using cryofixation, place this number closer to 63% (37, 38). This reduced astrocytic coverage of the capillary bed would provide even greater access to molecules entering from the PVS into the parenchyma. As the penetrating arteries branch into arterioles and capillaries, VRS disappear but CSF continues to flow into the perivascular spaces around arterioles. At capillaries, the extracellular matrix of the basal lamina is composed primarily of laminin, fibronectin, type IV collagen, heparin sulfate proteoglycan (11). Beyond the arteriolar perivascular space, the basal lamina provides the next low resistance fluid space from which the CSF moves into the parenchyma. AQP4 channels are highly expressed at the end-foot relative to the soma; this polarization to the abluminal vessel wall facilitates fluid influx into the parenchyma and efflux out of the brain (15, 20).
Enlarged Perivascular Spaces
Normal PVS are not typically seen on conventional structural MRI and can only be visualized when enlarged(2, 39). EPVS are considered one of the earliest and most consistent neuroimaging findings in SVD(3, 4, 39–41). They are spaces, less than 3 mm in diameter, filled with CSF-like fluid, which follow penetrating vessels. They are commonly found in the basal ganglia, centrum semiovale, and midbrain(3). Observing similar pathological findings in these different regions is intriguing because PVS anatomy varies by location. PVS in the cortex are smaller than in white matter(42), and PVS around superficial perforating arteries have only one layer of pia matter while PVS of deep perforating arteries in the basal ganglia have two(36). The role that anatomical differences play in susceptibility to enlargement are unknown, and a more in-depth characterization of regional differences in PVS is needed. Interestingly, the largest CSF influxes occur along large ventral perforating arteries of the basal ganglia, a common site for EPVS formation in SVD (20, 43).
Despite having relatively distinct etiologies, EPVS are widespread and present in almost all SVD types, it is an established observation in both sporadic type 1and 2 and hereditary type 2 and 3 forms of SVD(40, 44, 45). Although there are no confirmations of EPVS in the autosomal recessive form of CADASIL, CARASIL, there is some evidence of perivascular softening in this disease(46). EPVS are associated with WML, lacunar infarcts, and CMB(3, 40, 47–49). Basal ganglia and centrum semiovale EPVS are routinely seen together, implying a common cause(3). In addition, EPVS are independently associated with SVD risk factors including hypertension and advanced age(41, 47, 48). White matter lesions, comprised of myelin loss, scattered small infarcts, and astrogliosis, are an important contributor to the clinical picture of SVD(50–52). Interestingly, EPVS and WML are often spatially colocalized, and WML appear around EPVS(3). Additional studies have found the presence of perivenular edema associated with venous collagenosis –a type 5 SVD– of the deep medullary venules(53). These features suggest that EPVS may be a sign of glymphatic fluid stasis. Since current evidence indicates that EPVS play a role in the pathogenesis of SVD –as opposed to being the result of the disease– glymphatic dysfunction is likely to be an important contributor to SVD pathology(3, 4, 39–41). Whether perivascular fluid accumulation results in white matter edema, giving rise to WML remains to be addressed, and represents an interesting avenue of research.
Aquaporin-4
The clinical and neuroimaging picture of genetic forms of SVD closely resembles that of the sporadic forms. Most Mendelian forms of disease result from mutations in genes that encode proteins important to abluminal vascular function. This provides further evidence for the perivascular basis of SVD pathogenesis. In CADASIL, a mutated form of Notch3 is secreted by pericytes and vascular smooth muscle cells into the PVS(54, 55). Mutations in collagen type IV A1/A2, a principal component of the PVS extracellular matrix, results in a type 3 SVD. In CARASIL, the serine protease HTRA1 is secreted into the extracellular matrix of the PVS(8). To date, glymphatic function has not been characterized in any existing models of SVD, and the effect of EPVS on glymphatic function has not been described. The expression and polarization of AQP4 on the endfoot abutting the PVS is a strong regulator of normal glymphatic function – these channels cover approximately 50% of the surface area of the endfoot(56). A spatially distinct pattern of AQP4 expression is observed in the astroglial sheath with higher expression in the perivenous and pericapillary endfeet compared to the periarterial endfeet(20). The importance of AQP4 for glymphatic flow is highlighted by Aqp4−/− mice having significantly impaired CSF tracer influx compared to controls(20). These mice are hypothesized to lack a low resistance pathway for CSF flow into the parenchyma and for ISF out into the PVS. These changes in glymphatic transport in Aqp4−/− mice are evidenced by increased brain water content secondary to their inability to clear brain water during development(57). These aberations in brain water content result in larger extracellular space volume in these mice (ECS) (58). Recent theoretical models diverge with the experimental evidence of glymphatic function in Aqp4−/− mice, and additional experimental techniques will be required to draw firm conclusions(59).
A robust derangement of perivascular AQP4 also ocurrs in reactive astrogliosis(60). General expression of AQP4 is increased within regions of diffuse reactive glial fibrillary acidic protein (GFAP)-positive gliosis. A vascular dementia (VaD) model characterized by diffuse multiple microinfarction (MMI) was achieved by intracarotid injection of cholesterol crystals (40–70μm) in two recent studies(29, 30). Both of these studies demonstrated severe glymphatic system impairment initially, with glymphatic influx returing to baseline by 14 days after the injury. Interestingly, expression of AQP4 was elevated at 7 days post-injury, but normalized by day 14. Perhaps more important than increased AQP4 expression is the loss of perivascular AQP4 polarization. The polarization of AQP4 remains perturbed up to a month after injury. Data from traumatic brain injury (TBI), found that increased AQP4 expression and loss of perivascular polarization coincided with a decrease in intracranial pressure (ICP) (61). This redistribution of AQP4 may be a mechanism to protect against vasogenic edema further supported by the fact that Aqp4−/− mice are protected from a model of acute water intoxication(62).
The most applicable evidence for AQP4’s role in SVD is that in the cortex of old mice (18 months), general AQP4 expression is markedly increased, especially around penetrating arterioles, but shows almost no polarization to the astrocyte endfeet(28). In these mice, GFAP expression was also upregulated in arterioles and capillaries, and had a high degree of localization on endfeet processes(28). Perivascular AQP4 polarization in the brains of old mice was consistently and significantly reduced compared to the brains of young mice(28). CSF tracer penetration into the brains of young (2–3 months) controls was significantly increased compared to middle-aged mice (10–12 months), and even further increased compared to old-aged (18 months) mice. In the old mice, loss of cortical perivascular AQP4 polarization was a significant predictor of glymphatic influx impairment. GFAP expression did not show these relationships to influx impairment. The relationship between AQP4 and influx impairment was not maintained across all age groups, suggesting that there are additional age-related effects that alter glymphatic influx in addition to AQP4 polarization.
The role of AQP4 and glymphatics in Aβ clearance has recently garnered increased attention in the field of AD. In terms of SVD, there seems to be a connection between SVD and AD, as CAA pathology affects approximately 80–90% of AD patients(63). In a cohort of postmortem human brain specimens, age was positively correlated with increasing AQP4 expression in the frontal cortex, and the loss of perivascular AQP4 polarization was a predictor of AD status, amyloid plaque density, and Braak staging(64). Interestingly, AQP4 polarization was largely preserved in cognitively intact individuals over the age of 85. Vascular injury increased with age, but was not independently associated with perivascular AQP4 localization after controlling for the effects of age. This suggests that unknown abluminal mechanisms may be involved in perivascular AQP4 localization. Mislocalization of AQP4 at the astrocyte endfoot has also been confirmed in several mouse models of AD (32, 65, 66). To further extrapolate the role of perivascular AQP4 localization, a study using the same model of VaD as described above in rats, found that decreased perivascular AQP4 expression persisted up to 6 weeks after microinfarction(30). More importantly, perivascular AQP4 loss was positively correlated with cognitive impairment in three separate behavioral tasks (novel object recognition test, odor recognition, and Morris water maze)(30). Although an interesting relationship, the direct role that effective perivascular AQP4 polarization may play in cognitive function requires further exploration.
Despite no available evidence for the role of glymphatic function in models of SVD, there is literature evaluating the role of AQP4 expression in animal models of AHT (type 1 sporadic age- and vascular risk factor-related SVD) such as the spontaneously hypertensive rat (SHR) and the SHR-stroke prone (SHRSP). In SHR, increased expression of AQP4 was found in the frontal cortex, striatum, and hippocampus as early as 4 months when high blood pressure was still developing, and remained high until 6 months once hypertension was firmly established (67). This increase in AQP4 was followed by a later rise (starting at 6 months) in GFAP-positive astrogliosis in frontal cortex, occipital cortex, and striatum (68). This may suggest that in AHT, upregulation of AQP4 precludes astrogliosis, possibly in response to changes in brain water balance or blood-brain barrier (BBB) integrity. Similar findings were obtained in the SHRSP rat(69). AQP4 expression was significantly higher in 20 week- old SHRSP rats when blood pressure was already elevated to 226 ± 4.5 mmHg, while glucose transporter-1 (GLUT-1) on endothelial cells, vital for brain energy metabolism, was significantly reduced. Hormones relevant to arterial hypertension such as vasopressin and angiotensin II also influence the expression of AQP4(70). Unfortunately, these studies only quantified global expression and did not evaluate perivascular polarization of AQP4, but these data allow us to hypothesize how PVS glymphatic inflows may be altered.
In type 2 and 3 genetic SVD animal models including CAA and CADASIL models, the data suggests a different mechanism. In the APPSwDI mouse model of CAA (strain with high CAA levels), a stark decrease in AQP4-postive blood vessels was observed in the frontal cortex and hippocampus, in contrast to the observations in AHT models(71). However, these vessels also had evidence of reduced GFAP expression. Both these findings were confirmed in human tissue samples from a patient with a premortem diagnosis of severe CAA. This human tissue also showed a coinciding decrease of dystrophin expression, a protein that forms the alpha-syntrophin scaffolding complex that allows the polarization of AQP4 to the endfoot. These data, together with the almost ~50% decrease in vascular endfeet observed in this study suggests that in CAA, it is most likely that astrocyte endfeet become separated from the PVS basement membrane, compared to the perivascular reactive gliosis with redistribution of AQP4 seen in aging(71). Another postmortem human brain study of AD and CAA showed a paradoxical increase in AQP4 around blood vessels in AD cases with moderate to severe CAA(72). The previously described human study did not quantify the immunoreactivity, but quantified the expression changes at the mRNA level, while the latter quantified immunoreactivity and performed a qualitative analysis in terms of localization. Given the contradicting results, it is necessary to determine the perivascular expression of AQP4 in CAA further.
Additional evidence of the detachment of astrocytic endfeet can be seen in animal models of CADASIL. In the mouse model TgNotch3R169C, at 12 months there is a reduction in GFAP staining and a significant retraction (~70%) of the endfeet from the microvasculature. This pattern is followed by abnormally organized AQP4 distribution in the endfeet, and further supports the idea of loss of astrocytic coverage of the vasculature in CADASIL(55). The effects of this process in glymphatic function have not been described, and would provide an interesting avenue to study the cross-talk signaling mechanism that regulate flows within these spaces.
Protein Aggregates
The formation of protein aggregates is a hallmark of most neurodegenerative diseases: Aβ and tau in AD, alpha-synuclein in Parkinson’s disease, and huntingtin in Huntington’s disease. This is also the case in type 2 and 3 SVDs, and deposition occurs primarily on the abluminal surface of the PVS.
CADASIL is caused by a dominant mutation in Notch3 – a gene encoding a transmembrane receptor that is mainly expressed in vascular smooth muscle cells(54, 73). The Notch3 extracellular domain (Notch3ECD) accumulates, serving as a scaffold for the binding of tissue inhibitor of metalloproteinase 3 (TIMP3) and vitronectin resulting in the formation of GOM deposits (7, 74). Aggregation and deposition of GOM appears to be crucial for the development of CADASIL pathology(75). Notch3 aggregates can typically be found in tunica media, basement membranes and PVS of meningeal, pial and perforating small arteries, arterioles, and capillaries, especially around pericytes(76, 77).
The role of protein aggregation in the PVS on glymphatic system function has been recently tested and provides evidence supporting the role of glymphatic impairment in SVD pathogenesis. In a mouse model of subarachnoid hemorrhage (SAH), it was recently shown that SAH severely impaired glymphatic influx, even after bilateral craniectomy, suggesting that the impairment was independent of SAH-induced intracranial hypertension. The inability of a fluorescent-labeled dextran in the CSF to reach the PVS led them to identify the presence of fibrin/fibrinogen deposits(31). These fibrin clots occluded the perivascular spaces after SAH, and interestingly, glymphatic influx improved after the administration of a fibrinolytic agent (tissue-type plasminogen activator, tPA) (31). Whether the fibrin clot creates a sieving effect and prevents entry of the high molecular weight tracer but allows CSF entry, or if it infact blocks total CSF entry into the parenchyma has yet to be determined. The blockage of the PVS also slowed clearance of an MRI contrast agent from the striatum(31). Accumulation of plasma-derived proteins (i.e. fibrin/fibrinogen and albumin) are a hallmark of several SVD types and may likely affect glymphatic influx in a similar fashion.
It is well known that the key factor of the pathogenesis of AD and CAA is accumulation of different Aβ subtypes. Neuritic plaque formation in AD is primarily due to accumulation of Aβ1–42 (Aβ42) which contains 42–43 amino acid residues and is more insoluble and more prone to oligomerization than Aβ1–40 (Aβ40). Aβ40 contains 39–40 residues, and is responsible for cerebrovascular amyloid deposition prevalent in CAA(78). The effects of perivascular Aβ deposition on glymphatic function has recently been evaluated(32). Our recent study clearly demonstrated that both Aβ40 and Aβ42 circulate within the glymphatic pathway(32). Glymphatic influx of fluorescent-labeled Aβ40 was more efficient than that of Aβ42. Aβ40 was present within the parenchyma and taken up by neurons, while Aβ42 showed reduced glymphatic inflow, potentially reflecting its greater propensity to oligomerize and form fibrils(32). When Aβ was pre-infused into young wildtype mice (40 min), Aβ40 –but not Aβ42– reduced CSF influx of an inert dextran. This is particularly intriguing given that Aβ40 is the primary species found in perivascular deposits in CAA. The inhibitory effects of Aβ on CSF influx into the PVS was confirmed in a mouse model of AD (APP/PS1), that showed decreased inflow of CSF solutes in young AD mice (3–4 months), even prior to the 2 fold increase in soluble Aβ and development of plaque pathology that is seen starting at 6 months of age onward. It was also shown that CSF-derived Aβ could incorporate into established, endogenous Aβ plaques in older mice. Interestingly, a recently developed technique called “diffusion tensor image analysis along the perivascular space” (DTI-ALPS) was used to show that lower water diffusivity along the perivascular space of the medullary veins that run perpendicular to the lateral ventricle wall is negatively correlated with AD and mild cognitive impairment severity in humans(19). Although glymphatic function has never been formally studied in the context of CAA or CADASIL, taken together, these data point to a possible role for PVS obstruction secondary to protein aggregation in glymphatic impairment.
PVS Dynamics
Until recently, the PVS was considered a passive anatomical structure that most likely changed its volume secondary to transient vasoconstriction and vasodilation of the vessel it surrounded. A previous study using ultra-fast MR encephalography (MREG) in humans concluded that some CNS fluid pulsations could be attributed to very low- and low frequency waves(17). The authors surmised that these waves resulted from vasomotion modifying the PVS volume, and in turn modified its electrical conductance(17). However, a novel study used in vivo two photon laser scanning microscopy to image the PVS in βactin-GFP mice(33). Using orthogonal reconstruction from pial blood vessels, the authors found that periarterial spaces cross-sectional area were ~300 μm2 while paravenous spaces were smaller (~90μm2). They demonstrated that cortical spreading depression (CSD), commonly used as an animal model of migraine aura, induced a rapid and nearly complete closure of the paravascular space(33). PVS closure began in penetrating arteries and ended at pial vessels, lasted several minutes, and gradually reopened over a 30 min period. An offset in timing between vasomotion and the closure of the PVS suggests that this closure is not the result of changes in vessel diameter, but is instead due to neuronal and astrocytic swelling. The idea that astrocytic endfeet swelling is an active regulator of PVS glymphatic flow is appealing when considering potential mechanistic targets for interventions aimed at therapeutic modulation of the system. Astrocytes possess all the necessary machinery to modulate their cell volume largely via AQP4-dependent mechanisms(56). Nonetheless, the latter study used a model of pathology (pin-prick and KCl induction of CSD) that most likely does not reflect the physiological regulatory mechanisms. However, the previous MRI study attributed the very low wave frequencies to direct current-EEG (DC-EEG) which has a strong contribution from the BBB(17). For the BBB to account for these waves, there would have to be an oscillatory change in permeability at the BBB. Authors posited that this was unlikely, and attributed it to vasomotion, however transient changes in BBB permeability have not been convincingly disproven. In CSD, PVS closure causes a reduction in glymphatic clearance after intracortical dye injection. The selective and regionally specific closing of these spaces as a regulating mechanism for glymphatic flow is interesting. This is highly relevant in studying the glymphatics of CADASIL, as migraines with aura are present in 30–40% of patients as the earliest symptom, happening decades before stroke(6), implying that early treatment of migraines may improve the clearance of GOM deposits way before vascular pathology sets in.
2. Cerebrovascular Pulsatility
Arterial pulsatility secondary to the cardiac cycle has long been shown to contribute to perivascular and interstitial fluid flows(25, 26, 79). A previously mentioned study that used ultra-fast MR showed that pulsations derived from the R-wave of the cardiac cycle drive fluid movement in the periarterial spaces while oscillations due to the respiratory cycle drive perivenous flows(17). Respiratory pulsations function as a low-pressure counter-system that may provide a driving force for ISF convection (17). During inspiration, intrathoracic pressure becomes negative, reducing blood volume in the cerebral veins, which is hypothesized to increase the PVS allowing solute to accumulate. Exhalation does the converse, it raises intrathoracic pressure, increases cerebral venous blood volume, and closes the PVS, driving fluid out into the CSF(17). Although compelling, more research is needed to fully describe the roles that these physiological variables play in glymphatic system circulation. Vascular pathologies including arteriolosclerosis, fibrinoid necrosis, and lipohyalinosis may be found in all subtypes of SVD(4, 50). It is plausible that a connection between changes in vascular pulsatility in cerebrovascular disorders may have profound effects on glymphatic homeostasis.
In CAA, vascular Aβ deposition independently affects microvascular pulsatility. At the early stages CAA impairs leptomeningeal and cortical vessels and may later spread to the vessels of other brain regions(80), with the posterior areas of the cerebrum often heavily affected(81). CAA also leads to secondary vessel pathology involving loss of medial smooth muscle cells, fibrinoid necrosis, fibrohyalinosis, microaneurysms, thrombus formation, and luminal stenosis(81). Although these changes presumably lead to decreased arterial vasomotion and vascular reactivity due to permanent changes in the basement membrane, the loss in vessel wall compliance secondary to arteriosclerosis increases arterial pulsatility(77). Increased stiffness of large elastic arteries, also seen in aging, causes excessive propagation of the systolic pulse wave to the microcirculatory bed, increasing the pulsatility index (large and small artery cross-talk)(82–85).
Increased arterial pulsatility was shown to drive glymphatic influx by systemic administration of dobutamine (β1 adrenergic agonist) in mice(79). Dobutamine acutely increased systolic, diastolic, and mean arterial blood pressure. After 30 minutes, blood pressure returned to normal and CSF tracer was infused. Dobutamine caused a 60% increase in pulsatility and significantly increased CSF influx, without any changes to vessel diameter(79). Interestingly, the 60% increase in arterial pulsatility only caused about a 25% increase in CSF-ISF exchange, suggesting that there are additional factors driving influx(79). Similarly, in an independent study that increased blood pressure and heart rate by intravenous epinephrine in rats demonstrated faster interstitial convective clearance of fluorescently-labeled albumin, liposomes, and viral vectors(26). Of relevance to SVD, a recent study evaluated glymphatic function in DM2, a known vascular risk factor for arteriolosclerosis(3, 34). MRI analysis using contrast agent (Gd-DTPA) showed that DM2 rats had enhanced areas of perivascular arterial influx and increased signal intensity. Fluorescent tracers confirmed the MRI findings; more tracer accumulated in the periarterial spaces up to 6h later(34). This is extremely noteworthy as DM2 is the first instance of glymphatic influx enhancement, in contrast with all previously described studies of glymphatic pathology that have found reductions in glymphatic influx. This is a novel finding as it allows us to speculate that pathological glymphatic fluxes are not only on the side of inhibition, but also on the side of overstimulation. Curiously, cluster analysis of CSF-ISF exchange in DM2 rats(86), suggested that there was a significantly larger perivascular space in DM2 (34). Taking the latter into account, is it possible that EPVS may cause a paradoxical increase in glymphatic influx by reducing the resistance to flow in the PVS?
Despite an increase in arterial pulsatility due to changes in vascular wall elasticity, it would be wrong to ignore the heavily documented literature on the changes of cerebral blood flow seen in several SVD subtypes. One such example is the impaired reactivity of small vessels seen in CADASIL during increasing changes in blood pressure. Evidence shows that parenchymal arteries constrict less in response to the increased intravascular pressure due to upregulation of potassium channels(87). This ultimately alters the autoregulatory mechanisms of the cerebrovascular network causing hypoperfusion. Hypoperfusion causes reduced arterial pulsatility and reduced CSF-ISF bulk flow, as demonstrated by several groups including our own(25, 26, 79). Internal carotid artery ligation in rodents decreases arterial pulsatility by ~50% and shows markedly reduced CSF tracer influx (73%)(79). Experimental reduction of cranial artery pulse pressure by ligation of the brachiocephalic artery in dogs slowed paravascular CSF flow significantly(25). Similarly, decreasing blood pressure and heart rate by induction of hypovolemia in rats reduced ISF movement of solutes in the parenchyma(26). In support of these findings, and perhaps of glymphatic impairment in SVD, an MCAO model of embolic ischemic stroke in mice also found temporarily decreased paravascular CSF influx, that returned to normal by 24h. Normalization of CSF influx coincided with spontaneous arterial recanalization(31). Pulsatility of the deep penetrating arteries was also shown to be reduced by 27% in old mice (18 months), and this reduction correlated with the amount of CSF tracer influx compared to young (2–3 months) controls(28). Vascular wall compliance is reduced as a function of aging. A loss in compliance would be expected to result in an increase in pulsatility, counter to the results seen in old mice(28). It is possible that in cases of healthy aging without frank vascular pathology, age-related causes of glymphatic dysfunction reside in the pial sheath and AQP4 polarization on astroglial endfoot processes. It is important to note that DM2, an important cause of sporadic SVD, was shown to have larger PVS (on MRI cluster analysis, not confirmed by histology) and dramatically enhanced glymphatic influx. This model is perhaps the most useful in understanding the glymphatics of SVD to date. Although hypoperfusion maybe an important driver in SVD pathology, and with it, glymphatic inhibition, these most likely occur towards the later stages of disease. The DM2 model provides a better snapshot of the preclinical component of most SVD subtypes. This phase is likely characterized by vascular and PVS remodeling due to endothelial cell and BBB damage changing pulsatility without altering perfusion. AQP4 expression was not characterized in this study, but chronic inflammation in DM2 has shown to cause reactive astrogliosis and alter the expression of AQP4(88, 89). These changes will have small effects on overall brain function, but when compounded over years or decades (the average prodromal phase of SVD), it may be of significant clinical importance.
3. CNS Clearance
Efflux routes for glymphatic fluid after its exit from perivenous spaces, are still being elucidated. Among presumed pathways are clearance through the lymphatics in the nasal mucosa, perineural spaces around cranial and peripheral nerves, elements of the cervical neurovascular bundle and lymphatic vessels in the meninges(90–92). Tracers injected into CSF drain into superficial cervical lymph nodes (scLNs) via the cribriform plate (50%), and to the blood stream via arachnoid granulations in the dural sinuses(50%)(90, 93, 94). Arachnoid granulations are not found in rodents and recent studies have shown that a significant efflux site for CSF macromolecules in the rodent brain is meningeal lymphatics(92, 95). These lymphatic vessels travel parallel with the dural sinuses and meningeal arteries, exit the skull, and drain into the deep cervical lymph nodes (dcLNs) in the neck(92, 95). On the other hand, tracers injected directly into the brain exit primarily along two perivenous routes: the medial internal cerebral veins and the lateral-ventral caudal rhinal veins(20). In turn, the internal cerebral vein drains directly into the great vein of Galen and the straight sinus and the caudal rhinal vein drains into the transverse sinus (20). Tracers have also shown to be capable of exiting into the ventricular system, but these transependymal convective flows will require further description(96). Perivenous spaces are thought to drain directly into meningeal lymphatic vessels as they exit the skull but this still requires further elucidation (92, 95). The exact role that dysfunction of extra-CNS clearance pathways play in disease is unclear. However, mice lacking functional vascular endothelial growth factor receptor 3 (VEGFR3), a critical regulator of lymphangiogensis, develop LN hypoplasia and demonstrate decreased clearance of tracers injected into the brain(95). Reduced CSF clearance at the nasal mucosa in humans has also been linked to AD and increased Aβ burden(97). These data suggest that disruption of peripheral efflux sites significantly affect CNS solute clearance. A variety of solutes involved in brain energy metabolism (i.e. lactate and apoE) and toxic metabolic waste products (Aβ and tau) depend on physiological clearance via glymphatics(14, 20, 98, 99). However, fluorescent- or radio-labeled inert tracers like mannitol (182 Da), inulin (6 kDa), albumin (66 kDa), and dextrans (3–2000 kDa) have proved to be the most useful for studying this drainage. Not only can tracers be chosen by size, they also have no putative receptors or transporters that may alter measurements of bulk flow-mediated clearance via glymphatics. Intracortical injection of 3H-mannitol and 3H-dextran (10 kDa) show identical clearance rates in wildtype animals, indicating bulk flow ISF clearance(20, 100), a finding that had been previously confirmed(100, 101). Clearance rates after intrastriatal injection of 125I-Aβ40, exceeded that of 3H-mannitol or 3H-dextran (10 kDa), presumably because Aβ has specific transporters (e.g. RAGE and LRP-1) that can move it across the BBB(102). However, injection of fluorescently-tagged Aβ and tau show that both are transported through the glymphatic pathway and drain along subcortical white matter tracts and large-caliber draining veins (20, 99). Whether these pathways are modified as pathology progresses remains to be established. The role of AQP4 in CNS drainage was demonstrated by a dramatic reduction in clearance from the brain interstitium of about ~70% in Aqp4−/− mice(20). This same study identified that 75% of tracer injected into the brain parenchyma is cleared into the subarachnoid CSF, highlighting the importance of perivenous localization of AQP4.(20, 101). A separate study showed similar results, observing reduced clearance of an adeno-associated viral-9 (AAV9) vector in a different strain of Aqp4−/− mice(103). Aβ clearance was also reduced ~55% in Aqp4−/− mice, suggesting that glymphatics are responsible for about half of the clearance of soluble Aβ in the brain(20). Besides AQP4 manipulation, several other strategies have shown to reduce efflux to deep cervical lymphatics including intracranial depressurization (cisterna magna cisternotomy), acetazolamide (inhibitor of HCO3 anhydrase that blocks CSF production), head position (45° slope downward, neck flexion) and sleep deprivation (inhibits the increased convective flows seen during sleep)(98, 104).
Impaired clearance has been proposed as the main cause of Notch3 accumulation in CADASIL. There is no significant increase in Notch3 mRNA levels, suggesting that accumulation is either the result of impaired clearance or impaired degradation(75). A quantitative assay of protein clearance demonstrated that a CADASIL-inducing mutation caused a decrease in Notch3ECD clearance and turnover(105). Additionally, mutant Notch3ECD has decreased solubility, and an increased ability to spontaneously aggregate and form multimers(106, 107). Additional support for the hypothesized role of glymphatic impairment in accumulation processes is the increase of not only the insoluble bound form of TIMP3, but also a free active soluble form(108). Nevertheless, it has been clearly demonstrated that reducing TIMP3 or vitronectin levels can reduce pathological burden of CADASIL(108), providing a window for increased glymphatic clearance as a therapeutic target in this disease.
Clearance in models of SVD have not been formally studied. However, clearance of Aβ40 and inulin from the caudoputamen in the brain of aged mice was impaired by approximately 40%, providing evidence for the mechanism by which age acts as the single strongest risk factor for dementia(1). Interestingly, it is possible that certain cerebrovascular pathologies including AD affect clearance even before the aging process. Clearance of Aβ40 from the frontal cortex of young APP/PS1 mice showed a 2-fold reduction compared to littermate controls before neuropathology developed, suggesting a causal role for glymphatic impairment in plaque deposition(32). Indirect evidence of the role that glymphatic impairment plays in AD is seen by the genetic deletion of AQP4 in APP/PS1 mice. This additional mutation exacerbated the spatial learning and memory deficits normally seen in APP/PS1 mice(66). The knockout mutants also had more Aβ plaque pathology, and more Aβ deposition in cortical and leptomeningeal vessels (findings normally seen in CAA). These mice also had significantly more soluble and insoluble species of Aβ40 and Aβ42 despite having the same Aβ overexpressing mutations (APP/PS1), suggesting that AQP4 deletion either increased Aβ production or inhibited its clearance mechanisms (66). Further testing, demonstrated that AQP4 deletion did not alter the levels of proteins that play a role in Aβ formation or degradation, suggesting that AQP4 knockout only affected clearance pathways.
An important and not yet completely understood element of Aβ clearance and CAA pathogenesis is its association with apoE. ApoE binds and transports lipids and proteins including Aβ(109). The apoE ε4 allele is a risk factor for CAA(110, 111) because this form of apoE is not able to provide proper receptor-mediated clearance of Aβ(102). ApoE4 results in a shift in the amyloid deposition from parenchymal to vascular, increasing the ratio of Aβ40 to Aβ42(112). Recent evidence demonstrates that endogenous apoE has a spatial distribution within perivascular astrocytes that is higher around arteries and arterioles than around large veins(14). The non-lipidated form of apoE, preferentially to the lipidated form, radially distributes from arteries out into the parenchyma in an isoform-specific manner (apoE2>apoE3>apoE4). The fact that the apoE isoform with the lowest flow pattern is also the isoform that confers the highest degree of risk to AD, CAA, and other SVDs implies that effective transport within the glymphatic system may be an important component of normal apoE function. Of note, inflow of CSF-derived apoE is drastically reduced in Aqp4−/− mice and is likely also due to loss of perivascular AQP4 polarization(14). The authors hypothesize that decreased influx of apoE and reduced radial distribution of apoE4 causes the deposition of this isoform and its contents in the abluminal perivascular surface, in addition to its other well-known functions(102). More research is needed to gain a clear understanding of the role that glymphatic system function plays in apoE transport and its effects on Aβ clearance.
We will apply current evidence from an animal model of DM2 and VaD to understand how glymphatic clearance goes awry. In DM2 rats, there was a 3-fold increase in residual Gd-DTPA in the hippocampal interstitial space, suggesting slower clearance(34). These findings were confirmed by fluorescence imaging analysis as a Texas Red-dextran (3 kDa) was undetectable in the brain of non-DM2 rats at 6h but still quantifiable in the DM2 rats. This data demonstrates retention of both the Gd-DTPA and dextran (3 kDa) dextran despite the previously discussed increase in CSF influx. More importantly, accumulation of a 3 kDa dextran and residual intensity of Gd-DTPA in the hippocampus on MRI strongly correlated (r2 > 0.85) with performance on the Morris Water Maze and odor recognition tests(34). Whether a failure in glymphatic function plays a causal role in cognitive impairment remains to be explored. Authors had also found an increase in the perivascular spaces, and surmised that this larger accumulation of solutes in periarterial spaces may have been caused by reduced clearance. This idea proposes an alternative hypothesis to explain increased glymphatic influx. Although compelling, it is hard to envision that the influx of a determined volume of CSF into an arterial PVS is not matched by the efflux of an equal volume of ISF downstream. However, this could be explained by a sieving effect, in which increased CSF influx, drives more tracer into the PVS. However, due to alterations in PVS morphology (EPVS), AQP4 polarization, and/or changes in the molecular composition of the basal lamina, the tracer cannot leave the PVS. This state of “solute trapping” could also be occurring in the parenchyma caused by astrogliosis and changes in the extracellular space (volume and tortuosity). A recent study from our lab demonstrated the concept of “solute trapping” and glymphatic impairment in diffuse multiple microinfarcts (VaD)(29). We showed that 3 days after unilateral microinfarction, CSF tracer influx was virtually abolished in the ipsilateral cortex. Notably, CSF tracer movement in the contralateral hemisphere was also markedly reduced(29). We found that glymphatic function was predominately affected in the subcortical white matter of the corpus callosum and the hippocampus(60). The effect of multiple microinfarction on glymphatic influx was further reduced when aged animals were used. Normal glymphatic influx returned to baseline 14 days following injury. The exact mechanisms behind glymphatic impairment in this model are not completely understood, but the temporal features seem to fit with the resolution of mild cerebral edema (~2% increase in brain water content) and increased ICP(~4.2 mmH2O)(60). This in turn, coincides with the return to baseline patterns of AQP4 expression that normalized by 14 days, when glymphatic influx was recovered. This redistribution of AQP4 has been suggested to be a protective mechanism for the resolution of vasogenic cerebral edema, shown to also occur in this model(61, 62). A similar scenario is seen after TBI, where glymphatic function is reduced by 60% and lasts for at least 1 month after injury(99). This was attributed to an increase in AQP4 expression but a loss of perivascular AQP4 polarization. It is also possible, given the recent findings in CSD, that cerebral edema may cause transient PVS closure and therefore slow down glymphatic function until edema is resolved(33). The mechanisms underlying PVS closure depending on whether it is cytotoxic cell swelling or vasogenic edema will eventually need to be addressed. Focal solute trapping was seen when an ovalbumin (45 kDa) tracer was injected into the CSF 3 days after MMI. The tracer accumulated in the microinfarct cores (lacunes), and remained at 14 days(29). Focal solute trapping at the lesion cores persisted despite glymphatic function returning back to normal(29). Authors hypothesized that trapping of cytotoxic metabolites or cytokines could perpetuate a neuroinflammatory cycle and predispose to focal BBB disruption (29). A similar model of VaD in rats, found decreased clearance of a 3 kDa and 500 kDa dextran at 6 h after cisterna magna injection, suggesting impaired ISF clearance in VaD.
There is no direct evidence of glymphatic clearance failure in SVD to date. Nonetheless, there are several indirect findings that may indicate that clearance is also decreased in SVD. First and foremost, in cases of type 2 and 3 SVD including sporadic CAA and CADASIL, accumulation of protein deposits without evidence of increased production strongly suggest impairments in clearance. In a mouse model of CADASIL, the TgPAC-Notch3R169C line, has demonstrated to have progressive focal alterations in myelination, in part explaining the many WML found in this SVD. Coincidently, this strain had almost 4 times more myelin debris at 20 months of age than controls; this debris load increased 4-fold between 12 and 20 months, and was not accounted for by exaggerated oligodendrocyte cell death(113). Authors hypothesized that ineffective microglial phagocytosis was the basis for this accumulation, but only 6–13% of myelin debris was in contact with microglia. This could be indirect evidence of decreased clearance in CADASIL, and would support the recently proposed hypothesis that GOM deposition is the result of reduced clearance and not increased production (74). When discussing clearance in type I SVD, the contribution of hypertension on Aβ accumulation is also worth mentioning. Epidemiological data has found an association between hypertension and a strong predisposition for developing AD and Aβ plaque burden (114). Animal models of hypertension have also been shown to develop CAA deposition in the cortex and hippocampus without an amyloidogenic background(115). Hypertension can also aggravate white matter pathology in patients with CAA, in part by decreasing Aβ clearance (116). However, the exact mechanisms of such contribution remain unclear and further studies should be directed towards characterizing solute clearance in the CNS of SVD.
Altered Glymphatic Function in SVD?
Throughout this review we have covered the available literature pertaining to glymphatic impairment. A pervading theme in models of aging, ischemic stroke, SAH, TBI, and VaD is that glymphatic dysfunction occurs over the entire pathway, from CSF influx to ISF efflux. This makes sense as fluid inflow must be matched by an equal outflow to prevent edema. However, a recent paper testing the role of glymphatic impairment in DM2 found a paradoxical increase in CSF influx with a robust decrease in solute clearance. Most notably it happens to be a model that is of particular relevance to SVD. Perhaps one of the most comparable disease models is diffuse multiple microinfarcts. This model recapitulates most of the pathology of VaD seen in humans. The microinfarcts spread throughout the cortex, striatum, hippocampus, and subcortical white matter(60). They induced significant white matter rarefaction, axonal damage, and myelin loss in the corpus callosum and striatal white matter bundles(30). Despite the overlap with some features of SVD, VaD showed patterns of glymphatic dysfunction like those seen in acute ischemic stroke and TBI. However, the discrepancies between glymphatic impairment between VaD and DM2 highlight the issue of approaching SVD from a purely vasculopathic perspective. The most consistent finding in the field of glymphatics until now, in the context of pathology, is the loss of normal clearance. In line with this and the available literature, we surmise that most SVD types will be accompanied by a decrease in clearance mechanisms, characteristic of an aging brain. Moreover, we hypothesize that glymphatic influx will resemble that seen for DM2 more than a global decrease in CSF entry as seen in VaD. We expect that SVD pathogenesis occurs over decades, and is most likely a subclinical, insidious process as opposed to a repeated, acute ischemic process. Whether this is due to EPVS, changes in AQP4 expression, arterial pulsatility, reduced clearance, or a combination of these requires further study.
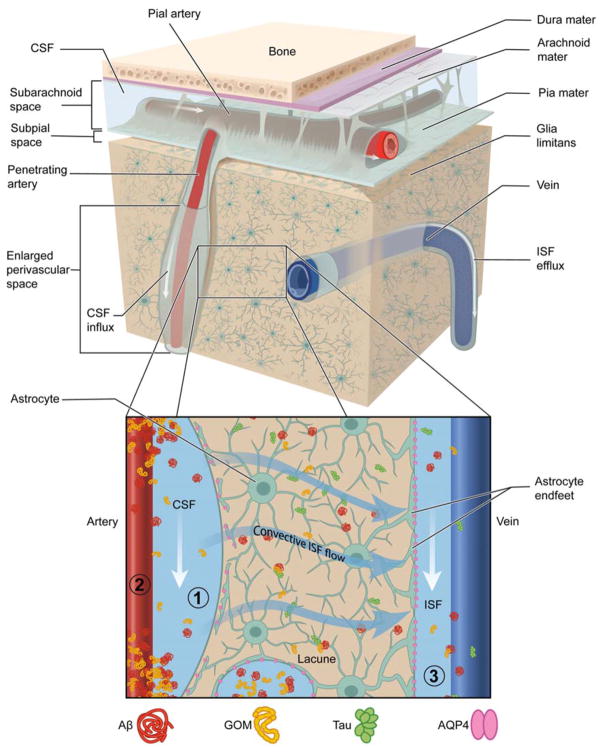
There are several limitations in understanding the potential role that glymphatics play in SVD (Figure 2). The unique evidence of glymphatic dysfunction prior to disease manifestation and the consequent disruption once pathology is established makes it a bidirectional risk factor, lying both upstream and downstream of SVD. It will be increasingly important to determine what factors cause early impairment and if chronic glymphatic failure could be a pathogenic driver for the deleterious effects on the vessel wall seen in these diseases.
Figure 2. Potential Mechanisms Driving Glymphatic System Impairment in SVD.
(1) PVS: Abluminal deposits of Aβ in CAA and GOM in CADASIL block PVS flows and may cause loss of AQP4 polarization and astrocyte endfoot and pericyte detachment. Increased PVS fluid and structural remodeling causes enlarged PVS. (2) Cerebrovascular pulsatility: Vascular pathology causes a decrease in vessel wall compliance increasing cerebrovascular pulsatility and mechanical stress against the PVS. Diffuse endothelial cell failure and impaired neurovascular unit cross-talk results in a net decrease in ISF formation offering a larger driving force for CSF entry. (3) CNS Clearance: Parenchymal changes associated to injury such as astrogliosis and lacunar infarcts, change the extracellular space volume and tortuosity, predisposing to solute trapping and reduced clearance of toxic metabolic byproducts (e.g. Aβ and tau). These changes would also decrease ISF efflux and cause fluid stasis. PVS inflammation due to extravasation of plasma proteins gives rise to perivascular edema and WML.
In recent years, an alternative to the traditional hypoperfusion-centric model has been presented based on diffuse cerebrovascular endothelial failure and consequent blood-brain barrier disruption(3). Despite the fact that this new model is more consistent with experimental data and clinical observations (35), little is known about the sequence of pathological events that lead up to BBB dysfunction and how this drives the clinical picture of SVD. It is still not clear whether BBB disruption is universal for all SVD types. Further work is necessary in fully describing the forces that govern the decoupling of influx and efflux mechanisms, as this will likely be relevant in studying subsequent SVD subtypes. An intriguing finding is the correlation of decreased clearance on cognitive function. In both VaD and DM2, the amount of tracer trapped in the brain parenchyma is positively correlated with cognitive deficits in several behavioral tests in rats(30, 34). This would be of interest to WML as these are also associated to cognitive performance(117). Is it possible that glymphatic clearance failure is correlated with WML burden? Evidence of EPVS and intramyelinic edema in pre-clinical mouse models of CADASIL in the absence of BBB disruption raises questions about glymphatic involvement and the possibility that CSF serves as a pool of water to drive edema(113). ISF stagnation or altered convective flows could cause increased cell death within the tissue (118). The novel idea of focal solute trapping could be of interest in lacunes, seen in several types of SVD(29). The recent discovery about the role of PVS closure opens several questions as to the role that astrocytes could play in the regional control of PVS flows and whether these are dependent on neuronal function or crucial for brain energy and ion homeostasis.
Targeting Glymphatics for Treatment and Prevention of SVD
The most notable regulating mechanism of glymphatic activity is its role in brain waste clearance during sleep(21). The rate of perivascular CSF circulation is reduced by ~95% in the awake state, while clearance of interstitial solutes such as Aβ is 2 times slower than in sleep. This appears to be attributable to a 60% expansion of the ECS volume(21). Interestingly, there is an association between SVD and sleep disorders. Sleep disturbances have been associated with WML burden(119–121). Subsequently, the presence of EPVS, negatively correlate with sleep efficiency(122). EPVS volume, especially in the basal ganglia, tends to be larger as duration of non-rapid eye movement stage 3 sleep decreases (122). All these findings are particularly interesting considering the strong connection between sleep and glymphatic system function. The exact relationship between sleep stage, extracellular space volume, and concentrations of different neurotransmitters with glymphatic function still remains unclear. Moreover, it is unknown whether glymphatic dysfunction can lead to sleep disturbances.
The therapeutic role of sleep improvement has not been comprehensively studied, and could potentially be tested using commonly prescribed sleep aids. To our knowledge, these have never been fully clinically tested in the context of SVD. A recent study demonstrated that obstructive sleep apnea (OSA) causes a reduction in the concentration of brain-derived proteins in CSF after slow wave sleep(123). The authors suggest that OSA causes alterations in CSF-ISF exchange causing these proteins to become trapped when they would normally be cleared out into the CSF. Independently, moderate to severe OSA has been associated with increased EPVS, WML burden, and CMB(124). Treatment of OSA and sleep improvement offer easily implemented strategies that may improve glymphatic function.
Exercise has long been shown to improve cognitive function in the aging brain. Recently, a study demonstrated that voluntary running for 6 weeks was able to significantly increase glymphatic influx and clearance in aged mice(125). The improvement in glymphatic function coincided with decreased Aβ concentrations (both Aβ40 and Aβ42) and higher synaptic integrity in the cortex and hippocampus of exercised mice. The authors attributed this to a reduction in reactive astro- and microgliosis and normalization in expression of AQP4 and its polarization(125). Of note was that this study found no difference in BBB permeability after quantifying the extravasation of an intravascular dye between the exercise and control groups. Interestingly, this study supported previous research by showing that the exercise group had significantly better spatial memory. This evidence might suggest that enhanced glymphatic function, and not a reduction in BBB permeability, is responsible for the improved cognitive performance seen after voluntary exercise. With recent studies showing the pathological increase of glymphatic system function it will be important to find therapies that aim to both increase and decrease different components of the glymphatic system pathways. Evidence for SAH showed benefit in treating rats with intraventricular tPA to induce fibrinolysis of fibrin clots in the PVS(31). Delivery into the glymphatic system could provide an optimal strategy to target the lysis of Aβ and GOM deposition in CAA and CADASIL, respectively.
Conclusion
There is a lack of understanding of how the gamut of pathological hallmarks of SVD relate to each other and drive progression. The accepted pathophysiology centered around vessel wall damage is not able to explain the complete picture; however, failure of a previously unappreciated brain-wide drainage system may be able to reconcile these missing links. Enlarged PVS, edema, accumulation of abluminal protein deposits (GOM and Aβ), and the association between SVD and sleep, suggest a potential role for glymphatic dysfuction and perivascular pathology as a crucial component of SVD. It is yet to be determined whether glymphatic failure is a primary cause or secondary effect of vascular pathology. Regardless of the extent of glymphatic involvement, enhancing brain clearance mechanisms may represent an important target for preventive and therapeutic strategies. The recent development of animal models that successfully recapitulate aspects of several SVD subtypes provide an exciting opportunity through which to approach these unresolved issues. In conclusion, further investigation into the factors that contribute to glymphatic system function and how these breakdown may yield a better understanding of SVD and offer the possibility of intervening decades before symptomatology has become apparent.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant numbers R01NS100366, R01AG048769 (to M.N.)]; the United States Department of Defense [grant number N00014-15-1-2016 (to M.N.)]; the Leducq Fondation [grant number FLQ 12CVD01]; the European Union’s Horizon 2020 Research and Innovation Programme (SVDs@target) [grant number 666881 (to M.N.)] and the National Institute of Neurological Disorders and Stroke (K08NS089830 to R.I.M.).
The authors thank Margaret McFarland for assistance with illustrations.
Abbreviations
- AD
Alzheimer’s disease
- AHT
arterial hypertension
- apoE
apolipoprotein E
- AQP4
aquaporin-4
- Aβ
amyloid-β
- Aβ40
amyloid-β1–40
- Aβ42
amyloid-β1–42
- BBB
blood-brain barrier
- CAA
cerebral amyloid angiopathy
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical ischemic strokes and leukoencephalopathy
- CARASIL
cerebral autosomal recessive arteriopathy with subcortical ischemic strokes and leukoencephalopathy
- CMB
cerebral microbleeds
- CSD
cortical spreading depression
- CSF
cerebrospinal fluid
- DM2
type 2 diabetes mellitus
- ECS
extracellular space
- EPVS
enlarged perivascular spaces
- GFAP
glial fibrillary acidic protein
- GOM
granular osmiophilic material
- ICH
intracerebral hemorrhage
- ICP
intracranial pressure
- ISF
interstitial fluid
- MCAO
middle cerebral artery occlusion
- MMI
multiple microinfarcts
- MREG
magnetic resonance encephalography
- MRI
magnetic resonance imaging
- Notch3ECD
Notch3 ectodomain
- OSA
obstructive sleep apnea
- PVS
perivascular space
- SAH
subarachnoid hemorrhage
- SAS
subarachnoid space
- SHR
spontaneous hypertensive rat
- SHRSP
spontaneous hypertensive rat-stroke prone
- SVD
small vessel disease
- TBI
traumatic brain injury
- tPA
tissue type plasminogen activator
- VaD
vascular dementia
- VRS
Virchow–Robin spaces
- WML
white matter lesions
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
H.M. and S.K. drafted the manuscript. RIM and MN critically revised the manuscript.
References
- 1.Hachinski V World Stroke O. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke. 2015;46(11):3039–40. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–97. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charidimou A, Pantoni L, Love S. The concept of sporadic cerebral small vessel disease: A road map on key definitions and current concepts. Int J Stroke. 2016;11(1):6–18. doi: 10.1177/1747493015607485. [DOI] [PubMed] [Google Scholar]
- 5.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 6.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 7.Joutel A, Faraci FM. Cerebral small vessel disease: insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2014;45(4):1215–21. doi: 10.1161/STROKEAHA.113.002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffner C, Malik R, Dichgans M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J Cereb Blood Flow Metab. 2016;36(1):158–71. doi: 10.1038/jcbfm.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R. White matter lesions in Fabry disease occur in ‘prior’ selectively hypometabolic and hyperperfused brain regions. Brain Res Bull. 2003;62(3):231–40. doi: 10.1016/j.brainresbull.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120(2):433–45. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40(12):2583–99. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun. 2015;6:6807. doi: 10.1038/ncomms7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep. 2013;3:2582. doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11(1):74. doi: 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6 Suppl 1):S93–5. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015;4(11):2058460115609635. doi: 10.1177/2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36(6):1033–45. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based Contrast Enhancement of the Perivascular Spaces in the Basal Ganglia. Magn Reson Med Sci. 2017;16(1):61–5. doi: 10.2463/mrms.mp.2016-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017 doi: 10.1007/s11604-017-0617-z. [DOI] [PubMed] [Google Scholar]
- 20.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015;35(31):11034–44. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545(1–2):103–13. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 24.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238(4):962–74. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–9. [PubMed] [Google Scholar]
- 26.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asgari M, de Zelicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–61. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.2112-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96–106. doi: 10.1016/j.neurobiolaging.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45(10):3092–6. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 32.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–25. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schain AJ, Melo A, Strassman AM, Burstein R. Cortical spreading depression closes the paravascular space and impairs glymphatic flow: Implications for migraine headache. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.3390-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow-Robin spaces. AJR Am J Roentgenol. 1988;151(3):551–8. doi: 10.2214/ajr.151.3.551. [DOI] [PubMed] [Google Scholar]
- 36.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191(Pt 3):337–46. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 38.Korogod N, Petersen CC, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife. 2015:4. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224–31. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Ihara M, Tham C, Low RW, Slade JY, Moss T, et al. Neuropathological correlates of temporal pole white matter hyperintensities in CADASIL. Stroke. 2009;40(6):2004–11. doi: 10.1161/STROKEAHA.108.528299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loos CM, Klarenbeek P, van Oostenbrugge RJ, Staals J. Association between Perivascular Spaces and Progression of White Matter Hyperintensities in Lacunar Stroke Patients. PLoS One. 2015;10(9):e0137323. doi: 10.1371/journal.pone.0137323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nonaka H, Akima M, Nagayama T, Hatori T, Zhang Z, Ihara F. Microvasculature of the human cerebral meninges. Neuropathology. 2003;23(2):129–35. doi: 10.1046/j.1440-1789.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Federico A, Di Donato I, Bianchi S, Di Palma C, Taglia I, Dotti MT. Hereditary cerebral small vessel diseases: a review. J Neurol Sci. 2012;322(1–2):25–30. doi: 10.1016/j.jns.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Yao M, Herve D, Jouvent E, Duering M, Reyes S, Godin O, et al. Dilated perivascular spaces in small-vessel disease: a study in CADASIL. Cerebrovasc Dis. 2014;37(3):155–63. doi: 10.1159/000356982. [DOI] [PubMed] [Google Scholar]
- 46.Nozaki H, Nishizawa M, Onodera O. Features of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2014;45(11):3447–53. doi: 10.1161/STROKEAHA.114.004236. [DOI] [PubMed] [Google Scholar]
- 47.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow–Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41(11):2483–90. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 48.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376–81. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450–4. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 50.Ihara M, Yamamoto Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke. 2016;47(2):554–60. doi: 10.1161/STROKEAHA.115.009627. [DOI] [PubMed] [Google Scholar]
- 51.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113(4):349–88. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 52.Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002;977:411–5. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 53.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 54.Fouillade C, Monet-Lepretre M, Baron-Menguy C, Joutel A. Notch signalling in smooth muscle cells during development and disease. Cardiovasc Res. 2012;95(2):138–46. doi: 10.1093/cvr/cvs019. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol. 2015;78(6):887–900. doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171–80. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A. 2011;108(43):17815–20. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao X, Hrabetova S, Nicholson C, Manley GT. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J Neurosci. 2008;28(21):5460–4. doi: 10.1523/JNEUROSCI.0257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol. 2016;148(6):489–501. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A, et al. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32(50):17948–60. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33(6):834–45. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–3. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 63.Yamada M. Risk factors for cerebral amyloid angiopathy in the elderly. Ann N Y Acad Sci. 2002;977:37–44. doi: 10.1111/j.1749-6632.2002.tb04797.x. [DOI] [PubMed] [Google Scholar]
- 64.Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, et al. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017;74(1):91–9. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Lunde LK, Nuntagij P, Oguchi T, Camassa LM, Nilsson LN, et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27(4):711–22. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- 66.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomassoni D, Bramanti V, Amenta F. Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats. Brain Res. 2010;1325:155–63. doi: 10.1016/j.brainres.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Tomassoni D, Avola R, Di Tullio MA, Sabbatini M, Vitaioli L, Amenta F. Increased expression of glial fibrillary acidic protein in the brain of spontaneously hypertensive rats. Clin Exp Hypertens. 2004;26(4):335–50. doi: 10.1081/ceh-120034138. [DOI] [PubMed] [Google Scholar]
- 69.Ishida H, Takemori K, Dote K, Ito H. Expression of glucose transporter-1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood-brain barrier function. Am J Hypertens. 2006;19(1):33–9. doi: 10.1016/j.amjhyper.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 70.Moeller HB, Fenton RA, Zeuthen T, Macaulay N. Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience. 2009;164(4):1674–84. doi: 10.1016/j.neuroscience.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 71.Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience. 2009;159(3):1055–69. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2010;69(12):1201–9. doi: 10.1097/NEN.0b013e3181fd252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 74.Monet-Lepretre M, Haddad I, Baron-Menguy C, Fouillot-Panchal M, Riani M, Domenga-Denier V, et al. Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain. 2013;136(Pt 6):1830–45. doi: 10.1093/brain/awt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105(5):597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto Y, Craggs LJ, Watanabe A, Booth T, Attems J, Low RW, et al. Brain microvascular accumulation and distribution of the NOTCH3 ectodomain and granular osmiophilic material in CADASIL. J Neuropathol Exp Neurol. 2013;72(5):416–31. doi: 10.1097/NEN.0b013e31829020b5. [DOI] [PubMed] [Google Scholar]
- 77.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117(1):1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 78.Prelli F, Castano E, Glenner GG, Frangione B. Differences between vascular and plaque core amyloid in Alzheimer’s disease. J Neurochem. 1988;51(2):648–51. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 79.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–9. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62(12):1287–301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 81.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30(5):637–49. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 82.Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. 2009;54(2):388–92. doi: 10.1161/HYPERTENSIONAHA.109.133116. [DOI] [PubMed] [Google Scholar]
- 83.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 84.Ghorbani A, Ahmadi MJ, Shemshaki H. The value of transcranial Doppler derived pulsatility index for diagnosing cerebral small-vessel disease. Adv Biomed Res. 2015;4:54. doi: 10.4103/2277-9175.151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging. 2001;11(3):229–35. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 86.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dabertrand F, Kroigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, et al. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci U S A. 2015;112(7):E796–805. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Xu G, Ling Q, Da C. Expression of aquaporin 4 and Kir4.1 in diabetic rat retina: treatment with minocycline. J Int Med Res. 2011;39(2):464–79. doi: 10.1177/147323001103900214. [DOI] [PubMed] [Google Scholar]
- 89.Baydas G, Nedzvetskii VS, Tuzcu M, Yasar A, Kirichenko SV. Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: effects of vitamin E. Eur J Pharmacol. 2003;462(1–3):67–71. doi: 10.1016/s0014-2999(03)01294-9. [DOI] [PubMed] [Google Scholar]
- 90.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1(1):2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnston M, Zakharov A, Koh L, Armstrong D. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol. 2005;31(6):632–40. doi: 10.1111/j.1365-2990.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 92.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519–34. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boulton M, Young A, Hay J, Armstrong D, Flessner M, Schwartz M, et al. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol. 1996;22(4):325–33. doi: 10.1111/j.1365-2990.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 95.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, et al. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS. 2015;12:23. doi: 10.1186/s12987-015-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Leon MJ, Li Y, Okamura N, Tsui WH, Saint Louis LA, Glodzik L, et al. CSF clearance in Alzheimer Disease measured with dynamic PET. J Nucl Med. 2017 doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–93. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240(4):F319–28. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 101.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246(6 Pt 2):F835–44. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 102.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118(12):4002–13. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murlidharan G, Crowther A, Reardon RA, Song J, Asokan A. Glymphatic fluid transport controls paravascular clearance of AAV vectors from the brain. JCI Insight. 2016;1(14):e88034. doi: 10.1172/jci.insight.88034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35(2):518–26. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meng H, Zhang X, Yu G, Lee SJ, Chen YE, Prudovsky I, et al. Biochemical characterization and cellular effects of CADASIL mutants of NOTCH3. PLoS One. 2012;7(9):e44964. doi: 10.1371/journal.pone.0044964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18(22):2730–5. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duering M, Karpinska A, Rosner S, Hopfner F, Zechmeister M, Peters N, et al. Co-aggregate formation of CADASIL-mutant NOTCH3: a single-particle analysis. Hum Mol Genet. 2011;20(16):3256–65. doi: 10.1093/hmg/ddr237. [DOI] [PubMed] [Google Scholar]
- 108.Capone C, Cognat E, Ghezali L, Baron-Menguy C, Aubin D, Mesnard L, et al. Reducing Timp3 or vitronectin ameliorates disease manifestations in CADASIL mice. Ann Neurol. 2016;79(3):387–403. doi: 10.1002/ana.24573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(17):8098–102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148(6):2083–95. [PMC free article] [PubMed] [Google Scholar]
- 111.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38(2):254–9. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 112.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25(11):2803–10. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cognat E, Cleophax S, Domenga-Denier V, Joutel A. Early white matter changes in CADASIL: evidence of segmental intramyelinic oedema in a pre-clinical mouse model. Acta Neuropathol Commun. 2014;2:49. doi: 10.1186/2051-5960-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59(4):780–6. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60(1):188–97. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63(9):1606–12. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 117.Pavlovic AM, Pekmezovic T, Zidverc Trajkovic J, Svabic Medjedovic T, Veselinovic N, Radojicic A, et al. Baseline characteristic of patients presenting with lacunar stroke and cerebral small vessel disease may predict future development of depression. Int J Geriatr Psychiatry. 2016;31(1):58–65. doi: 10.1002/gps.4289. [DOI] [PubMed] [Google Scholar]
- 118.Maneshi MM, Maki B, Gnanasambandam R, Belin S, Popescu GK, Sachs F, et al. Mechanical stress activates NMDA receptors in the absence of agonists. Sci Rep. 2017;7:39610. doi: 10.1038/srep39610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanda A, Matsui T, Ebihara S, Arai H, Sasaki H. Periventricular white matter lesions and sleep alteration in older people. J Am Geriatr Soc. 2003;51(3):432–3. doi: 10.1046/j.1532-5415.2003.51125.x. [DOI] [PubMed] [Google Scholar]
- 120.Cheng CY, Tsai CF, Wang SJ, Hsu CY, Fuh JL. Sleep disturbance correlates with white matter hyperintensity in patients with subcortical ischemic vascular dementia. J Geriatr Psychiatry Neurol. 2013;26(3):158–64. doi: 10.1177/0891988713493503. [DOI] [PubMed] [Google Scholar]
- 121.Del Brutto OH, Mera RM, Zambrano M, Lama J, Del Brutto VJ, Castillo PR. Poor sleep quality and silent markers of cerebral small vessel disease: a population-based study in community-dwelling older adults (The Atahualpa Project) Sleep Med. 2015;16(3):428–31. doi: 10.1016/j.sleep.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 122.Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, et al. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015;38(6):853–8. doi: 10.5665/sleep.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol. 2016;80(1):154–9. doi: 10.1002/ana.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song TJ, Park JH, Choi KH, Chang Y, Moon J, Kim JH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36–42. doi: 10.1016/j.sleep.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 125.He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, et al. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci. 2017;10:144. doi: 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340(6140):1529–30. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]