Abstract
Exercise is presumed to be a potentially helpful smoking cessation adjunct reputed to attenuate the negative effects of deprivation. The present study examined the effectiveness of moderate within-session exercise to reduce four key symptoms of smoking deprivation during three 72-hour nicotine abstinence blocks in both male and female smokers. Forty-nine (25 male, 24 female) sedentary smokers abstained from smoking for three consecutive days on three separate occasions. At each session, smokers’ abstinence-induced craving, cue-induced craving, negative mood, and withdrawal symptom severity were assessed prior to and after either exercise (AM exercise, PM Exercise) or a sedentary control activity (Magazine reading). Abstinence-induced craving and negative mood differed as a function of condition, F(2,385)=21, p< 0.0001, and F(2,385) = 3.38, p= 0.03. Planned contrasts revealed no difference between AM and PM exercise, but exercise overall led to greater pre-post reduction in abstinence-induced craving, t(385)=6.23, p< 0.0001, effect size Cohen’s d = 0.64, and negative mood, t(385)= 2.25, p= 0.03, d = 0.23. Overall exercise also led to a larger pre-post reduction in cue-induced craving in response to smoking cues, F(2,387) = 8.94, p = 0.0002, and withdrawal severity, F(2,385) = 3.8, p= 0.02. Unlike the other three measures, PM exercise reduced withdrawal severity over control, t(385) = 2.64, p = .009, d = 0.27, whereas AM exercise did not. The results support the clinical potential of exercise to assist smokers in managing common and robust negative symptoms experienced during the first 3 days of abstinence.
Keywords: smoking, abstinence, exercise, craving, withdrawal
Eighty percent of the 17 million Americans who try to quit each year relapse within the first month (U.S. Department of Health and Human Services, 2014) and only 3% remain abstinent six months post-quit (Benowitz, 2008). To increase cessation success, considerable research and clinical work has focused on diminishing negative symptoms experienced upon quitting smoking, most commonly abstinence-induced craving to smoke and negative mood. Although nicotine replacement (NRT) studies show that NRT significantly attenuates both of these symptoms, relief is not complete and varies considerably (Dale et al., 1995; Killen, Fortmann, Davis, Strausberg, & Varady, 1999; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006). Moreover, upwards of 70% of smokers using NRT to quit still relapse (Jolicoeur, Richter, Ahluwalia, Mosier, & Resnicow, 2003; Shiffman et al., 2002). Exacerbating the difficulty of quitting, smokers readily report that confrontation with cues associated with smoking is another vital aspect of their inability to stay quit (Shiffman et al., 2007). Unfortunately, past attempts to attenuate such cue-reactivity through nicotine replacement and/or behavioral exposure treatment have consistently failed (Conklin & Tiffany, 2002a; Ferguson & Shiffman, 2009; Martin, LaRowe, & Malcolm, 2010). Therefore, treatment adjuncts capable of reducing not only abstinence-induced craving and negative mood, but cue-induced craving as well, may significantly enhance current smoking cessation treatments.
One promising behavioral adjunct for smoking cessation is exercise. Several studies suggests that short bouts of moderate-intensity exercise can reduce abstinence-induced craving in briefly-deprived smokers (Janse Van Rensburg & Taylor, 2008; Taylor, Katomeri, & Ussher, 2005; Taylor, Ussher, & Faulkner, 2007; Taylor & Katomeri, 2006, 2007), as well as reduce cue-induced craving and increase time-to-next cigarette (Elibero, Van Rensburg, & Drobes, 2011; Fong, De Jesus, Bray, & Prapavessis, 2014; Taylor & Katomeri, 2007). Several reviews of the acute impact of physical activity on abstinence-induced craving (Haasova et al., 2014; Roberts, Maddison, Simpson, Bullen, & Prapavessis, 2012; Taylor et al., 2007) and withdrawal symptoms (Roberts et al., 2012; Taylor et al., 2007) have revealed significant evidence of craving attenuation among briefly deprived smokers (ranging 30 minutes to 24 hrs). However, whether exercise can attenuate both abstinent- and cue-induced craving repeatedly over longer deprivation, such as the first 3 days of abstinence when negative symptoms of quitting smoking often peak, remains untested.
Finding more effective methods of reducing elevations in negative mood, also commonly experienced during nicotine withdrawal (Diagnostic and Statistical Manual of Mental Disorders Source Information, 2015; Hughes, 1992; Hughes, 2007; West, Hajek, & Belcher, 1989), may further aid efforts to quit. Although nicotine replacement therapies improve negative mood, they fail to completely suppress affective dysregulation in quitting smokers (Dale et al., 1995; Killen et al., 1999; Shiffman et al., 2006). Exercise may be one behavioral method to improve mood during abstinence as it has been shown to have a positive impact on negative mood escalation experienced by female smokers upon quitting (Bock, Marcus, King, Borrelli, & Roberts, 1999). Among other smoking cessation trials, there is evidence of exercise attenuating both abstinence-induced craving and negative mood (Ussher, Taylor, & Faulkner, 2012a), but assessment has often focused changes from baseline to end of treatment, or at the end of each week of a several week treatment (e.g., Bock et al., 1999) thus limiting understanding of the acute impact of exercise on these measures. Moreover, the specificity of exercise to attenuate negative symptoms during withdrawal is often hard to determine in cessation studies due to the frequent use of NRT, which alone reduces variable levels of abstinence-induced craving and negative mood (Ussher, Taylor, & Faulkner, 2012b). Thus, whether exercise relieves abstinence-induced craving, cue-induced craving, and negative mood during extended deprivation in the early stages of abstinence, for example consistently over the first three days of peak negative symptoms, has not been examined. Moreover, no studies have examined the acute effects of exercise over a longer period of deprivation in both male and female smokers.
Also unknown is the best time of day to exercise in order to get maximal relief from negative symptoms occurring early in the process of smoking cessation. Nicotine withdrawal intensity can have considerable diurnal variability, particularly craving, which studies have shown can be high in the morning and dip lowest at midday with the potential to peak again in the evening (Chandra, Scharf, & Shiffman, 2011a; Dunbar, Scharf, Kirchner, & Shiffman, 2010; Perkins, Briski, Fonte, Scott, & Lerman, 2009). Using exercise to attenuate these symptoms may be more effective at different times of day, or among smokers with different circadian patterns. Thus, examinations of exercise at different times of days to reduce negative symptoms experienced during the first days of smoking deprivation among both sexes would provide more nuanced information about the potential of exercise to help smokers quit.
The goal of the present study was to examine the impact of daily moderate AM versus PM exercise on 4 key consequences of nicotine abstinence; namely, increased abstinence-induced craving, negative affect, cue-induced craving, and withdrawal severity, during a 3 day (72 hours) smoking deprivation period in male and female smokers. It was hypothesized that exercise overall, compared to control activity, would lead to reductions in pre- post-levels of abstinence-induced craving, negative affect, cue-induced craving and withdrawal severity. Additionally, the study examined differences in the efficacy of exercise to relieve each measure as a function of sex as well as exercise time of day (AM/PM).
METHODS
Study design
A within-participants design was used to examine three exercise conditions (AM, PM, No exercise: Magazine control) on abstinence-induced craving, cue-induced craving, negative mood, and withdrawal severity among 72-hour abstinent, non-treatment seeking, sedentary smokers. The data presented here are part of a study exploring the effects of exercise on outcome measures presented here, as well as on sleep changes brought on by nicotine deprivation (Soreca et al., n.d.). Thus the study took place in the Neuroscience Clinical Translational Research Center (N-CTRC) at Western Psychiatric Institute and Clinic, where participants slept at night during each 3-day/2 night experimental block. All participants abstained from smoking for three consecutive days for each of the three exercise condition blocks. In between experimental blocks, participants were instructed to return to regular smoking for one week. Exercise condition was counterbalanced and stratified by gender such that all condition orders occurred an equal number of times across all participants and equally across male and female participants. To increase adherence to abstinence and compensate for their time, participants were paid for each phase of the study as detailed in Table 1. All methods were approved by the University of Pittsburgh Institutional Review Board.
Table 1.
Participant compensation
| SCID & Assessments | $25 |
| Medical Exam & Labs | $25 |
| Apnea Screen & Baseline Night | $150 |
| Graded Exercise Test | $25 |
| Block 1 | $300 |
| Block 2 | $300 |
| Block 3 | $300 + $100 completion bonus |
Participants
Participants were men and women age 18 to 45 who met criteria for DSM-IV-TR nicotine dependence (American Psychiatric Association, 2000). They had been smoking for at least one year, had smoked at least 10 cigarettes a day for the past six months, had not attempted to quit for longer than one week in the previous month, and were not taking smoking cessation medication. In addition, individuals were eligible to enter the study if they met sedentary criteria by not regularly exercising (defined as exercising fewer than three times per week and for no more than 20 minutes each time), were free of medical illness and able to exercise (as determined by medical history, EKG, and blood tests), had not met criteria for any psychiatric disorders in the past year, assessed via the Structured Clinical Interview (Non-patient Version) for DSM-IV (SCID-NP), were not positive for any recreational drug (confirmed by urine drug screen) and, to confirm regular smoking, gave an expired-air carbon monoxide breath sample >10ppm (Vitalograph Inc., Lenexa, KS) and urine cotinine level > 100ng/ml, measured as a 3 or greater on the NicAlert urine test (Nymox Corporation, Hasbrouck Heights, NJ). The also completed a Smoking history form with demographic information and the Composite Scale (Smith, Reilly, & Midkiff, 1989) to assess chronotype eveningness/morningness.
Self-report Measures
Chronotype/Eveniningness
Chronotype was assessed with the Composite Scale (CS; Smith, Reilly, & Midkiff, 1989) a self-report measure of habitual timing preference for activities, as well as circadian rhythms of energy and productivity. Low scores on the CS indicate tendency toward eveningness, while high scores are consistent with a morning chronotype. The CS was completed once at the screening session.
Cue-induced Craving
Post-trial craving during cue-reactivity was assessed via the 4-item Brief Questionnaire on Smoking Urges (Carter & Tiffany, 2001). This 4-item scale is derived from a widely used clinical and laboratory measure of urges to smoke (Tiffany & Drobes, 1991). Rated on a scale of 0–100, the four items include: “Nothing would be better than smoking a cigarette right now,” “I have an urge for a cigarette,” “All I want now is a cigarette,” and “I crave a cigarette right now.” Cronbach’s alpha values for this four-item version of the Questionnaire on Smoking Urges ranged from .93 to .95.
Abstinence-induced craving
The Questionnaire on Smoking Urges (Cox, Tiffany, & Christen, 2001) is a 10-item version of the larger 32-item QSU (Tiffany & Drobes, 1991). The QSU-Brief yields a general craving score with excellent reliability (alpha=.97) and has been used extensively in smoking cessation research. The QSU-Brief was completed pre- and post-exercise.
Negative mood
The Negative Affect items of the Mood Form (Diener & Emmons, 1984) were used to assess negative mood. Items include: depressed/blue, unhappy, frustrated, worried/anxious, and angry/hostile, which are rated on a 0 (not at all) to 100 (very much) visual analog scale and yield a negative affect score. The Negative Affect scale is internally consistent, with Cronbach’s alpha values ranging from .81 to .85.
Withdrawal
The Minnesota Withdrawal Scale-Revised (MWS; Hughes & Hatsukami, 1986) assesses general withdrawal symptoms and is comprised of 9 items rated on a scale from 0 to 4 exploring craving for tobacco, irritability, anxiety, difficulty concentrating, restlessness impatience, somatic complaints, insomnia, increased hunger. The MWS was completed pre- and post-exercise.
Rated perceived exertion RPE
RPE (Borg, 1998) was assessed throughout exercise at 5-minute intervals. RPE is a basic measure of exercise intensity used to assess an individual’s perceived level of exertion during physical activity. When prompted by the experimenter, who held up a paper copy of the scale, participants verbally assigned a number from 6–20 (e.g., 6=no exertion, 13=somewhat hard, 20 = maximal exertion) to their level of exertion.
Baseline
After meeting initial screening criteria, all participants completed a baseline session to allow for completion of study assessments during their regular, nondeprived, smoking state. They were instructed to smoke freely prior to and during this session. Upon arrival at the laboratory, they completed self-report measures of withdrawal (Minnesota Withdrawal Scale- MWS; Hughes & Hatsukami, 1986) mood (Diener & Emmons, 1984), and craving (Brief Questionnaire of Smoking Urges-QSU-Brief; Cox, Tiffany, & Christen, 2001). They then underwent a 15-minute cue reactivity protocol (CR; described below) assessing self-report responses to smoking and neutral pictorial cues (Conklin et al., 2015). They were then told they could relax and watch TV or read; and, if they wanted to smoke they could ask an attendant to take them to a nearby smoking room. They were given dinner two hours prior to their bedtime, which was based on average self-reported bedtime during the previous two weeks. In the morning, they repeated the assessments, including CR and were then free to leave the laboratory.
Experimental Blocks
Participants were instructed to abstain from smoking 6-hours prior to their first 72-hour abstinence block session. This was verified by a CO breath sample, which after 6 hours abstinent, was expected to be at least 50% reduced from their baseline CO level (acquired during the screening process). Participants were instructed that they were not allowed to use nicotine replacement products to aid abstinence. Abstinence during the three-day experimental sessions was monitored via CO and urine cotinine at each visit to the laboratory. Although urine cotinine was not necessarily expected to decrease during the three-day block, anything other than no change or a reduction in cotinine during that time was recorded as having smoked. If a participant failed to meet the CO cutoff the first day of a block, they were given one chance to reschedule. Participants who failed to remain abstinent after starting a 3-day block (n=5) were excluded from the study. In between the three 3-day experimental periods, participants were told to resume regular smoking. Individuals who expressed a desire to permanently quit smoking were given educational material on smoking cessation, referred to specialty counseling, and excluded from the study (n=1). Participants slept in the laboratory each night during the 3 three-day abstinence blocks. Each day participants left the laboratory and returned 5 hours prior to their bedtime. Physical activity outside of sessions was recorded with actigraphy watches and evaluated for compliance with sedentary criteria. No participant increase out-of-session activity over the course of the study beyond that allowed under the established sedentary criteria (i.e., exercising fewer than three times per week and for no more than 20 minutes each time). Protocol for the three exercise block conditions were as follows:
PM Exercise
Participants arrived at the laboratory 5 hours before bedtime, completed pre-exercise assessments and CR, followed by exercise (as described below) and post-exercise assessments and CR. They were then served dinner and could watch TV or read until bedtime. The following morning, they repeated the ratings and CR within 30 minutes of their wake up time and were then free to leave the laboratory for the day.
AM Exercise
Participants arrived at the laboratory 5 hours prior to their habitual bedtime and completed initial ratings and CR. They were then free to eat dinner, watch TV or read until their bedtime. The following morning they completed the pre-exercise self-report assessments and CR within 30 minutes of their wake up time followed by exercise and post-exercise assessments and CR. They were then free to leave the laboratory.
Magazine Control
Participants followed the same protocol described for the exercise sessions, but they were instructed to sit quietly and read magazines in place of exercise. Half of the participants were randomized to AM reading and half to PM reading.
Exercise protocol
Participants walked briskly on a treadmill with HR monitoring (Polar Electro Inc., Lake Success, NY) to ensure that 60% of their age adjusted maximum heart rate (AAMHR), computed as (220-age)*0.60, was maintained. A 5-minute warm up with the treadmill set at 2.5 MPH, 0% grade was followed by an increase to 3 MPH. Mean heart rate over each subsequent 5-minute period was used to determine if HR was at 60%±5 bpm of AAMHR, with adjustments made to grade (and speed if necessary) if HR was outside of this prescribed range. Participants also gave verbal RPE (Borg, 1998) ratings at each 5-minute interval. Each 30-minute session was followed by a 5-minute cool-down.
Cue Reactivity (CR)
CR consisted of a computer-automated 6-trial cue reactivity protocol involving 40 seconds of relaxation, 40 seconds of cue presentation, and self-report ratings. The cue presentation period started with a screen instructing the participant to “focus on the following item and think about actually having it with you right now” followed by a series of four pictures of the same cue from four different angles, with each angle presented for 10 seconds. Smoking cues included objects such as a cigarette burning in an ashtray, lighters, etc. Neutral cues included stimuli not related to smoking such as, a pen and pad of paper, lip balms, etc. (Conklin et al., 2015). Smoking and neutral cue trials were presented in a counterbalanced order such that no more than two of any cue type (smoking, neutral) occurred consecutively. After each 40-second cue presentation period, participants completed a computer-automated 4-item craving rating before the next trial began with relaxation.
Data analysis
All data analyses were performed using SAS (version 9.4, SAS Institute, Inc., Cary NC, 2013). Eighty-eight participants entered the study, with 49 study completers contributing data to the final analysis. The demographics and baseline clinical variables between participants who completed all the assessments (i.e., Completers), and those who entered the study, but did not complete (Non-completers), were compared using Fisher’s exact tests or Chi-squared tests for categorical variables, and t-tests for continuous variables. The primary dependent variables were self-report ratings of abstinence-induced craving, cue-induced craving, withdrawal severity, and negative mood, measured pre- and post-exercise. The primary analytical strategy was repeated measures mixed model for continuous outcomes. In all models, model diagnostics were examined to identify influential observations and to assess the model fit. All hypothesis tests were two-sided and at the .05 level.
First, mixed-effect models were applied to pre-post session reduction in the four primary outcomes that were measured repeatedly on three days in each exercise block. The fixed terms included exercise condition (AM, PM, Mag), block (1, 2, 3) and day (1, 2, 3), as well as factors known to be related to the outcomes such as age, number of cigarettes, and nicotine dependence. Sex, eveningness, the exercise by block interaction, and the exercise by day interaction were considered for inclusion in the models, but none of these terms were significant and were therefore not included in the final models. Random terms included exercise sequence (e.g., PM-AM-No exercise, PM-No-AM exercise, etc), and participants, which were nested in sequence to account for dependence among individuals who received the same treatment sequence and dependence in repeated measures from the same participant. The Kenward and Roger adjustment was used for computing denominator degrees of freedom in F tests.
Significant condition effects were followed up by contrast tests to determine if active exercise led to greater reduction in acute symptoms than the non-exercise condition by comparing the averaged change scores from the two exercise conditions with those taken during the control (magazine) inactive period. As done in past studies examining cue-reactivity (e.g., Brandon et al., 2011), we also conducted a comparison of group differences in cue-induced craving to smoking cues while controlling for craving induced by neutral cues. Contrasts were also set up to examine the effects of morning exercise versus evening exercise on withdrawal, mood, abstinence-induced craving and cue-induced craving. Given evidence of heightened craving and negative affect during morning hours (Chandra, Scharf, & Shiffman, 2011b), it was hypothesized that morning exercise would lead to greater pre- post-exercise reduction in withdrawal, negative mood, abstinence-induced craving, cue-induced craving to smoking related cues compared to evening exercise. Additional tests were conducted to examine whether the ratings on percent exertion (RPE) were associated with pre-post reduction in cravings, negative mood and withdrawal during exercise sessions. Similar mixed-effect models were applied to pre-post reductions measured repeatedly over the three days during the AM exercise and PM exercise. The maximum RPE during exercise was included as a fixed term.
RESULTS
Eighty-eight participants were consented for the study. Forty-nine participants completed the study. As shown in Table 2, completers did not differ from those who entered the study but failed to complete on any of the demographic and clinical variables. The final analyses were conducted on the 49 completers only. Of the non-completers (n=39), the majority (n=32) dropped out or were excluded immediately after the baseline night. Reasons for non-completion included: Failing a drug test (n=19), inability to maintain abstinence (n=10), and personal decision (n=10).
Table 2.
Completer and Non-completer Demographics
| Completers N=49 | Entered but not completers N = 39 | P value | |
|---|---|---|---|
| Female (%) | 46.9 | 35.9 | 0.30 |
| Non Hispanic/Latino (%) | 100 | 94.9 | 0.20 |
| Age (mean ± SD) | 27.5 ± 6.6 | 28.5 ± 7.2 | 0.50 |
| CPD (mean ± SD) | 14.3 ±4.1 | 16.4 ± 7.1 | 0.13 |
| FTND (mean ± SD) | 4.8 ± 1.7 | 5.3 ± 1.9 | 0.27 |
Changes in withdrawal symptoms as a function of exercise condition
(1) Abstinence-induced craving
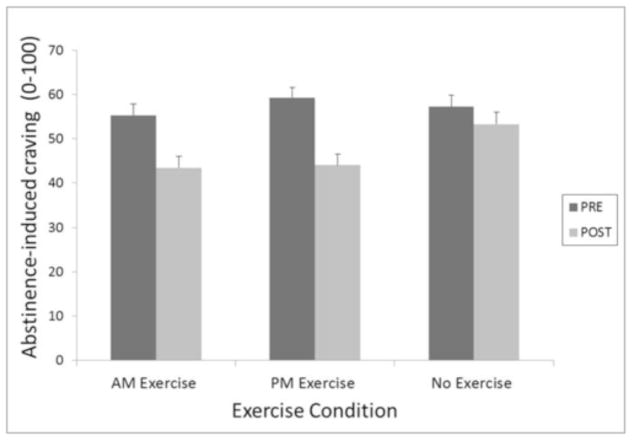
Abstinence-induced craving, pre- to post-exercise was significantly attenuated by 30-minutes of in-session moderate exercise. Specifically, abstinence-induced craving differed as a function of condition F(2,385)=21, p< 0.0001. Planned contrasts revealed significant reductions in abstinence-induced craving both between PM exercise and control (est = 11.2, t(385) = 6.28, p < 0.0001, d = 0.64) and between AM exercise and control (est = 8, t(385) = 4.51, p < 0.0001, d = 0.46), but no difference between AM and PM exercise.
(2) Cue-induced craving
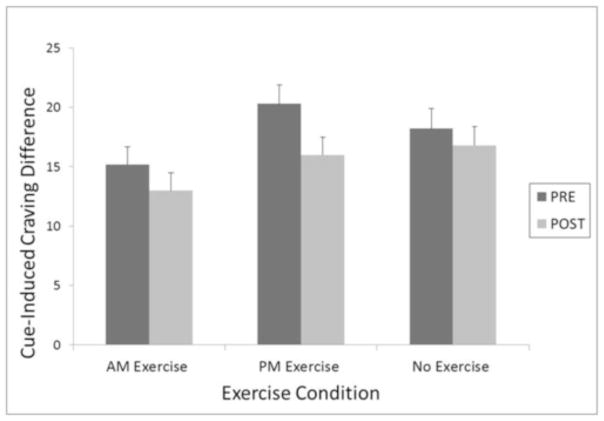
Compared to the sedentary condition, exercise led to a larger pre-post reduction in cue-induced craving in response to smoking-related stimuli measured via the QSU-4 following cue exposure trials (est = 9.4, t(385)=7.5, p < 0.0001, d = 0.76). A weaker, but still significant effect was found for reactivity to neutral cues (est = 8.7, t(384) = 6.7, p < 0.001, d = 0.68). We then compared group differences in smoking cues while controlling for neutral cues, which revealed a significant effect of exercise, F(2,387) = 8.94, p = 0.0002. Planned contrasts revealed significant reductions in smoking cues between exercise and control (est = 4.16, t(383) = 3.87, p = 0.0001, d = 0.39), with larger reduction between PM exercise and control (est = 5.27, t(379) = 4.33, p < 0.0001, d = 0.44), smaller reduction between AM exercise and control (est = 3.05, t(383) = 2.47, p = 0.01, d = 0.25), with the difference between AM and PM exercise nearing significance (est = 02.21, t (374) = −1.87, p= 0.06, d = 0.19).
(3) Negative mood
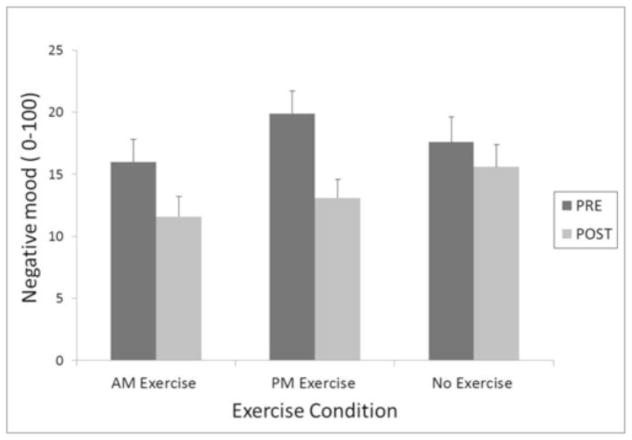
Negative mood was also significantly attenuated by exercise. Reduction in negative mood differed as a function of condition (AM, PM exercise or control), F(2, 385) = 3.38, p=0.04. Again, no difference between AM and PM exercise was revealed, but planned contrasts showed a significantly greater pre-post reduction in negative mood following exercise compared to control activity, t(385)= 2.25, p=.03, d = 0.23.
(4) Withdrawal Severity
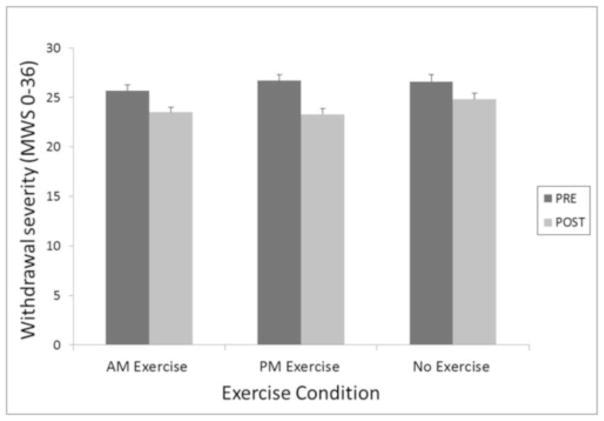
There was a significant effect of condition on withdrawal severity (F(2, 385) = 3.8, p = 0.02). Further, unlike the other three symptoms, PM exercise was associated with larger reduction in withdrawal severity compared to the control condition (t(385) = 2.64, p = 0.009, d = 0.27), while AM exercise was not different from control. However, the difference between AM and PM only neared significance t(385) = −2.01, p = 0.07, d= 0.2.
Changes in withdrawal symptoms as a function of RPE
After controlling for the timing of exercise (AM vs. PM), block, day, age, number of cigarettes, and nicotine dependence, maximum ratings on percent exertion were not associated with pre-post reduction in cravings, negative mood and withdrawal severity during exercise sessions (all p’s >0.13). Exercise worked equally well to attenuate all four abstinence-related symptoms regardless of how easy or difficult individuals perceived it to be.
DISCUSSION
Results support moderate exercise to attenuate abstinence-induced craving, cue-induced craving, negative mood and withdrawal severity in both male and female smokers over 3 continuous days (72 hours) of smoking deprivation. Exercise reliably reduced all four symptoms each day of the three-day abstinence periods without evidence of habituation. These effects occurred regardless of participant sex or level of perceived exertion (RPE) while exercising. In contrast to our expectation, there was no differential impact of AM versus PM exercise on abstinence-induced craving, cue-induced craving, or negative mood. However, PM exercise was more effective than control activity in in reducing withdrawal severity, which was not true of AM exercise.
The finding that moderate exercise reduced abstinence-induced craving and withdrawal severity is consistent with recent meta-analytic reviews examining the impact of physical activity on these measures during brief abstinence from smoking (Haasova et al., 2014; Roberts et al., 2012). Unique to the present study is the finding that moderate exercise reduced cue-induced craving to smoking cues during 3 consecutive days of deprivation. The effect of exercise on reducing reactivity to smoking cues was greater than reduction during exposure to neutral cues. Although both were significant, evaluation of group differences in cue-induced craving to smoking cues while controlling for neutral cues revealed a significant effect of exercise to attenuate cue-induced craving to smoking-related stimuli. This suggests potential for exercise to aid smokers in coping with cue-induced craving where other methods have failed, an important possibility given that cue-induced craving is not alleviated by nicotine replacement (Tiffany, Cox, & Elash, 2000) and can continue much longer than abstinence-induced withdrawal. Moreover, although two studies have demonstrated reduction in cue-induced craving during treatment with varenicline (Brandon et al., 2011; Franklin et al., 2011) other studies have not found this effect (e.g., Hitsman et al., 2013); and, behavioral techniques aimed at reducing cue-induced craving have also consistently failed (Conklin & Tiffany, 2002a, 2002b; Martin et al., 2010). The current finding is in line with other studies examining the impact of physical activity during very brief abstinence (several hours) on cue-induced craving. One such study demonstrated attenuation of cue-provoked craving via brief walking during 2 hours of abstinence (Taylor & Katomeri, 2007), one showed that cardiovascular activity reduced cue-specific craving relative to both Hatha yoga and no exercise control (Elibero et al., 2011), and a third demonstrated reductions in stressor-induced craving using moderate exercise (Fong et al., 2014). The present findings extend that work to show that exercise may acutely reduce cue-induced craving several days into nicotine abstinence when the negative effects of quitting are likely at peak. A larger trial is needed to realize the full potential of exercise to reduce cue-induced craving during actual quitting.
Timing of exercise had limited differential impact on negative symptoms with only PM exercise proving more effective in reducing subjective withdrawal severity relative to control activity. Although individuals prone to “eveningness” over “morningness” are more likely to be smokers (Negriff, Dorn, Pabst, & Susman, 2011; Urbán, Magyaródi, & Rigó, 2011), we found no moderating effect of chronotype on measures of interest in the present study. There are several possibilities for why exercising in the evening may be more effective than control activity for reducing perceived withdrawal severity. First, as public smoking bans increase, smoking may occur much more frequently in specific environments (Chandra, Shiffman, Scharf, Dang, & Shadel, 2007; Conklin, 2006), most notably, the home where many wakeful hours often occur in the evening. Second, smokers may benefit from more distractions from smoking during daytime hours, when they are actively engaged in work or activities. Third, the decision to refrain from smoking requires cognitive effort (Tiffany, 1990) at the expense of other cognitively demanding tasks. By day’s end, the effort to remain cognitively vigilant may wane with fatigue making withdrawal more difficult to combat and the benefits of exercise more robust.
Of note, this was not a clinical trial and only non-treatment seeking smokers were recruited. The study goal was to examine the negative effects of smoking abstinence during the first 3 days of deprivation when symptoms are likely peaking. A significant number of participants initially accepted into the study dropped out after baseline night, and only study completers contributed to the final analysis. Therefore, the findings may not generalize to all smokers, but rather to those able/willing to remain abstinent for 3 day periods outside of a quit attempt. Further, to increase compliance with repeated 3-day abstinence blocks, participants were paid substantially. In a treatment study, smokers would not be given such monetary compensation for quitting; however, treatment-seeking smokers would likely have considerable internal motivation to remain abstinent, something our participants were specifically lacking. Although the present study precludes conclusions on the impact of moderate exercise during actual smoking cessation, the findings do speak to the feasibility of testing exercise as a beneficial adjunct in future cessation trials in several ways. First, exercise was well tolerated. No participants were excluded from the study or dropped out because of difficulty exercising. Participants average Rated Perceived Exertion (RPE; Borg, 1998) was a 12, which is midway between fairly light and somewhat hard work, suggesting variance in perceived difficulty, but no reports that it was too taxing. Second, RPE did not predict level of reduction in any of the withdrawal variables, suggesting that regardless of perceived exertion, moderate walking was equally effective in attenuating the four negative effects of smoking deprivation we studied. Third, the impact of time of day was modest, with PM exercise reducing withdrawal severity over control; however, both AM and PM exercise equally reduced abstinence- and cue-induced craving, as well as negative mood. Therefore, there may be considerable schedule flexibility without sacrificing much efficacy when implementing this potential treatment technique, flexibility many smokers’ may require in a treatment plan. Fourth, there was no evidence of habituation to exercise over the three day abstinence period as it continued to acutely reduce negative symptoms each day. Therefore, exercise may function well over a longer course of abstinence as a daily aid to help smokers cope with negative symptoms that arise during cessation. Fifth, no sex differences were revealed, suggesting that exercise may work equally well for male and female smokers to reduce negative symptoms of abstinence.
There is adequate initial evidence, from the present study and others, to suggest that this simple non-medication based behavioral technique may significantly aid cessation, particularly during early abstinence, a highly relapse-vulnerable time when 80% of quitting smokers return to smoking (Benowitz, 2008). It also suggests the need for further investigation into the ability of exercise to attenuate cue-provoked urges during abstinence and to examine the potential of an exercise adjunct to improve cessation rates achieved with existing treatments for both male and female smokers.
Figure 1.
Abstinence-induced craving pre- and post-exercise averaged across 72 hours of abstinence as a function of exercise condition.
Figure 2.
Cue-induced craving difference score (craving to smoking stimuli minus craving to neutral stimuli) pre- and post-exercise averaged across 72 hours of abstinence as a function of exercise condition.
Figure 3.
Negative mood pre- and post-exercise averaged across 72 hours of abstinence as a function of exercise condition.
Figure 4.
Withdrawal severity pre- and post-exercise averaged across 72 hours of abstinence as a function of exercise condition.
Relevance/Public health significance.
Smoking cessation remains an unattained goal for many smokers attempting to quit. One potential means of augmenting cessation success is through behavioral treatment adjuncts that target negative symptoms experienced during the initial stage of quitting. Results of this study suggest that daily moderate exercise may be one such method as it acutely reduces craving, negative mood, and withdrawal severity consistently over the first three days of smoking deprivation.
Acknowledgments
This work was financially supported by grants from the National Institute on Drug Abuse (DA027508 & DA023646) awarded to Cynthia A. Conklin, Ph.D.
The authors thank Sofia Pikalova, Nicholas Capozzi, Nicholas Rossi, Autumn Keller, Danielle Carns, and Allison Domzalski for their help in running the study.
Footnotes
Disclosures
All authors contributed significantly to the manuscript and have read and approved it.
The authors have no conflicts of interest to report.
References
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. The American Journal of Medicine. 2008;121(4 Suppl 1):S3–10. doi: 10.1016/j.amjmed.2008.01.015. https://doi.org/10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24(3):399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg’s perceived exertion and pain scales. Human kinetics; 1998. Retrieved from http://psycnet.apa.org/psycinfo/1998-07179-000. [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, … Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011;218(2):391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Chandra S, Scharf D, Shiffman S. Within-Day Temporal Patterns of Smoking, Withdrawal Symptoms, and Craving. Drug and Alcohol Dependence. 2011a;117(2–3):118–125. doi: 10.1016/j.drugalcdep.2010.12.027. https://doi.org/10.1016/j.drugalcdep.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Scharf D, Shiffman S. Within-Day Temporal Patterns of Smoking, Withdrawal Symptoms, and Craving. Drug and Alcohol Dependence. 2011b;117(2–3):118–125. doi: 10.1016/j.drugalcdep.2010.12.027. https://doi.org/10.1016/j.drugalcdep.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Shiffman S, Scharf DM, Dang Q, Shadel WG. Daily smoking patterns, their determinants, and implications for quitting. Experimental and Clinical Psychopharmacology. 2007;15(1):67. doi: 10.1037/1064-1297.15.1.67. [DOI] [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Experimental and Clinical Psychopharmacology. 2006;14(1):12. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction (Abingdon, England) 2002a;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Cue-Exposure Treatment: Time for Change. Addiction. 2002b;97(9):1219–1221. doi: 10.1046/j.1360-0443.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Vella EJ, Joyce CJ, Salkeld RP, Perkins KA, Parzynski CS. Examining the relationship between cue-induced craving and actual smoking. Experimental and Clinical Psychopharmacology. 2015;23(2):90. doi: 10.1037/a0038826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, Schroeder DR. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA. 1995;274(17):1353–1358. [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders Source Information. Statistics and Reports. 2015 Jun 2; Retrieved June 2, 2015, from http://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/DSM4/
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47(5):1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Dunbar MS, Scharf D, Kirchner T, Shiffman S. Do smokers crave cigarettes in some smoking situations more than others? Situational correlates of craving when smoking. Nicotine & Tobacco Research. 2010;12(3):226–234. doi: 10.1093/ntr/ntp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elibero A, Van Rensburg KJ, Drobes DJ. Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine & Tobacco Research. 2011:ntr163. doi: 10.1093/ntr/ntr163. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fong AJ, De Jesus S, Bray SR, Prapavessis H. Effect of exercise on cigarette cravings and ad libitum smoking following concurrent stressors. Addictive Behaviors. 2014;39(10):1516–1521. doi: 10.1016/j.addbeh.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, … Childress AR. Effects of Varenicline on Smoking Cue–Triggered Neural and Craving Responses. Archives of General Psychiatry. 2011;68(5):516–526. doi: 10.1001/archgenpsychiatry.2010.190. https://doi.org/10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Taylor AH. The acute effects of physical activity on cigarette cravings: exploration of potential moderators, mediators and physical activity attributes using individual participant data (IDP) meta-analyses. Psychopharmacology. 2014;231(7):1267–1275. doi: 10.1007/s00213-014-3450-4. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Hogarth L, Tseng LJ, Teige JC, Shadel WG, DiBenedetti DB, … Niaura R. Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug and Alcohol Dependence. 2013;130(1):135–141. doi: 10.1016/j.drugalcdep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology. 1992;60(5):689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2007;9(3):315–327. doi: 10.1080/14622200701188919. https://doi.org/10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg K, Taylor AH. The effects of acute exercise on cognitive functioning and cigarette cravings during temporary abstinence from smoking. Human Psychopharmacology. 2008;23(3):193–199. doi: 10.1002/hup.925. https://doi.org/10.1002/hup.925. [DOI] [PubMed] [Google Scholar]
- Jolicoeur DG, Richter KP, Ahluwalia JS, Mosier MC, Resnicow K. Smoking cessation, smoking reduction, and delayed quitting among smokers given nicotine patches and a self-help pamphlet. Substance Abuse. 2003;24(2):101–106. doi: 10.1080/08897070309511538. https://doi.org/10.1080/08897070309511538. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology. 1999;7(3):226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Martin T, LaRowe S, Malcolm R. Progress in cue exposure therapy for the treatment of addictive disorders: a review update. Open Addict J. 2010;3:92–101. [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185(3):408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Briski J, Fonte C, Scott J, Lerman C. Severity of tobacco abstinence symptoms varies by time of day. Nicotine & Tobacco Research. 2009:ntn003. doi: 10.1093/ntr/ntn003. https://doi.org/10.1093/ntr/ntn003. [DOI] [PMC free article] [PubMed]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology. 2012;222(1):1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, … Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91(2–3):159–168. doi: 10.1016/j.drugalcdep.2007.05.017. https://doi.org/10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184(3–4):637–644. doi: 10.1007/s00213-005-0184-3. https://doi.org/10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Rolf CN, Hellebusch SJ, Gorsline J, Gorodetzky CW, Chiang YK, … Di Marino ME. Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction (Abingdon, England) 2002;97(5):505–516. doi: 10.1046/j.1360-0443.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- Soreca I, Conklin CA, Kupfer DJ, Jakicic JM, Salkeld RP, Mumma Joel. Does exercise attenuate sleep disturbances during acute nicotine withdrawal? n.d doi: 10.1037/pha0000390. Manuscript under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Katomeri M, Ussher M. Acute effects of self-paced walking on urges to smoke during temporary smoking abstinence. Psychopharmacology. 2005;181(1):1–7. doi: 10.1007/s00213-005-2216-4. https://doi.org/10.1007/s00213-005-2216-4. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction (Abingdon, England) 2007;102(4):534–543. doi: 10.1111/j.1360-0443.2006.01739.x. https://doi.org/10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Taylor A, Katomeri M. Effects of a brisk walk on blood pressure responses to the Stroop, a speech task and a smoking cue among temporarily abstinent smokers. Psychopharmacology. 2006;184(2):247–253. doi: 10.1007/s00213-005-0275-1. https://doi.org/10.1007/s00213-005-0275-1. [DOI] [PubMed] [Google Scholar]
- Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2007;9(11):1183–1190. doi: 10.1080/14622200701648896. https://doi.org/10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68(2):233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Urbán R, Magyaródi T, Rigó A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28(3):238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2014. [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. The Cochrane Database of Systematic Reviews. 2012a;1:CD002295. doi: 10.1002/14651858.CD002295.pub4. https://doi.org/10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012b:1. doi: 10.1002/14651858.CD002295.pub4. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002295.pub4/pdf/ [DOI] [PubMed]
- West R, Hajek P, Belcher M. Time course of cigarette withdrawal symptoms while using nicotine gum. Psychopharmacology. 1989;99(1):143–145. doi: 10.1007/BF00634470. [DOI] [PubMed] [Google Scholar]