Abstract
Importance
Comparisons of the relative effect of three anti-vascular endothelial growth factor (anti-VEGF) agents to treat DME warrant further assessment.
Objective
P rovide additional outcomes from a randomized trial evaluating three anti-VEGF agents for DME within subgroups based on baseline visual acuity (VA) and optical coherence tomography central subfield thickness (CST).
Design/Setting
Post-hoc, exploratory analyses of randomized trial data.
Participants
660 adults with DME and decreased VA (~Snellen equivalent 20/32 to 20/320).
Interventions
Repeated intravitreous 2.0-mg aflibercept (N=224), 1.25-mg bevacizumab (N=218), or 0.3-mg ranibizumab (N=218) as needed per protocol.
Main Outcome Measures
1-year VA and CST outcomes within pre-specified subgroups based on both baseline VA and CST thresholds: “worse” (20/50 or worse) or “better” (20/32 to 20/40) VA, “thicker” (≥400 microns) or “thinner” (250 to 399 microns) CST.
Results
In the worse baseline VA subgroup, irrespective of baseline CST, aflibercept showed greater improvement than bevacizumab or ranibizumab for several VA outcomes. With better VA and thinner CST at baseline (n=61 to 73 across 3 treatment groups), visual acuity outcomes showed little differences between groups; mean change was +7.2, +8.4, and +7.6 letters in the aflibercept, bevacizumab, and ranibizumab groups, respectively. However, with better VA and thicker CST at baseline (n=31 to 43), there was a suggestion of worse VA outcomes in the bevacizumab group; mean change from baseline to one year was +9.5, +5.4, and +9.5 letters in the aflibercept, bevacizumab, and ranibizumab groups, respectively; at one-year VA letter score was >84 (~20/20) in 21 (64%), 7 (23%), and 21 (49%) eyes, respectively. The adjusted differences and 95% confidence intervals for aflibercept versus bevacizumab was 39%(17% to 60%), for ranibizumab versus bevacizumab was 25% (5% to 46%) and for aflibercept versus ranibizumab was 13% (−8% to 35%).
Conclusions and Relevance
These post-hoc secondary findings suggest that for eyes with better initial VA and thicker CST, some VA outcomes may be worse in the bevacizumab group than in the other two groups. Given the exploratory nature of these analyses and the small sample size within subgroups, caution is suggested when using the data to guide treatment considerations for an individual patient.
INTRODUCTION
The Diabetic Retinopathy Clinical Research Network (DRCR.net) conducted a comparative effectiveness randomized clinical trial with 660 study participants to compare treatment outcomes with intravitreous aflibercept (Eylea, Regeneron Pharmaceuticals, Inc.), bevacizumab (Avastin, Genentech, Inc.), or ranibizumab (Lucentis, Genentech, Inc.) in eyes with center-involving diabetic macular edema (CI-DME) associated with best corrected visual acuity (VA) of 20/32 to 20/320.1 A standardized treatment protocol which limited investigator discretion was used with treatment decisions based on VA and central subfield thickness (CST) evaluated by optical coherence tomography (OCT) at each study visit.
Mean VA improvement was seen at one month with each anti-vascular endothelial growth factor (VEGF) agent, and substantial improvement, on average, was noted at the one-year primary outcome for all three drugs.1 For the primary outcome at 1 year for the entire cohort, average improvement was 2 to 3 letters greater with aflibercept than ranibizumab or bevacizumab. However, the magnitude of aflibercept’s greater effect was dependent on initial visual acuity.1 The worse the initial VA, the greater the relative advantage of aflibercept over the other two agents on VA outcomes. When the initial VA was a Snellen equivalent of 20/32 to 20/40, which represented about half of the study eyes, on average there was little difference in one-year VA change from baseline among the three agents. The relative treatment effect on VA also varied according to initial CST; the worse the initial CST, the greater the relative advantage of aflibercept over the other two agents.1 These findings prompted evaluation of other clinically relevant post-hoc, exploratory analyses including the following: (1) outcomes within subgroups combining baseline VA and CST and (2) additional outcomes and treatments administered that were not previously reported within the worse and better initial VA subgroups.
METHODS
The study procedures and statistical methods have been reported1 and are only summarized briefly herein. The study adhered to the tenets of the Declaration of Helsinki. The protocol and Health Insurance Portability and Accountability Act compliant informed consent forms were approved by multiple institutional review boards. The study is accessible on www.clinicaltrials.gov (NCT01627249 website registration date 06-21-2012) and the protocol is available on the DRCR.net website (www.drcr.net, accessed November 1, 2014).
The study included 660 individuals ≥18 years old. Each participant had one study eye only with best-corrected visual acuity letter score of 78 to 24, (approximate Snellen equivalent 20/32 to 20/320) due to DME with CST equivalent to ≥250 microns on Stratus time domain OCT (Carl Zeiss Meditec, Dublin, CA). CST values usually were obtained on a spectral domain OCT (96%) and translated2 to Stratus OCT equivalent thickness values based on a validated conversion equation.
Pre-specified subgroups for baseline VA included eyes with letter score 78 to 69 (20/32 to 20/40 - “better” VA subgroup) or 68 to 24 (20/50 to 20/320 - “worse VA” subgroup), while those for baseline CST included eyes with thickness >=400 microns (“thicker” subgroup) and 250 to 399 microns (“thinner” subgroup). The subgroups were defined based on pre-study anticipated median VA of 20/50 and median CST of 400 microns. Among the 660 participants, 329 (50%) eyes were in the worse VA subgroup and 331 (50%) were in the better VA subgroup, while 299 (46%) and 353 (54%) eyes were in the thicker and thinner subgroups, respectively. Eight eyes had missing baseline CST. Among the 323 eyes in the worse VA group, 185 (62%) were in the thicker group and 138 (39%) in the thinner group. Among the 329 eyes in the better VA group, 114 (38%) were in the thicker group and 215 (61%) in the thinner group (eFigure 1).
The study eye was assigned randomly to 0.05-mL intravitreous injection of 2.0-mg aflibercept, 1.25-mg bevacizumab, or 0.3-mg ranibizumab. Injections were given at baseline and 4 weeks. Subsequent injections were performed every 4 weeks unless one or more of the following occurred: (1) before the 24-week visit, visual acuity letter score was ≥84 (20/20 or better) with CST <250 microns time-domain-equivalent (thinner than the eligibility threshold) and no “improvement” (at least a 5 letter gain or at least a 10% reduction in CST) from the last two injections, referred to as “treatment success”, or (2) starting at the 24-week visit, no “improvement” and no “worsening” (at least a 5-letter change or 10% change in CST) after two consecutive injections, referred to as “stability”. Injections were resumed following deferral of treatment if visual acuity or CST subsequently worsened. For persistent DME at or after the 24-week visit, focal/grid laser was given if there were thickened areas to treat within the macula and if the CST was ≥250 microns or there was edema threatening the fovea and the eye did not improve on VA or CST from the last two consecutive injections. Retreatment with focal/grid laser was given unless one of the following criteria were met: 1) less than 13 weeks had passed since the last focal/grid laser, 2) in the investigator’s judgment, complete focal/grid laser had been given, 3) the CST was <250 microns and there was no edema threatening the fovea on clinical exam, or 4) the eye had improved since the last focal/grid laser treatment.
Statistical Methods
Analyses included only participants having 1-year outcome data (94% 1-year completion rate which was similar across groups). Outlying VA changes were truncated to three standard deviations from the mean change (+11 ± 33) in VA at 1 year. Area under the curve (AUC) for change in VA from baseline up to 1 year was calculated using the trapezoidal rule. Mean change in VA, CST and AUC from baseline to 1 year, were estimated and compared among treatment groups using an analysis of covariance (ANCOVA) model and binary VA outcomes were analyzed using a binomial regression model.3 VA and CST analyses included adjustment for baseline VA, baseline CST, the three-way interaction of baseline VA, baseline CST and treatment, and all two-way interactions. When both VA and CST and the interactions with treatment were included in the mean change in VA model, the treatment effect still varied according to initial VA (P = 0.006 for interaction with visual acuity) but there was less confidence in the interaction with CST (P = 0.11; P = 0.82 for the three-way interaction of VA, CST and treatment group). For the mean change in CST model, the treatment effect still varied according to the baseline CST (P<0.001) but neither a two-way interaction of treatment and baseline VA nor the three-way interaction of treatment, CST and VA were identified (P = 0.84 and 0.92 respectively). Differences in least squares means or binary proportions with corresponding 95% confidence intervals are reported for the models including three-way and all lower-order interactions.
Means are reported with standard deviations as all analyses were considered exploratory. All reported P values are two-sided and no corrections were made for multiple comparisons unless otherwise stated in the table footnotes. Analyses used SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics within VA and OCT subgroups are given in eTable 1 and eTable2. eFigure 1 online provides a scatter plot of the distribution of baseline VA and baseline CST. Eyes in the worse VA subgroup were slightly more likely to be in the thicker CST subgroup than thinner subgroup, while eyes in the better VA subgroup were more likely to be in the thinner CST subgroup than thicker subgroup (r=−0.36, P<0.001). Regardless, there still were many eyes with thinner CST and worse VA (lower right quadrant of eFigure 1) or thicker CST and better VA (upper left quadrant of eFigure1).
VA and CST Outcomes by Baseline VA and Baseline CST Subgroups
Tables 1 and 2 provide VA outcomes at 1 year stratified by baseline VA and baseline CST. Figures 1 – 2, eFigure 2 and eFigure 3 show VA outcomes within the four subgroups over time. As shown in Table 1 and 2, for eyes in the worse baseline VA subgroup, for both thicker and thinner baseline CST (n=50 to 61 and n=39 to 49, respectively, across the 3 anti-VEGF agents), mean VA letter score change from baseline was greater in the aflibercept-treated eyes than in the bevacizumab- or ranibizumab-treated eyes. The difference in the area under the curve (essentially the average visual acuity letter score change over the 1 year) was also greater in the aflibercept-treated eyes than in the bevacizumab- or ranibizumab-treated eyes. Comparing AUC with ranibizumab to bevacizumab, within the worse VA subgroup at baseline, slight differences were noted in favor of ranibizumab with thicker but not thinner CST at baseline.
Table 1.
Visual Acuity Outcomes at 1 Year by Baseline Central Subfield Thickness and Visual Acuity
| Observed Data | Treatment Group Comparisons Adjusted Differences in Mean Change (95% CI)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aflibercept | Bevacizumab | Ranibizumab | Aflibercept | Bevacizumab | Ranibizumab | A vs B | A vs R | R vs B | A vs B | A vs R | R vs B | ||
| ≥400 microns | <400 microns | ≥400 microns | <400 microns | ||||||||||
| Baseline Visual Acuity 20/50 or Worse (Letter Score <69) | |||||||||||||
| N = 61 | N = 61 | N = 50 | N = 40 | N = 39 | N = 49 | ||||||||
| Baseline | |||||||||||||
| Mean ± SD | 54.9 ± 11.0 | 55.6 ± 10.3 | 55.5 ± 10.5 | 59.0 ± 10.0 | 59.4 ± 9.7 | 57.7 ± 9.1 | |||||||
| ~Snellen Equivalent | 20/80 | 20/80 | 20/80 | 20/63 | 20/63 | 20/80 | |||||||
| 1 Year | |||||||||||||
| Mean ± SD | 74.7 ± 11.1 | 66.8 ± 15.2 | 70.4 ± 13.0 | 76.6 ± 10.1 | 71.0 ± 10.7 | 71.1 ± 11.1 | |||||||
| ~Snellen Equivalent | 20/32 | 20/50 | 20/40 | 20/32 | 20/40 | 20/40 | |||||||
| Change from baseline (letter score improvement) | |||||||||||||
| Mean AUC± SD | 15.5 ± 8.9 | 10.5 ± 9.3 | 12.5± 8.7 | 13.3± 9.8 | 9.6 ± 7.3 | 10.0 ± 8.3 | |||||||
| Mean ± SD | 19.7 ± 11.7 | 11.3 ± 13.2 | 14.9 ± 10.0 | 17.5± 11.3 | 11.6 ± 8.8 | 13.3 ± 11.3 | |||||||
| ≥5 letter improvement | 55 (90%) | 46 (75%) | 43 (86%) | 35 (88%) | 30 (77%) | 41 (84%) | +16% (+3% to +28%) | +5% (−7% to +17%) | +11% (−4% to +25%) | +9% (−8% to +26%) | +3% (−11% to +18%) | +6% (−11% to +23%) | |
| ≥ 10 letter improvement | 50 (82%) | 36 (59%) | 36 (72%) | 28 (70%) | 23 (59%) | 33 (67%) | +23% (+8% to +38%) | +10% (−5% to +25%) | +14% (−3% to +31%) | +9% (−11% to +30%) | +2% (−17% to +21%) | +7% (−13% to +28%) | |
| ≥ 15 letter improvement | 44 (72%) | 26 (43%) | 26 (52%) | 23 (58%) | 14 (36%) | 23 (47%) | +30% (+14% to +45%) | +20% (+3% to +37%) | +10% (−8% to +27%) | +20% (−1% to +41%) | +13% (−7% to +33%) | +7% (−13% to +27%) | |
| ≥ 84 (20/20) | 12 (20%) | 3 (5%) | 6 (12%) | 11 (28%) | 4 (10%) | 7 (14%) | 15% (+3% to +26%) | +8% (−6% to +21%) | +7% (−3% to +18%) | +17% (0% to +33%) | +15% (−2% to +32%) | +2% (−12% to +16%) | |
| Baseline Visual Acuity 20/32–20/40 (Letter Score 78–69) | |||||||||||||
| N = 33 | N = 31 | N = 43 | N = 72 | N = 73 | N = 61 | ||||||||
| Baseline | |||||||||||||
| Mean ± SD | 73.2 ± 2.7 | 72.4 ± 2.7 | 72.8 ± 2.7 | 73.6 ± 2.5 | 73.0 ± 2.9 | 73.7 ± 2.7 | |||||||
| ~Snellen Equivalent | 20/40 | 20/40 | 20/40 | 20/32 | 20/40 | 20/32 | |||||||
| 1 Year | |||||||||||||
| Mean ± SD | 82.6 ± 8.4 | 76.1 ± 15.6 | 82.3 ± 6.1 | 80.7 ± 8.2 | 81.5 ± 6.0 | 81.3 ± 7.3 | |||||||
| ~Snellen Equivalent | 20/25 | 20/32 | 20/25 | 20/25 | 20/25 | 20/25 | |||||||
| Change from baseline (letter score improvement) | |||||||||||||
| Mean AUC± SD | 8.2± 5.1 | 4.7 ± 5.2 | 6.8 ± 4.3 | 6.3 ± 4.7 | 5.6 ± 4.8 | 6.3 ± 4.8 | |||||||
| Mean ± SD | 9.5±8.4 | 5.4±8.6 | 9.5±6.7 | 7.2±7.2 | 8.4±6.6 | 7.6±6.8 | |||||||
| ≥ 5 letter improvement | 29 (88%) | 17 (55%) | 33 (77%) | 48 (67%) | 52 (71%) | 46 (75%) | +35% (+15% to +56%) | +13% (−4% to +29%) | +23% (+1% to +44%) | −4% (−19% to +11%) | −8% (−23% to +7%) | +4% (−11% to +19%) | |
| ≥ 10 letter improvement | 21 (64%) | 11 (35%) | 25 (58%) | 31 (43%) | 36 (49%) | 27 (44%) | +32% (+8% to +55%) | +7% (−15% to +30%) | +25% (+2% to +47%) | −4% (−20% to +12%) | 0% (−16% to +16%) | −4% (−21% to +12%) | |
| ≥ 15 letter improvement | 9 (27%) | 4 (13%) | 10 (23%) | 10 (14%) | 13 (18%) | 6 (10%) | +11% (−7% to +29%) | +6% (−14% to +25%) | +6% (−10% to +22%) | −1% (−12% to +10%) | +3% (−6% to +13%) | −4% (−15% to +6%) | |
| ≥ 84 (20/20) | 21 (64%) | 7 (23%) | 21 (49%) | 31 (43%) | 27 (37%) | 30 (49%) | +39% (+17% to +60%) | +13% (−8% to +35%) | +25% (5% to +46%) | +5% (−11% to +21%) | −7% (−24% to +10%) | +12% (−5% to +29%) | |
SD = standard deviation; CI = confidence interval; A = aflibercept; B = bevacizumab; R = ranibizumab
CST values usually were translated2 to an equivalent thickness based on a Stratus time domain OCT.
Treatment group comparisons are means from binomial regression models adjusted for baseline visual acuity (categorical), baseline central subfield thickness (continuous with the exception of the ≥84 outcome), the three-way interaction of baseline visual acuity (categorical), baseline central subfield thickness (categorical) and treatment and all two-way interactions (categorical).
Baseline OCT data unavailable for 2 eyes in the aflibercept group, 2 eyes in the bevacizumab group, and 3 eyes in the ranibizumab group.
One-year visit data unavailable for 16 eyes in the aflibercept group, 12 eyes in the bevacizumab group, and 12 eyes in the ranibizumab group.
Table 2.
Treatment Group Comparisons of Change in Visual Acuity and Central Subfield Thickness from Baseline to 1 Year
| Baseline VA 20/50 or Worse (Letter Score <69) | Baseline CST ≥400 microns | Baseline CST <400 microns |
|---|---|---|
| Adjusted Differences in Mean Change (95% CI)* | ||
| A vs. B | (N= 61 vs 61) | (N= 40 vs 39) |
| VA (Letter Score) | 8.1 (CI: 4.9, 11.3) | 5.7 (CI: 1.8, 9.7) |
| VA AUC† (Letter Score) | 4.8 (CI: 2.4, 7.1) | 3.5 (CI: 0.6, 6.4) |
| CST (microns) | −123 (CI: −155, −91) | −31 (CI: −70, 8) |
| A vs. R | (N= 61 vs 50) | (N= 40 vs 49) |
| VA (Letter Score) | 4.5 (CI: 1.2, 7.8) | 4.7 (CI: 0.9, 8.4) |
| VA AUC† (Letter Score) | 2.8 (CI: 0.3, 5.2) | 3.7 (CI: 1.0, 6.5) |
| CST (microns) | −24 (CI: −57, 10) | −7 (CI: −45, 30) |
| R vs. B | (N= 50 vs 61) | (N= 49 vs 39) |
| VA (Letter Score) | 3.6 (CI: 0.3, 7.0) | 1.1 (CI: −2.7, 4.8) |
| VA AUC† (Letter Score) | 2.0 (CI: −0.5, 4.4) | −0.2 (CI: −3.0, 2.5) |
| CST (microns) | −99 (CI: −133, −66) | −24 (CI: −61, 14) |
| Baseline VA 20/32–20/40 (Letter Score 78–69) | Baseline CST ≥400 microns | Baseline CST <400 microns |
| Adjusted Differences in Mean Change (95% CI)* | ||
| A vs. B | (N= 32 vs 30) | (N= 72 vs 73) |
| VA (Letter Score) | 3.7 (CI: −0.8, 8.1) | −1.0 (CI: −3.9, 1.9) |
| VA AUC† (Letter Score) | 3.6 (CI: 0.4, 6.9) | 1.0 (CI: −1.2, 3.1) |
| CST (microns) | −112 (CI: −157, −68) | −32 (CI: −61, −3) |
| A vs. R | (N= 32 vs 43) | (N= 72 vs 59) |
| VA (Letter Score) | 0.2 (CI: −3.8, 4.3) | −0.4 (CI: −3.5, 2.7) |
| VA AUC† (Letter Score) | 1.6 (CI: −1.4, 4.6) | 0.0 (CI: −2.2, 2.3) |
| CST (microns) | −22 (CI: −63, 19) | −20 (CI: −51, 11) |
| R vs. B | (N= 43 vs 30) | (N= 59 vs 73) |
| VA (Letter Score) | 3.4 (CI: −0.8, 7.6) | −0.6 (CI: −3.7, 2.4) |
| VA AUC† (Letter Score) | 2.0 (CI: −1.1, 5.0) | 1.0 (CI: −1.3, 3.2) |
| CST (microns) | −90 (CI: −132, −48) | −12 (CI: −43, 19) |
VA= visual acuity; CST = central subfield thickness; CI = confidence interval; A = aflibercept; B = bevacizumab; R = ranibizumab; AUC = area under curve
CST values usually were translated2 to an equivalent thickness based on a Stratus time domain OCT.
Treatment group comparisons are means from ANCOVA models adjusted for baseline visual acuity (continuous), baseline central subfield thickness (continuous), the three-way interaction of baseline visual acuity (categorical), baseline central subfield thickness (categorical) and treatment and all two-way interactions (categorical).
Baseline central subfield thickness data missing for 2 eyes in the aflibercept group, 2 eyes in the bevacizumab group and 3 eyes in the ranibizumab.
One-year central subfield thickness data missing for 1 eye in the aflibercept group, 1 eye in the bevacizumab group and 2 eyes in the ranibizumab.
One-year visit data unavailable for 16 eyes in the aflibercept group, 12 eyes in the bevacizumab group, and 12 eyes in the ranibizumab group.
Weighted average based on total area under the curve divided by number of days.
Figure 1.
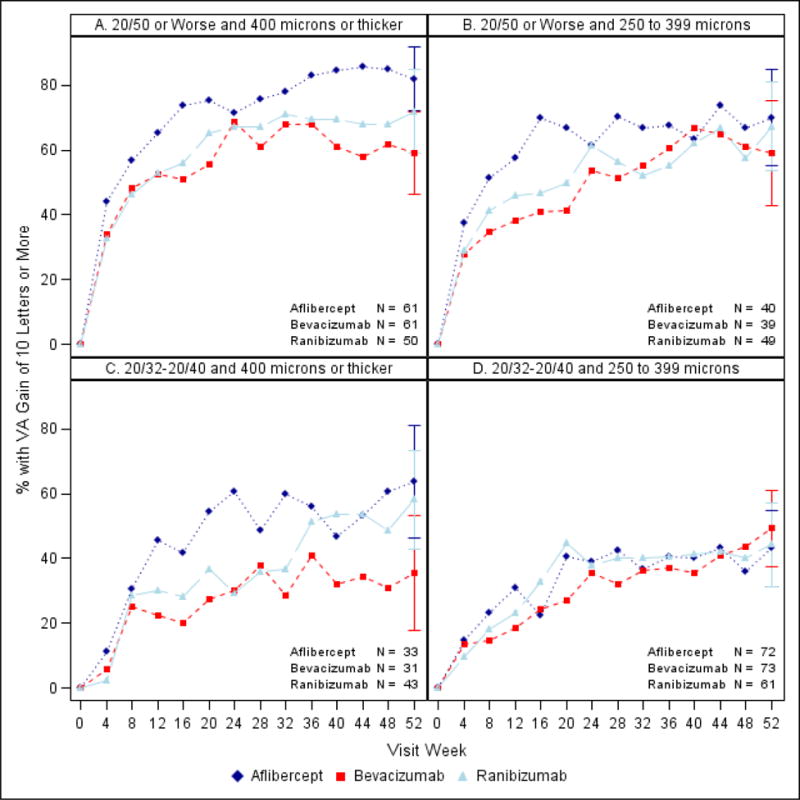
Percentage of eyes with ten or more letter improvement stratified by baseline VA and CST A. Worse and thicker. B. Worse and thinner C. Better and thicker. D. Better and thinner. CST values were translated2 to a Stratus equivalent thickness. All available data shown at each visit; Ns for 52 week visit.
Figure 2.
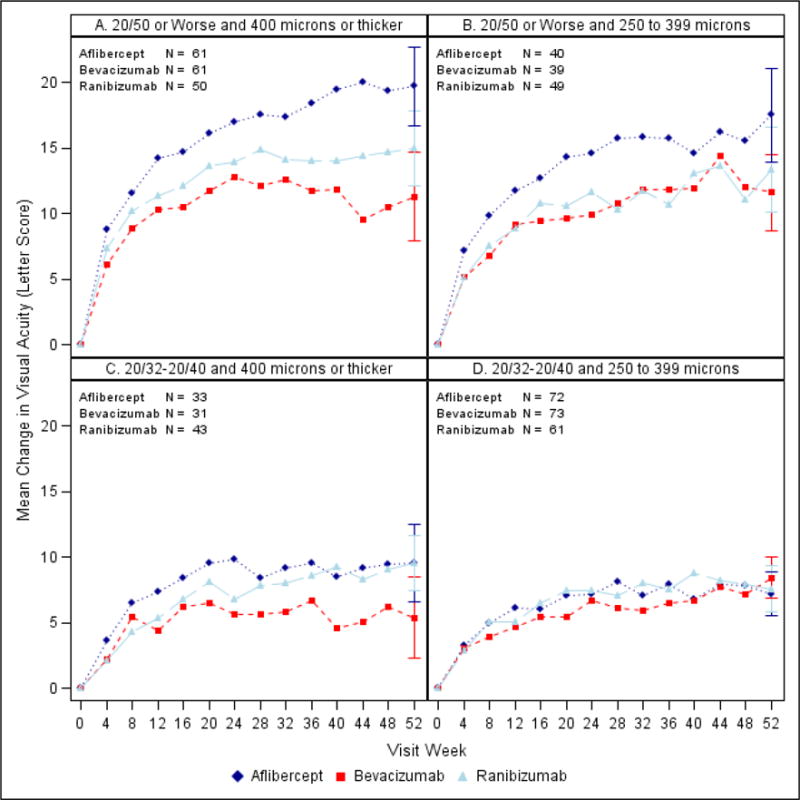
Mean change in VA over time by baseline VA and CST. A. Worse and thicker. B. Worse and thinner C. Better and thicker. D. Better and thinner. CST values were translated2 to a Stratus equivalent thickness. All available data shown at each visit; Ns for 52 week visit.
In the better baseline VA subgroup with thicker baseline CST (n = 31 to 43 across the 3 anti-VEGF agents), there was a suggestion of less VA improvement in the bevacizumab group than the other two groups on several VA outcome measures, especially the percentage of eyes with VA letter score ≥84 (20/20) at one year (64%, 23%, and 49% in the aflibercept, bevacizumab, and ranibizumab groups, respectively). In the subgroup with better baseline VA and thinner CST (n = 61 to 73 across the 3 anti-VEGF agents), differences among the three anti-VEGF agents in VA outcomes were small, with the mean change from baseline to 1 year in VA letter score of +7.2, +8.4, and +7.6 in the aflibercept, bevacizumab, and ranibizumab groups respectively (Table 1).
Outcomes based on change in OCT at one year for the four subgroups were in the same direction to those seen for visual acuity (Tables 2 and 3). In general the reduction in retinal thickening from baseline to 1 year was greater in the aflibercept and ranibizumab groups compared with the bevacizumab group [mean differences (95% CI) −123 (−155, −91) and −99 (−133, −66) microns] among the thicker eyes with worse VA at baseline (Table 2). As demonstrated in Table 2, the reductions in retinal thickening among the thinner eyes with worse or better VA were less in the bevacizumab group, but these treatment differences could not be considered to be definitively different as the lower limit of the 95% confidence intervals crossed 0 except for aflibercept versus bevacizumab in which the upper limit of the difference approached 0 (equal to −3 microns) in the worse VA subgroup.
Table 3.
Optical Coherence Tomography Central Subfield Thickness Outcomes by Baseline Central Subfield Thickness and Visual Acuity
| ≥400 microns | <400 microns | |||||
|---|---|---|---|---|---|---|
| Baseline Visual Acuity 20/50 or Worse (Letter Score <69) | ||||||
| Aflibercept (N = 61) |
Bevacizumab (N = 61) |
Ranibizumab (N = 50) |
Aflibercept (N = 40) |
Bevacizumab (N = 39) |
Ranibizumab (N = 49) |
|
| Baseline CSF (μm) | ||||||
| Mean ± SD | 534 ± 128 | 558 ± 130 | 540 ± 106 | 327 ± 44 | 323 ± 39 | 320 ± 50 |
| 1 Year (μm) | ||||||
| Mean ± SD | 244 ± 96 | 373 ± 175 | 269 ± 109 | 229 ± 51 | 258 ± 71 | 234 ± 68 |
| Change from baseline (μm) | ||||||
| Mean ± SD | −203 ± 285 | −181 ± 170 | −266 ± 152 | −97 ± 60 | −63 ± 77 | −85 ± 78 |
| Baseline Visual Acuity 20/32–20/40 (Letter Score 78–69) | ||||||
|
Aflibercept (N = 32) |
Bevacizumab (N = 30) |
Ranibizumab (N = 43) |
Aflibercept (N = 72) |
Bevacizumab (N = 73) |
Ranibizumab (N = 59) |
|
| Baseline CSF (μm) | ||||||
| Mean ± SD | 499 ± 103 | 471 ± 75 | 479 ± 68 | 317 ± 45 | 318 ± 44 | 314 ± 45 |
| 1 Year (μm) | ||||||
| Mean ± SD | 260 ± 73 | 365 ± 100 | 277 ± 103 | 233 ± 46 | 265 ± 50 | 253 ± 65 |
| Change from baseline (μm) | ||||||
| Mean ± SD | −235 ± 134 | −104 ± 86 | −198 ± 106 | −82 ± 50 | −52 ± 47 | −61 ± 66 |
SD = standard deviation; CI = confidence interval
CST values usually were translated2 to an equivalent thickness based on a Stratus time domain OCT.
Treatment group comparisons are means from ANCOVA models adjusted for baseline visual acuity (continuous), baseline central subfield thickness (continuous), the three-way interaction of baseline visual acuity (categorical), baseline central subfield thickness (categorical) and treatment and all two-way interactions (categorical).
Baseline central subfield thickness data missing for 2 eyes in the aflibercept group, 2 eyes in the bevacizumab group and 3 eyes in the ranibizumab.
One-year central subfield thickness data missing for 1 eye in the aflibercept group, 1 eye in the bevacizumab group and 2 eyes in the ranibizumab.
One-year visit data unavailable for 16 eyes in the aflibercept group, 12 eyes in the bevacizumab group, and 12 eyes in the ranibizumab group.
Outcomes Stratified by Either Baseline Visual Acuity or CST Subgroups
DME treatment by VA subgroup are presented in eTable 3 and eTable 4 and by central subfield thickness subgroup in eTable 5 and eTable 6. Visual acuity outcomes by baseline visual acuity subgroups, including percentage of eyes achieving 20/20 visual acuity, full distribution of 1-year visual acuity letter scores and changes in visual acuity letters, and area under the curve are presented in eTable 7 and eFigure 4. eFigures 5 through 8 and eTable 8 present visual acuity outcomes by baseline CST instead of VA.
DISCUSSION
In a comparative effectiveness trial evaluating 3 different anti-VEGF agents for DME, treatment with aflibercept resulted in greater mean improvements in VA than bevacizumab or ranibizumab. However, a significant interaction between treatment group and baseline visual acuity showed that the worse the initial VA, the greater the relative advantage of aflibercept over the other two agents for VA outcomes.1 Although there were separate interactions of baseline VA with treatment group and baseline CST with treatment group, when both two-way interactions were included in the model, the interaction with CST was no longer significant. This suggests that baseline VA is more effective than baseline CST at explaining the differences in VA outcomes across the three anti-VEGF agents at 1 year. The post-hoc findings in this report support the initial publication that the worse the initial VA, the greater the relative advantage of aflibercept on visual acuity outcomes at 1 year.1 These superior aflibercept outcomes, on average, were not due to a greater number of intravitreous injections in that arm nor an increased number of injections in the fellow eye which theoretically could have caused a cross-over effect on the study eye.1 An additional finding in this report among eyes in the better baseline VA subgroup with thicker CST (albeit based on a relatively small sample size of 31 to 43 eyes across the 3 anti-VEGF agents) was less VA improvement in several VA outcome measures in the bevacizumab group than with the other agents, particularly the percentage of eyes with VA letter score ≥84 (~20/20 or better) at one year (66%, 23%, and 49% in the aflibercept, bevacizumab, and ranibizumab groups, respectively).
These findings need to be interpreted with caution. The strengths of this study include the randomized study design and the standardized retreatment protocol that incorporated the principle of visits every 4 weeks in the first year to assess whether anti-VEGF treatment should be repeated or focal/grid laser should be performed. However, given the post-hoc nature of these exploratory analyses and wide confidence intervals that result from variability of the measurements and limited number of participants within subgroups, there is a limit to the confidence in the differences reported herein. When considering applications of these data to clinical practice, one also should consider that adjustments were not made for the multiple analyses undertaken. Also, while the study participants and OCT reading center personnel were masked to treatment assignment at all visits, and visual acuity and OCT examiners were masked at least at the 1 year visit, the treating ophthalmologists were not masked. While most of the treatment protocol decisions were based on VA and CST that avoided investigator discretion, biases may have been introduced when making subjective determinations, for example, whether complete focal/grid laser had been given. In view of the exploratory nature of these analyses and the small sample sizes, particularly in the analyses stratified by VA and CST, caution is suggested when using the data to guide treatment considerations for an individual patient.
Supplementary Material
Acknowledgments
Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817.
Financial Conflicts of Interest: The following information is accurate, complete, and up-to-date and is consistent with that reported in each authors ICMJE disclosure forms.
John A. Wells, MD: Regeneron, Genentech (Clinical or lab research grants)
Adam R. Glassman, MS: National Institutes of Health (Grant); Genentech/Roche (Grant); Regeneron (Grant)
Lee M. Jampol, MD: Janssen/QLT (Data Monitoring)
Lloyd Paul Aiello, MD, PhD: Genentech (Consultancy)
Andrew N. Antoszyk, MD: Allegro (Grant), Allergan (Grant, personal fees, nonfinancial support), Regeneron (Grant, Non-Financial Support), Genentech (Grant, personal fees, nonfinancial support), Alimera (Grant, personal fees), Quark (Grant), Valeant (personal fees), Novartis (personal fees)
Carl W. Baker, MD: Genentech (Clinical or lab research grants)
Neil M. Bressler, MD: Northwestern University (subcontract from National Eye Institute) Genentech/Roche, Lumenis, Bayer, Novartis, Regeneron (Clinical or lab research grants)
David J. Browning, MD: Alcon, Pfizer, Aerpio, Regeneron, Novartis (Clinical or lab research grants); Alimera (personal fees), springer (royalties)
Crystal G. Connor, MS, MPH: none
Michael J. Elman, MD: None
Frederick L. Ferris, MD: None
Scott M. Friedman, MD: Alson, Allergan, Regeneron, Genentech (Grants)
Michele Melia, ScM: National Eye Institute (Grant); Alimera Sciences (DSMB Membership)
Dante J. Pieramici, MD: Genentech, Regeneron (Consultancy, Clinical or lab research grants, other non-clinical or lab research grants)
Jennifer K. Sun, MD, MPH: Genentech (Clinical or lab research grants)
Roy W. Beck, MD, PhD: Genentech, Regeneron (Non-financial support, drug provided); NIH (Grant)
Footnotes
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Trial Registration: NCT01627249
Author Contributions:
Study concept and design: Bressler, Glassman, Jampol, Wells, Beck, Ferris, Aiello
Acquisition of data: 1John A. Wells, MD; 4Lloyd Paul Aiello, MD, PhD; 5Andrew N. Antoszyk, MD; 6Carl W. Baker, MD; 7Neil M. Bressler, MD; 5David J. Browning, MD; 8Michael J. Elman, MD;; 10Scott M. Friedman, MD; 11Dante J. Pieramici, MD; 4Jennifer K. Sun, MD
Analysis and interpretation of data: Connor, Glassman, Melia
Drafting of the manuscript: Bressler, Connor, Glassman, Beck
Critical revision of the manuscript for important intellectual content: Wells, Glassman, Connor, Jampol, Aiello, Antoszyk, Baker, Bressler, Browning, Elman, Ferris, Friedman, Melia, Pieramici, Sun, Beck
Obtained funding: Bressler, Glassman, Jampol
Administrative, technical, and material support: Connor, Glassman
Crystal Connor and Adam Glassman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Contributions: Regeneron Pharmaceutical provided the aflibercept and Genentech provided the ranibizumab for the study. Genentech also provided funding for blood pressure cuffs and the collection of serum and urine that are not part of the main study results reported herein. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publicationRole of the Sponsor: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript or the decision to submit the manuscript for publication.
Disclaimer: Dr. Neil Bressler is the JAMA Ophthalmology Editor-in-Chief but was not involved in the review process or the acceptance of the manuscript.
References
- 1.Diabetic Retinopathy Clinical Research Network.*. Comparative effectiveness randomized clinical trial of aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. New Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetic Retinopathy Clinical Research Network. Reproducibility of spectral domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132:1113–22. doi: 10.1001/jamaophthalmol.2014.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Edwards AR, Beck RW, Bressler NM, Davis MD, Ferris F, Glassman AR, Ip MS, Miller KM, for the Diabetic Retinopathy Clinical Research Network Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2010;117:946–53. doi: 10.1016/j.ophtha.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


