Abstract
We examined the effect of intravitreal injections of D1-like and D2-like dopamine receptor agonists and antagonists and D4 receptor drugs on form-deprivation myopia (FDM) in tree shrews, mammals closely related to primates. In eleven groups (n = 7 per group), we measured the amount of FDM produced by monocular form deprivation (FD) over an 11-day treatment period. The untreated fellow eye served as a control. Animals also received daily 5 μL intravitreal injections in the FD eye. The reference group received 0.85% NaCl vehicle. Four groups received a higher, or lower, dose of a D1-like receptor agonist (SKF38393) or antagonist (SCH23390). Four groups received a higher, or lower, dose of a D2-like receptor agonist (quinpirole) or antagonist (spiperone). Two groups received the D4 receptor agonist (PD168077) or antagonist (PD168568). Refractions were measured daily; axial component dimensions were measured on day 1 (before treatment) and day 12. We found that in groups receiving the D1-like receptor agonist or antagonist, the development of FDM and altered ocular component dimensions did not differ from the NaCl group. Groups receiving the D2-like receptor agonist or antagonist at the higher dose developed significantly less FDM and had shorter vitreous chambers than the NaCl group. The D4 receptor agonist, but not the antagonist, was nearly as effective as the D2-like agonist in reducing FDM. Thus, using intravitreally-administered agents, we did not find evidence supporting a role for the D1-like receptor pathway in reducing FDM in tree shrews. The reduction of FDM by the dopamine D2-like agonist supported a role for the D2-like receptor pathway in the control of FDM. The reduction of FDM by the D4 receptor agonist, but not the D4 antagonist, suggests an important role for activation of the dopamine D4 receptor in the control of axial elongation and refractive development.
Keywords: Refractive development, Form-deprivation myopia, Emmetropization, Intravitreal injection, Dopamine
Introduction
At birth, the axial length of the eyes of many animals, including most humans, is shorter than the location of the focal plane, where light is focused by the cornea and crystalline lens. This discrepancy creates hyperopic refractive error. During postnatal development, an emmetropization feedback mechanism uses this hyperopic refractive error to modulate the growth of the eye, moving the retina toward the focal plane and reducing the refractive error so that the eyes gradually become nearly emmetropic (distant objects in focus without accommodation). It is generally accepted that the retina detects the refractive error, either hyperopia or myopia (eye too long for the focal plane), and generates either GO signals, if the eye needs to elongate to reduce hyperopic refractive error, or STOP signals, if the eye needs to slow its growth to reduce myopic refractive error (Schaeffel & Howland, 1991; Rohrer & Stell, 1994; Norton, 1999; He et al., 2014).
In young animals, placing a translucent diffuser in front of an eye, removing clear images but only slightly reducing overall retinal illuminance (form deprivation, FD), appears to stimulate retinal GO signaling which increases the axial elongation rate, moving the retina behind the focal plane. Measured with the diffuser removed, the eye develops form-deprivation myopia (FDM) (Sherman et al., 1977; Wiesel & Raviola, 1977; Wallman et al., 1978; Troilo & Judge, 1993).
Although defocus information, such as that produced by a diffuser, is sent to central visual structures, creating the perception of blur, there also is a direct signaling cascade within the eye in which retinally-generated signals pass into the retinal pigment epithelium (RPE), then into the choroid and reach the sclera where they modulate scleral biochemistry, mRNA levels, and protein levels to increase the axial elongation rate in response to a diffuser (Gao et al., 2011; Guo et al., 2014; He et al., 2014; McBrien et al., 2001; Moring et al., 2007; Norton et al., 1994; Norton & Rada, 1995; Siegwart, Jr. &; Norton, 1999; Troilo et al., 1987; Wildsoet & Pettigrew, 1988; Wildsoet & Schmid, 2000).
Dopamine is a transmitter/neuromodulator that is widely present throughout the brain. In the retina, dopamine is used by a subgroup of amacrine cells, some or all of which are interplexiform cells (Dowling & Ehinger, 1975; Mariani & Hokoc, 1988; Muller & Peichl, 1991; Nguyen-Legros et al., 1997b; Zhang et al., 2008). The interplexiform cells have axons that reach the outer retina where they have the potential to modulate retinal signals that are related to emmetropization. Dopamine release follows a circadian rhythm; it is high during the day and low at night, which is inversely related to melatonin production (Dubocovich, 1983; Boatright et al., 1989; Cahill & Besharse, 1991; Behrens et al., 2000; Ribelayga et al., 2004). The high dopamine levels in the day are involved in light adaptation, reducing connections between horizontal cells, and reducing the sensitivity of the retina to light (Dowling, 2012).
Dopamine acts through five receptor subtypes that are grouped into two families: the D1-like (D1 and D5) receptors and the D2-like (D2, D3, and D4) receptors (Gingrich & Caron, 1993; Seeman & Van Tol, 1994). The main functional difference is that the D1-like receptors are linked to activation of adenylate cyclase thus increasing intracellular cAMP, whereas activation of D2-like receptors leads to inhibition of adenylate cyclase followed by subsequent decreasing intracellular cAMP. Although dopaminergic neurons have been found in tree shrew retina (Muller & Peichl, 1991), dopamine receptor types have not been examined. However, the distribution of dopamine receptor types in tree shrew brain (Mijnster et al., 1999) was similar to other species, suggesting a phylogenetic conservation of types and their location. D1 receptors have been found on bipolar, horizontal, amacrine, and ganglion cells (Veruki & Wassle, 1996; Nguyen-Legros et al., 1997a); D5 receptors occur on retinal pigment epithelium cells (Versaux-Botteri et al., 1997). D2 receptors are expressed by all dopaminergic cells (Derouiche & Asan, 1999) and have been localized to photoreceptor inner and outer segments, the outer and inner plexiform layers, cells in the inner nuclear layer, and the ganglion cell layer in several species (Muresan & Besharse, 1993; Wagner et al., 1993; Rohrer & Stell, 1995). D3 receptors, and mRNA encoding D3 receptors, have not been found in the retina (Cohen et al., 1992; Derouiche & Asan, 1999). D4 receptors are present on the inner segment of photoreceptors (Cohen et al., 1992; Li et al., 2013) as well as in the inner nuclear and ganglion cell layers. An important route by which dopamine can reach receptors is through paracrine diffusion (Witkovsky, 2004).
The first indication that dopamine might be involved in retinal signaling in the emmetropization mechanism came from the discovery that levels of dopamine (and its metabolite DOPAC) are reduced in monocularly form-deprived eyes of monkeys and chicks relative to the untreated fellow control eye (Stone et al., 1989; Stone et al., 1990; Iuvone et al., 1991). This suggested that raising dopamine activity in FD eyes might slow or prevent axial elongation and myopia. Indeed, topical or sub-conjunctival administration of apomorphine, which activates all dopamine receptor types (but D2-like more than D1-like) (Creese et al., 1976), reduced FDM in monkeys (Iuvone et al., 1991) and chicks (Stone et al., 1989; Stone et al., 1990; Rohrer et al., 1993; Nickla et al., 2010). Peribulbar (Jiang et al., 2014) or intravitreal (Dong et al., 2011) administration of apomorphine had a similar effect in guinea pigs.
These and other studies have led to the hypothesis that high levels of light increase dopaminergic activity which then retards axial elongation and prevents myopia development, whilst lower levels of dopamine activity facilitate elongation and myopia (Morgan & Boelen, 1996; Norton & Siegwart, Jr., 2013; McCarthy et al., 2007; Ashby et al., 2009; Ashby & Schaeffel, 2010; Cohen et al., 2011; Cohen et al., 2012; Smith et al., 2012; Smith et al., 2013) (Brainard & Morgan, 1987). For example, Cohen et al. (2011) showed that chicks raised in cages with 10,000 lux emmetropized normally but animals raised in 50 lux became myopic. They also found that dopamine activity (DOPAC) was high in the 10,000 lux animals and was reduced in the myopic chicks exposed to the 50 lux illuminance (Cohen et al., 2012). Karouta and Ashby (2015) found that exposing form-deprived chicks to 40,000 lux for six hours per day almost completely blocked the development of myopia. Increased dopamine activity may contribute to the effect of outdoor activity in reducing myopia incidence (He et al., 2015) and prevalence (Rose et al., 2008; Wu et al., 2013).
It appears that dopamine's role in axial elongation and refractive development depends on dopamine levels being modulated within a range. The lowered levels of dopamine in FD might suggest that removing dopamine would increase axial elongation and FDM. However, Schaeffel and colleagues found that drastically reducing dopamine levels with reserpine or 6-OHDA actually reduced FDM in chicks (Schaeffel et al., 1994; Schaeffel et al., 1995). In a knockout mouse model lacking D2 receptors, FDM was reduced by 50% (Huang et al., 2014). It is unclear how lowering dopamine levels (6-OHDA) or knocking out D2 receptors (mouse) can all produce a reduction in FDM. No doubt, other neurotransmitter systems are involved in modulating eye growth, whose activity may be altered as a side effect of these manipulations. These may include vasoactive intestinal polypeptide (Stone et al., 1988) or (in chicks) glucagon (Feldkaemper & Schaeffel, 2002; Vessey et al., 2005). Nonetheless, it is clear that dopamine activity levels are importantly involved in refractive development.
Numerous studies have applied a variety of approaches in several species to learn if the reduction of FDM by elevated dopamine activity is mediated through the dopamine D1-like or the D2-like pathways. Similar to the studies mentioned above, where apomorphine was introduced, investigators asked which pathway, the D1-like or D2-like, would reduce myopia development. Much of this work has used monocularly form-deprived chicks and has generally implicated the D2-like receptor pathway. McCarthy et al. (2007) and Nickla and colleagues (Nickla et al., 2010; Nickla et al., 2013) used a straightforward approach of intravitreally injecting the D2-like agonist, quinpirole, into FD chicks and found that it reduced myopia development. Administering the D2-like antagonist spiperone in FD animals did not affect FDM, perhaps because FD had already reduced dopamine activity. McCarthy et al. (2007) found that neither a D1-like agonist nor antagonist had an effect on FDM suggesting that this pathway is not involved in FDM. However, Stone et al. (1989) found that subconjunctival administration of haloperidol reduced FDM in chicks.
Another approach has been to combine a myopiagenic condition (form deprivation or a minus lens) with an anti-myopiagenic stimulus that reduced the amount of myopia, and then use dopamine antagonists to block the protective effects of the anti-myopiagenic stimuli. One such antimyopiagenic stimulus is the short-term removal of a diffuser (Napper et al., 1995; Smith, III et al., 2002) or minus lens (Schmid et al., 1999; Shaikh et al., 1999). This stimulus can reduce myopia progression by 50% when applied just one hour per day. When McCarthy et al. (2007) applied the D2 antagonist spiperone, it blocked the effect of removing the diffuser. Another antimyopia stimulus in FD animals is elevated light levels (ELL) (Ashby & Schaeffel, 2010). Administering spiperone blocked the protective effect of ELL, increasing the amount of myopia in comparison to animals only treated with ELL. These results in chick support the hypothesis that the D2-like receptor pathway is involved in the regulation of axial length.
In some mammals (guinea pigs, monkeys, and mice), dopamine drugs have been administered through subconjunctival, peri-bulbar, or intraperitoneal routes (Iuvone et al., 1991; Dong et al., 2011; Huang et al., 2014; Jiang et al., 2014). These routes are indirect compared with intravitreal administration because, to reach the retina, the drugs must first penetrate the sclera, choroid, and RPE. Nonetheless, the results of most of these studies have also been consistent with a role for the D2-like receptor pathway rather than the D1-like pathway. Exceptions include a study that used albino, spontaneously myopic guinea pigs (Jiang et al., 2014). The present study applied the intravitreal injection approach, as used in chick, to tree shrews, mammals closely related to the primate line (Luckett, 1980). In addition, we used an agonist and antagonist that only bind the dopamine D4 receptor with high affinity (Glase et al., 1997; Belliotti et al., 1998; Clifford & Waddington, 2000; Gu et al., 2006).
Materials and methods
Experimental groups
As in previous studies from this laboratory (McBrien & Norton, 1992; Guo et al., 2013; He et al., 2014), juvenile northern tree shrews (Tupaia glis belangeri) were raised in our breeding colony by their mothers on a 14 h light/10 h dark cycle. Tree shrews are small (150–200 g) mammals (dichromats) with excellent vision for their size (2–4 cyc/deg). All procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The first day both eyes are open, which occurs about three weeks after birth, is considered the first day of visual experience (DVE). Experimental groups were balanced to include both males and females and avoided pups from the same parents wherever possible. However, two pups shared the same mother in the D1-like antagonist (higher-dose) group and in the D2-like antagonist (higher-dose) group. Which eye was form deprived was balanced between the right and left eyes in each group.
Experimental procedures
The eleven groups in this study (n = 7 per group) are summarized in Table 1. The animals in all groups received monocular form deprivation (FD) for 11 days with a translucent diffuser held in a goggle frame. On day 1 of treatment, after pre-treatment refractive and axial component measures were made, the animals were anesthetized with 5% isoflurane. After instilling a drop of topical anesthetic in the to-be treated eye, an opening was made in the temporal conjunctiva, allowing access to the sclera, which was then punctured temporally near the ora serrata with a sterile 33-gauge needle. The needle was then removed and a sterile glass pipette, pulled to a diameter smaller than the scleral opening and filled with the substance to be injected, was inserted to a depth of approximately 3–4 mm, taking care to keep the tip behind the lens and in front of the retina. The pipette was held in place for approximately 20 s, while 5 μL of either 0.85% NaCl or the dopamine drugs listed in Table 1 (dissolved in 0.85% NaCl) was injected into the vitreous chamber. Delivery was controlled by a 25 μL Hamilton micro syringe (Hamilton Company, Reno, NV) connected to the pipette through PE-90 tubing. Upon removal of the pipette, no back-flow of liquid was observed after any of the injections. After replacing the monocular diffuser, the animal recovered from anesthesia in a nest box before it was returned to its cage in the animal colony. This procedure was repeated daily for 10 more days; typically, the puncture hole from the previous day could be located and the pipette inserted through the existing opening. Injections were made at about the same time each day, approximately 1.5 to 2.5 h after the onset of colony lighting. The final refractive and axial measures were made on day 12, 24 h after the 11th injection.
Table 1.
Dopamine agonists and antagonists.
| Substance | Action | Intravitreal conc. (μM) | Intravitreal conc. (μM) in other studies |
|---|---|---|---|
| NaCI | Vehicle | … | |
|
| |||
| SKF38393 | D1-like agonist | 26.3 (lower dose) | 62.5; McCarthy et al., 2007 |
| 263 (higher dose) | 58.8; Nickla et al., 2010 | ||
|
| |||
| SCH23390 | D1-like antagonist | 57.7 (lower dose) | 15.4, 154.2, 308.4; Schaeffel et al., 1995 |
| 577 (higher dose) | 31.3; McCarthy et al., 2007 | ||
| 31.3; Nickla et al., 2010 | |||
|
| |||
| Quinpirole | D2-like agonist | 30.1 (lower dose) | 62.5; McCarthy et al., 2007 |
| 301 (higher dose) | 58.8; Nickla et al., 2010 | ||
| 61.1; Schmid et al., 2013 | |||
|
| |||
| Spiperone | D2-like antagonist | 7.7 (lower dose) | 31.3; McCarthy et al., 2007 |
| 77 (higher dose) | 31.3; Nickla et al., 2010 | ||
| 38.5; Ashby and Schaeffel 2010 | |||
| 28.9; Schmid et al., 2013 | |||
|
| |||
| PD168077 | D4 agonist | 301* | |
|
| |||
| PD168568 | D4 antagonist | 77* | |
Calculations for final vitreous molarity by species vitreous volume: tree shrew (∼125 μl) and chick (∼150 μl).
indicates doses matched to higher doses of D2-like agonist and antagonist.
Injected solutions
Vehicle solution (0.85% NaCl) was prepared using sterile water obtained from an ultrapure system with a 0.22 μm final filter (Millipore Corporation, Billerica, MA). SKF38393 (catalog #D047), SCH23390 (catalog #D054), quinpirole (catalog #Q102), and PD168077 (catalog #P233) were all obtained from Sigma-Aldrich, St. Louis, MO. PD168568 (catalog # 3529) was obtained from Tocris Bioscience, Bristol, United Kingdom. Spiperone (catalog #ab120549) was obtained from Abcam, Cambridge, United Kingdom. All solutions were made in advance, divided into 50 μL aliquots, and stored at −20°C until use. Doses were calculated assuming a 125 μL vitreous volume for tree shrews at 21 DVE. The concentrations of the D1 and D2 drugs were similar to those used in previous studies (Table 1) (Schaeffel et al., 1995; McCarthy et al., 2007; Ashby & Schaeffel, 2010; Nickla et al., 2010; Schmid et al., 2013). The micromolar concentrations in Table 1 were calculated from information provided in those papers, using the formula weight of the drugs and indicate the concentration immediately after the injection. The selective D4 drugs have not previously been used in form-deprivation studies. The D4 agonist has been used in retinal studies (Pozdeyev et al., 2008; Jackson et al., 2011). The D4 antagonist has been shown to have high selectivity for D4 receptors [Ki 8.8nM] vs. D2 receptors [Ki 1842nM] (Belliotti et al., 1998). The molarity of these drugs was matched to that of the higher dose of the D2-like agonist and antagonist (Table 1).
Many studies using intravitreal injections of dopamine or dopamine drugs have used ascorbic acid as an antioxidant (Rohrer et al., 1993 ; Schaeffel et al., 1994; Schaeffel et al., 1995; Schmid & Wildsoet, 2004). However, ascorbic acid is abundant in the retina and is actively involved in the dopamine pathways as a neuromodulator that reduces the uptake of dopamine by reducing voltage-dependent K + currents ( Fan & Yazulla, 1999b). Ward et al. (2016) found that ascorbic acid, added to NaCl, slightly reduced the amount of FDM in tree shrews. We therefore avoided adding ascorbic acid to the solutions in this study and instead only used 0.85% NaCl as a solvent. A 0.85% NaCl solution was effective in dissolving all drugs. However, the higher dose of spiperone (80 μM) required minor warming in a water bath for approximately 30 s after the aliquot was thawed to re-dissolve fully.
Form deprivation
To produce FDM, all animals wore a goggle frame that contained a monocular translucent diffuser that covered the treated eye (Norton & Rada, 1995). The other (control) eye had unobstructed vision through an open goggle frame. The goggle was clipped to a dental acrylic pedestal that was attached to the skull at 21 ± 1 DVE (Siegwart & Norton, 1994 ). For pedestal installation, each animal was anesthetized with ketamine (100 mg/kg) and xylazine (7.5 mg/kg), supplemented with 0.5–2.0% isoflurane as needed. After pedestal installation and recovery from anesthesia, the animals were placed in individual cages (or occasionally pair-housed) with fluorescent lighting (GE, F34CW-RS-WM-ECO lamps), 100–300 lux measured on the floor of the cage. Three days later (day 1), the goggle frame holding the monocular diffuser was clipped to the pedestal, firmly holding the diffuser in front of the treated eye. Goggles were removed daily for cleaning. The eyes were examined periodically with an indirect ophthalmoscope for signs of inflammation and lenticular or retinal damage, which was not observed in any animal in this study.
Refractive and axial measures
Immediately before the start of FD (day 1), and every morning for the next 11 days, awake, noncycloplegic refractive measures were taken with a Nidek ARK-700A infrared auto-refractor (Marco Ophthalmic, Jacksonville, FL) (Norton et al., 2003). The daily measures were taken approximately 1.5 to 2.5 h after the onset of colony lights, followed by subsequent injection procedures. Since atropine may interfere with retino-scleral signaling, cycloplegic refractive measures were not performed (McKanna & Casagrande, 1981). However, previous studies have shown that noncycloplegic measures provide a valid estimate of the refractive state, and of induced myopia, in tree shrews (Norton et al., 2000; Norton et al., 2003; Norton et al., 2006). All refractive values were corrected to the corneal plane and adjusted for the small eye artifact (Glickstein & Millodot, 1970), previously shown to be approximately +4 D in tree shrews (Norton et al., 2003). The final refractive measure, on day 12, occurred 24 h after the final anesthesia and injection.
On day 1 and day 12, a Lenstar LS-900 optical biometer (Haag-Streit USA, Mason, OH) was used, in awake animals, to measure corneal thickness, anterior chamber depth, lens thickness, vitreous chamber depth, retinal thickness, and choroidal thickness. Off-line, the vitreous chamber depth was measured with the retinal cursors; the anterior retinal cursor was placed on the peak that denoted the posterior surface of the crystalline lens while the posterior retinal cursor was located at the front of the retina. The same cursors were relocated to measure the retinal and choroidal thicknesses. Fig. 1 presents a Lenstar waveform showing the retinal and choroidal profiles. In almost all cases, the Lenstar was able to distinguish the posterior edge of the retina (front of choroid) and also the back of the choroid in both the treated and control eyes. At the start of FD, none of the ocular component dimensions differed significantly across groups (1-way analysis of variance, P > 0.05).
Fig. 1.
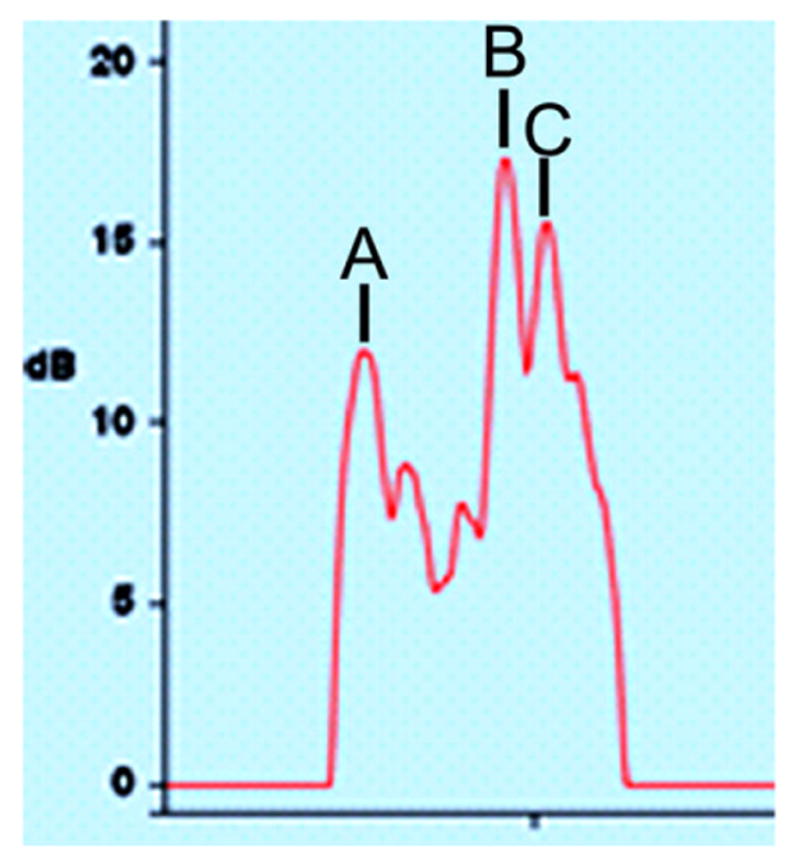
Waveform of retina and choroid. Waveform produced by the Lenstar optical biometer in the control eye of a tree shrew. (A) indicates the anterior surface of the retina. (B) indicates the posterior retina/front of the choroid. (C) marks the back of the choroid. In this example, the retinal thickness, from (A to B), was 173 μm. The choroidal thickness (B to C) was 52 μm.
Post-injection measures
To assess if any of the injected substances might have permanently interfered with the development of FDM, FD (without intravitreal injections) was continued for variable periods of time, typically in animals that developed little myopia. Some animals were not followed because they were used for other measures on day 12. Recovery from FD was followed in some animals to verify that the retina was also able to guide eye growth back toward emmetropia.
Mass spectrometry
Ascorbic acid has traditionally been added to intravitreal solutions of dopamine drugs to prevent oxidation. However, it was not clear that the synthetic dopamine drugs used in this study required an antioxidant to remain stable. To examine this directly, we placed a solution of spiperone dissolved in 0.85% NaCl on a counter in the laboratory near a window at room temperature for 24 h and compared its mass spectrometry profile with that of a freshly made solution. There was no evidence of oxidative products of spiperone after 24 h, suggesting that an antioxidant was not needed.
We were also interested in learning the duration of the dopamine drugs in the vitreous chamber. As an example, we injected the higher-dose spiperone solution into tree shrew eyes and collected the vitreous after 10 min or 60 min. Vitreous was also collected from an animal that had received no injections. All samples were analyzed via liquid chromatography mass spectrometry using standard conditions. Spiperone was detected in the vitreous chamber 10 min after injection but not after 60 min.
Data analysis
Refractive values for each eye and the refractive differences (treated eye–control eye) from each animal for each day were examined in Excel spreadsheets. To determine the rate at which the groups developed FDM, slope values (D/day of increased myopia) were calculated for each animal by fitting a linear regression to the refractive differences (treated–control eye values). The rates were analyzed for the entire 11-day period (days 1–12), the first 5 days of FD (days 1–6), the last 7 days (days 6–12), and, for animals in which FD continued after injections stopped, the 4-day post-injection period (days 12–16). The mean squared error of the linear regression fits for all these time-periods did not differ significantly across groups (1-way analysis of variance, P > 0.05). One-way analysis of variance with the Fisher LSD post-hoc test (ANOVA; IBM SPSS Statistics 22) was used to assess group differences in the final amount of FDM (day 12 values), the slope values, and the axial component dimensions; P < 0.05 was considered significant. A repeated measures ANOVA was conducted on daily refractions to determine if there was : (1) an effect of treatment regardless of day, (2) an effect of day regardless of treatment, and (3) if the effect of treatment and effect of day depended on each other. For groups in which these three conditions were satisfied, post-hoc tests (Fisher LSD) were conducted on the daily refractions from treatment day 1–12. Paired t-tests were used to assess if there was a significant difference between treatment days 6–12 and post-injection days 12–21 for the D2-like and D4 drug groups and also to assess if there were significant differences in ocular component dimension in the treated eyes vs. control eyes as a result of the form-deprivation myopia across all 11 groups.
Results
The refractive and axial component data are reported here as the difference between the treated eye and the untreated fellow control eye (treated eye–control eye). There were no significant differences between the control eyes across all groups and the control eye vitreous chamber depth was not significantly different from a group of seven normal animals. Thus, the change in refraction was due to treated eyes only, which, when analyzed alone, showed similar results to the treated eye–control eye values. The NaCl group was the reference group.
Dopamine D1-like drugs
Daily intravitreal injections of neither the D1-like agonist nor the D1-like antagonist significantly affected the development of FDM compared with the group that received the NaCl vehicle. As shown in Fig. 2A, the group that received the dopamine D1-like agonist, SKF38393, developed a similar degree of myopia throughout the 11-day period as did the NaCl group. On day 12 (Fig. 2B), the amount of myopia that developed in the group that received the lower (mean ± s.e.m., −5.6 ± 1.1 D) or the higher (−4.2 ± 0.8 D) dose of SKF38393 was not significantly different from the myopia in the NaCl group (−4.7 ± 0.6 D). Although the lower-dose group developed more myopia than the higher-dose group, the FDM in these groups did not differ significantly from each other at any time during the 11-day treatment period.
Fig. 2.
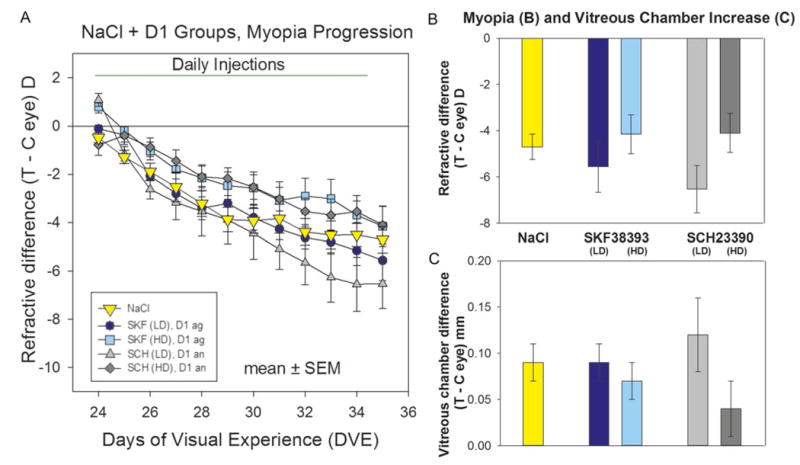
Development of FDM and day 12 measures in the NaCl and the D1-like receptor agonist and antagonist groups. (A) The refractive difference (treated eye–control eye) throughout the 11-day period of FD and daily administration of the D1-like receptor agonist or antagonist. The rst measure (day 1) was made immediately before the first intravitreal injection and the start of FD; the day 12 measures were made 24 h after the last intravitreal injection. (B) Refractive differences on day 12. (C) Elongation of the vitreous chamber on day 12. Error bars indicate standard error of the mean (s.e.m.). The color of each bar in (B) and (C) matches the symbol color in (A). Abbreviations: SKF = SKF38393, SCH = SCH23390, Ag = agonist, An = antagonist, LD = lower dose, HD = higher dose.
The developmental time-course of the FDM in the groups receiving the dopamine D1-like antagonist, SCH23390, was also not significantly different from the NaCl group throughout the treatment (Fig. 2A). On day 12 (Fig. 2B), the myopia in the lower-dose group was −6.5 ± 1.0 D; in the higher-dose group it was −4.1 ± 0.8 D. As with the D1-like agonist groups, the higher-dose antagonist group developed less myopia than the lower-dose group, but the amount of myopia did not differ significantly from each other at any time during the 11-day treatment period.
Examination of the rates of myopia progression during days 1–6 and 6–12 found that there were no significant differences across the five groups. The vitreous chamber elongation, measured on day 12 (Fig. 2C), also was not significantly different from that in the NaCl group. The treated-control eye differences of none of the other ocular component dimensions (cornea thickness, anterior chamber depth, lens thickness, retinal thickness, or choroid thickness) differed across groups.
Dopamine D2-like drugs
As shown in Fig. 3, daily intravitreal administration of D2-like receptor agonists and antagonists, at the higher doses, reduced the development of FDM compared with the NaCl group. For the first three days, there was no significant difference in the degree of myopia induced in any group. Starting on day 5 (after 4 days of intravitreal injections), the rate of FDM development slowed in the groups receiving the higher doses of quinpirole and spiperone. In contrast, the FDM in the lower-dose groups continued to develop at a similar rate to the NaCl vehicle group. At the end of the intravitreal injection series (day 12), the amount of myopia in the quinpirole higher-dose group (−2.4 ± 0.7 D) and the spiperone higher-dose group (−2.5 ± 0.7 D) was significantly less than the myopia in the NaCl group (−4.7 ± 0.6 D; crosses in Fig. 3B). For these two higher-dose groups, there was a main effect of drug treatment, regardless of day. In addition, during days 6–12, the rate of myopia development in the higher-dose groups receiving quinpirole (0.0 ± 0.1 D/day) and spiperone (−0.1 ± 0.1 D/day), although slower, was not significantly different from the rate of myopia development in the NaCl group (−0.2 ± 0.1 D/day). The elongation of the vitreous chamber in the form-deprived eye (on day 12, Fig. 3C) in the groups receiving the higher-dose quinpirole (0.02 ± 0.02 mm) and spiperone (0.01 ± 0.02 mm) was significantly less than the vitreous elongation in the NaCl vehicle group (0.09 ± 0.02 mm; P < 0.05 for both groups, indicated by the asterisks).
Fig. 3.
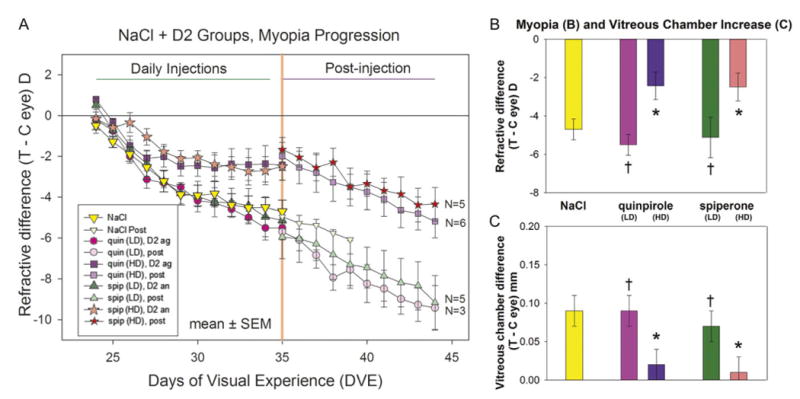
Development of FDM and day 12 measures in the NaCl and the D2-like receptor agonist and antagonist groups. (A) The refractive difference (treated eye–control eye) throughout the 11-day period of FD and daily administration of dopamine D2-like receptor agonists or antagonists. The first measure (day 1) was made immediately before the first intravitreal injection and the start of FD. The final measure (day 12) was made 24 h after the last intravitreal injection. The refractive response to continued FD, without injections, is shown to the right of the vertical line at 35 DVE. N = number of animals in which myopia development was followed post-injection (after the intravitreal injections ceased). (B) Refractive differences on day 12. (C) Elongation of the vitreous chamber on day 12. Error bars indicate standard error of the mean (s.e.m.). The color of each bar in (B) and (C) matches the symbol color in (A). Asterisks (*) in (B) and (C) indicate significant differences between the higher-dose groups and the NaCl group. Crosses (†) in (B) and (C) indicate that the lower-dose groups were signicantly different from the higher-dose groups. Abbreviations: Quin = quinpirole, Spip = spiperone, Ag = agonist, An = antagonist, LD = lower dose, HD = higher dose, Post = post-injection.
After the daily intravitreal injections were discontinued, the development of FDM was examined in most of the animals in the higher-dose spiperone and quinpirole groups and in some animals in the lower-dose groups. The rate of myopia progression in the lower-dose groups on days 12–21 [quinpirole (lower dose), −0.4 ± 0.1 D/day; spiperone (lower dose), −0.4 ± 0.0 D/day] was not different after injections stopped than it was during days 6–12 [quinpirole (lower dose), −0.4 ± 0.0 D/day; spiperone (lower dose), −0.3 ± 0.1 D/day]. In contrast, the rate in the groups that received higher doses increased significantly when FD continued without daily intravitreal injections (t-test, P < 0.05). In the six animals that were followed during days 12–21 in the quinpirole higher-dose group, the rate increased from −0.1 ± 0.1 D/day to −0.3 ± 0.1 D/day (t-test, P < 0.05). For the five animals followed during days 12–21 in the higher-dose spiperone group, the rate increased from −0.0 ± 0.1 D/day to −0.3 ± 0.0 D/day (t-test, P < 0.05), indicating that the retina and emmetropization mechanism were still capable of producing a high rate of myopia development.
Dopamine D4 drugs
As shown in Fig. 4, during the first 4 days of FDM with intravitreal injections (days 1–5), the group that received the D4 agonist (PD168077) and the group that received the D4 antagonist (PD168568) did not differ significantly in the amount of FDM, relative to the NaCl group. After that, the average amount of myopia remained nearly constant in the D4 agonist group. It had significantly less myopia than the NaCl group on days 6, 7, 9, and 10, and less myopia than the group receiving the D4 antagonist on days 6 through 11 but not on day 12. The D4 antagonist group did not differ from the NaCl group. On day 12, the amount of FDM in the D4 agonist group (−2.7 ± 1.1 D) was very similar to that in the D2-like agonist (quinpirole) group (−2.4 ± 0.7 D). Consistent with the reduced myopia (Fig. 4B), there was little elongation of the vitreous chamber (Fig. 4C) in the D4 agonist group relative to the NaCl group; the vitreous elongation of the D4 agonist group was not significantly different from the NaCl group at the end of treatment (t-test, P > 0.05). None of the other ocular components differed across the three groups.
Fig. 4.
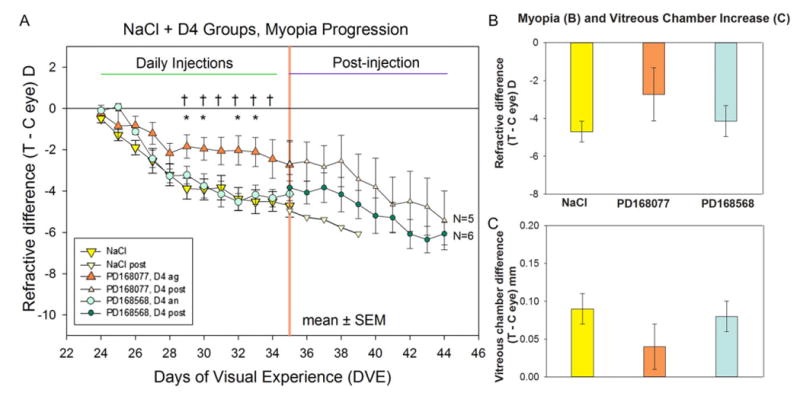
Development of FDM and vitreous chamber depth in the NaCl and the D4 receptor agonist and antagonist groups. (A) The refractive difference (treated eye–control eye) throughout the 11-day period of FD and daily administration of dopamine D4 receptor agonist or antagonist. The first measure (day 1), was made immediately before the first intravitreal injection and the start of FD. The final measure (day 12) was made 24 h after the last intravitreal injection. The refractive response to continued FD, without injections, is shown to the right of the orange vertical line. Asterisks (*) indicate days on which the amount of myopia in the D4 agonist (PD168077) group differed significantly from the myopia in the NaCl group. Crosses (†) indicate significant differences between the D4 agonist (PD168077) group and the D4 antagonist (PD168568) group. N = number of animals in which myopia development was followed post-injection (after the intravitreal injections ceased, starting at day 12). (B) Refractive differences on day 12. (C) Elongation of the vitreous chamber on day 12. Error bars indicate standard error of the mean (s.e.m.). The color of each bar in (B) and (C) matches the symbol color in (A). Abbreviations: Ag = agonist, An = antagonist, Post = post-injection.
Fig. 4 (triangles) plots the average FDM of the seven animals that received the D4 agonist. Fig. 5 shows the daily refractive measures in each of these animals individually. These are shown individually because, although each animal gave a consistent response to the D4 agonist, there was considerable variation across animals. One of the seven (circles, Fig. 5A) developed no myopia in the FD eye compared with the untreated fellow control eye. Due to a broken diffuser, this animal was followed for only two days after injections ceased, during which refraction in the FD eye remained nearly unchanged. In four of the seven animals (erect and inverted triangles in Fig. 5A and 5B), the D4 agonist strongly reduced the development of FDM throughout the 11-day treatment period. When the daily intravitreal injections ceased, the rate of development of FDM increased in three of the four animals after 1–4 days. FDM development in two animals (squares and diamonds in Fig. 5A) seemed unaffected by the D4 agonist; they developed substantial FDM, comparable to the largest FDM found in the NaCl group. One of these (diamonds) was followed for nine days post-injection. FDM development continued at nearly the same rapid rate, suggesting that the D4 agonist had not affected FDM development. In the group that received the D2-like drugs, there also was some variability between animals in the amount of FDM that developed (Fig. 3); however, unlike the D4 agonist group, none of the animals in these groups showed either complete reduction of FDM or developed FDM at a high rate over the treatment period.
Fig. 5.
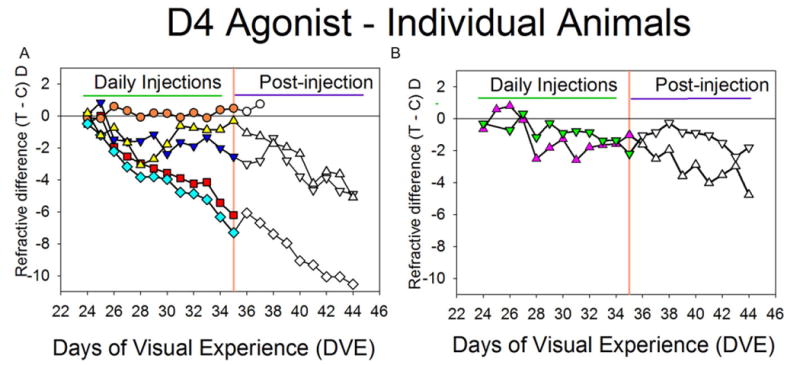
Refractive responses of individual animals in the D4 agonist group. (A) Five of the animals. In one animal (circles), FDM was completely blocked. In two animals (squares and diamonds), the D4 agonist produced no reduction of FDM. In two other animals (triangles), FDM was substantially reduced. (B) The remaining two animals in which FDM was also substantially reduced, displayed separately for clarity. In both (A) and (B), the refractive response to continued FD, without injections, is shown to the right of the orange vertical lines.
Overall treated vs. control ocular component changes
The focus of this study was to compare refractive and axial component changes in the groups receiving dopamine agonists or antagonists against the changes found in the NaCl reference group. As reported in the foregoing sections, the primary ocular component that changed was the vitreous chamber, which was significantly different between the spiperone (higher dose) and quinpirole (higher dose) groups in comparison to the NaCl group. Another ocular component, the thickness of the choroid, was consistently thinner in the treated eye than in the control eye across all groups. For the 77 animals (11 groups), the control eye choroid was measured at 0.066 ± 0.001 mm (mean ± s.e.m.). The treated-eye choroid was thinner, averaging 0.060 ± 0.001 mm. In contrast, the crystalline lens did not differ significantly in treated eyes (3.313 ± 0.006 mm) compared with control eyes (3.317 ± 0.006 mm). The retinal thickness in the treated eyes (0.185 ± 0.001 mm) was identical to the control-eye retinal thickness. Thinning of the choroid during the development of form-deprivation myopia has been observed in chicks using A-scan ultrasound (Wallman et al., 1995). It was not possible for us to detect this in the thinner tree shrew choroid until the Lenstar optical biometer was employed for the first time in this study.
Discussion
In tree shrews, our data using intravitreal administration of dopamine agonists and antagonists support the conclusion from studies in chick (Rohrer et al., 1993; McCarthy et al., 2007; Ashby & Schaeffel, 2010; Nickla et al., 2010; Nickla & Totonelly, 2011; Nickla et al., 2013) that the dopamine D2-like receptor pathway participates in reducing the axial elongation and myopia produced by FD. The D2-like receptor agonist quinpirole and the D4 receptor agonist PD168077 both reduced the development of FDM. Indeed, the reduction in FDM by the D4 agonist (Fig. 4) was very similar to the effect of the D2-like agonist quinpirole (Fig. 3). D3 receptors are absent in the retina of other species (Cohen et al., 1992; Derouiche & Asan, 1999). Assuming they also are not present in tree shrew retina, and that the quinpirole activated both dopamine D2 and D4 receptors, then the similar effect of the dopamine D4 agonist and D2-like agonist on FDM may have been due to activation of the dopamine D4 receptors. We did not find support for involvement of the D1-like receptor pathway on FDM using intravitreal administration.
Because this is the first examination of the effects of dopamine receptor pathways on FDM in tree shrews, and because the pharmacokinetics of intravitreally administered drugs have not been studied in tree shrews, consideration must be given to the doses used in comparison with intravitreally-administered drugs in chick. An advantage of intravitreal administration is that all of the administered substance is already inside the eye where it should be moved by fluid flow to, and through, the retina (Steinberg, 1986). Beyond that, little is known in any species about dispersion within the eye or the rate at which the drugs reach, and eventually leave, the retina. For the D1-like and D2-like agonists and antagonists, we initially delivered drug concentrations similar to those found effective in chicks (lower dose, Table 1). When these all were ineffective, we utilized a “higher dose” that was increased by one order of magnitude, a typical increase in establishing a dose-response curve. The D1-like drugs were ineffective at the higher dose, but both the D2-like agonist and antagonist at higher doses reduced FDM.
The change from no effect of the D2-like drugs at the lower dose to a measureable effect at the higher dose suggests that the lower dose was too low and that the higher dose was in the effective range. A potentially important reason that a higher dose was required in this study is that we avoided using ascorbic acid in the vehicle solution because it is an active dopaminergic substance (Tolbert et al., 1992; Fan & Yazulla, 1999a) and, by itself, reduces FDM (Rohrer et al., 1993; Ward et al., 2016). Using only one dopaminergically-active substance may have raised the amount required to have an effect.
Because neither dose of the D1-like agonist or antagonist produced an effect on FDM, it is impossible to know if a higher dose would have been effective. This seems unlikely to us. Our lower dose was higher than those shown to be effective in guinea pigs using retrobulbar drug administration (Jiang et al., 2014), even assuming that all of the drug administered outside the eye had reached the retina. In chicks, subconjunctival injections of the D1-like antagonist SCH23390 also were found to have an effect when administered in conjunction with apomorphine in FD animals (Stone et al., 1990), blocking the effect of apomorphine. In addition, also in chick, with intravitreal injections similar to our lower dose, Nickla et al. (2010) found that the D1-like antagonist SCH23390 was effective in reducing the axial elongation produced by a negative lens, suggesting a role for the D1-like pathway in chick.
Could our D1 drug doses have been too high and therefore ineffective due to retinal toxicity? Our information is limited, but there was no obvious toxicity based on the fact the eyes responded to the FD with increased elongation and myopia after injections ceased. Two animals that received the D1-like antagonist and were offered the opportunity to recover after FD was discontinued showed refractive recovery toward emmetropia. This indicated that the emmetropization mechanism could detect the absence of FD and slow axial elongation to produce recovery. Thirteen animals treated with the D2-like drugs also recovered.
The results with the D4 agonist and antagonist are based on a single dose, matched in molarity to the effective doses of the D2-like agonist and antagonist on the assumption that the similar, very large, number of molecules would have a similar effect. The D4 agonist PD169077 [Ki 8.7nM] (Glase et al., 1997; Clifford & Waddington, 2000; Gu et al., 2006) binds more effectively at that receptor than does quinpirole [Ki 30nM] (Seeman & Van Tol, 1994). If binding was a factor, the D4 agonist dose might have been too high, compared with quinpirole. This did not seem to be the case; the variability in the response of the animals within the group suggests that the dose was on the lower edge of the effective range, so that animals with a higher individual threshold showed less of an effect on FDM. The selected dose of the D4 antagonist PD168568 [Ki 8.8nM] (Belliotti et al., 1998) did not affect FDM. We cannot know if a higher dose of the D4 antagonist might have affected FDM. However, the spiperone [Ki 0.08 nM at D4 receptors; Ki 0.06 nM at D2 receptors] (Hoyer et al., 1994; Seeman & Van Tol, 1994) would have bound to two receptor types, whereas the PD168568 is strongly selective for the D4 receptor [Ki 1842 at the D2]. It seems more likely to us that the D4 receptor antagonist had no effect, but the spiperone did, because the spiperone can stimulate serotonergic (Hoyer et al., 1994; George et al., 2005) or adrenergic (Yoshio et al., 2001) receptors which may play a role in the control of axial elongation, in addition to the dopaminergic pathways.
An advantage of the daily refractive measures in this study is that, in addition to the usual pre- and post-treatment measures, the progression of FDM throughout the treatment period could be followed. In the group receiving NaCl, and also the groups receiving the dopamine D1-like drugs (Fig. 2), FD produced a progressive, monotonic increase in myopia throughout the 11-day treatment period, and low variability between animals. The daily refractive measures allowed us to see that the FDM in the groups receiving the D2-like agonist quinpirole and the D2-like antagonist spiperone (both, the higher doses), FDM initially followed a similar course (Fig. 3, days 1–5). Subsequently, the rate of FDM development slowed and the average amount of FDM remained relatively stable for the remainder of the treatment period. The utility of daily refractive measures was especially important in examining the effect of the D4 agonist. Significant reductions in myopia were seen on days 6, 7, 9, and 10, but not on the last day. If the assessment of the D4 agonist had depended on the day 12 measures, the conclusion would have been that there was no D4 agonist effect.
Although it was somewhat surprising that spiperone reduced the amount of FDM, a reduction of FDM by dopamine antagonists is not unique. Iuvone et al. (1991) and Stone et al. (1990) found that haloperidol, a dopamine D2-like antagonist, strongly reduced FDM in monkeys and chicks. The reason for this effect remains unknown. One may wonder if the haloperidol blocks dopamine from activating dopaminergic synapses, removing any dopaminergic influence on axial elongation in response to FD. This is consistent with the result, also in chicks, of intravitreal administration of 6-OHDA, which impairs the function of dopaminergic neurons in the retina (Maguire & Smith, 1985), or reserpine, which depletes dopamine. Both reduced the development of FDM (Schaeffel et al., 1995). Taken together, it seems reasonable to state that dopamine D2-like agonists generate biological activity in the signaling pathway whereas antagonists block that activity, but that there must be at least some activity in order for the dopaminergic system to exert an influence on axial elongation during FDM.
Although the average curve of FDM development for the group that received the D4 agonist (dose matched to the higher-dose of the D2 agonist) was similar to that of the D2-like agonist group, the daily refractive measures of the individual animals within the D4 agonist group showed three distinct patterns (Fig. 5), including complete reduction of FDM, substantial reduction, and no apparent effect. Two aspects of this pattern are of interest: (1) the consistency of the pattern within each animal across the 11 days of treatment suggesting no increase or decrease in effect across the 11 days of treatment for individual animals and (2) the source of the variability across animals, suggesting that the selected dose was on the lower edge of the effective dose.
Within-animal consistency
The consistency of the effect of the D4 agonist within animals is of interest because so little is known about the pharmacokinetics of this agonist, or any of the dopamine drugs used in this study. Evidently, the effect of the D4 agonist persisted long enough in the retina so that the once-daily dose produced nearly the same effect from day-to-day throughout the 11-day treatment period. For instance, in the D4 agonist-treated animal that developed no FDM (circles in Fig. 5A), the refraction of the treated eye never deviated from that of its fellow eye. In three of the four animals in which the injections strongly reduced FDM, the amount of myopia fluctuated little (around 1 D) from day-to-day; the deviations may have reflected slight waxing and waning of the effective dose of the D4 agonist. In the fourth animal (triangles in Fig. 5A), FDM of over −2 D developed over the first four days of treatment and then decreased to less than one diopter, suggesting a cumulative effect over time. That D4 receptor agonist administration produced a range of responses suggests that the dose was not excessive.
To produce the complete reduction of FDM seen day after day in one animal (Fig. 5A), it may not have been necessary for the D4 agonist to remain effective in the retina for more than two hours. With both FD and negative-lens wear in tree shrews, chicks, and monkeys, removal of the diffuser (or lens) for two hours each day is sufficient to prevent myopia from developing (Napper et al., 1995; Schmid & Wildsoet, 1996; Napper et al., 1997; Shaikh et al., 1999; Smith, III et al., 2002; Kee et al., 2007). Indeed, the substantial, but incomplete, reduction of FDM in four animals by the D4 agonist could have been achieved by a shorter period of bioactivity, in the range of 30 min to one hour each day (Shaikh et al., 1999). It appears that the daily dose was sufficient, in each animal, to produce the same effect on FDM throughout the 11-day treatment period. Whatever the effective duration, there is no obvious basis for it to have varied between animals receiving the same dose.
Between-animal variability
Although each of the seven animals in the D4 agonist group responded in a consistent manner throughout the 11-day treatment period, there was a large range of responses (no myopia, limited myopia, or full myopia). This suggests to us that there was variability in sensitivity (or threshold) to the D4 agonist across animals. To the best of our ability to achieve it, the amount of the D4 agonist administered each day (in micrograms) to each animal was the same. The animals were all about the same weight at the start of treatment and the initial ocular component dimensions (especially the vitreous chamber depth) did not vary greatly (1-way ANOVA, P > 0.05), suggesting that the volume of the vitreous chamber and, therefore, the intravitreal concentration of the D4 agonist, should have been very similar. Interestingly, animals in the D4 agonist group with deeper vitreous chamber depth at the start of treatment (suggesting larger vitreous chamber volume and lower drug concentration) developed significantly less myopia than animals with a smaller vitreous chamber depth. To the extent that drug delivery was consistent across animals, the variability suggests that our dose was on the lower end of the effective range of the D4 agonist.
Emmetropization normally involves a feedback loop in which refractive error is used to adjust the axial elongation rate to achieve or maintain low refractive error. FD produces an open loop situation in which there is no feedback; there is nothing in FD to signal, as eyes elongate, that elongation should be slowed. In this situation, the rate at which individual animals develop myopia depends on the “gain” of each animal's emmetropization feedback loop. In animals with a low intrinsic gain (and, therefore high sensitivity to the D4 agonist), our dose of the D4 agonist may have been sufficient to stop the development of myopia, as seen in one case. Perhaps the animals that developed a great deal of FDM had higher intrinsic gain, such that the same dose of the D4 agonist was insufficient to slow FDM development. The four animals which developed a small amount of myopia may have had intermediate intrinsic gain. Future studies examining how dopamine agonists and antagonists could affect not only refraction and other ocular components, but also the expression levels of dopamine receptors, could provide answers to some of these questions and possibly provide evidence for a link between dopamine circadian rhythms and refraction.
In summary, intravitreal administration of dopamine drugs in monocularly form-deprived tree shrews produced a similar result as did intravitreally-administered dopamine drugs in chick. These results lead to the conclusion that, in the retina, dopamine acts to reduce the development of axial elongation and myopia through the D2-like receptor pathway and, indeed, may act primarily through the D4 receptor specifically.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, National Eye Institute (R01 EY005922) and by a Vision Core Grant (P30 EY003039). This work was performed in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Alabama at Birmingham (Alexander H. Ward). Preliminary results were presented in abstract form at the 2014 International Society for Eye Research meeting. We thank Dr. Karen Gamble for statistical guidance. We also thank Dr. James Mobley and Dr. Steven Harville for assistance with the mass spectrometry measures.
References
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology & Visual Science. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Investigative Ophthalmology & Visual Science. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Behrens UD, Douglas RH, Sugden D, Davies DJ, Wagner HJ. Effect of melatonin agonists and antagonists on horizontal cell spinule formation and dopamine release in a fish retina. Cell & Tissue Research. 2000;299:299–306. doi: 10.1007/s004419900161. [DOI] [PubMed] [Google Scholar]
- Belliotti TR, Brink WA, Kesten SR, Rubin JR, Wustrow DJ, Zoski KT, Whetzel SZ, Corbin AE, Pugsley TA, Heffner TG, Wise LD. Isoindolinone enantiomers having affinity for the dopamine D4 receptor. Bioorganic & Medicinal Chemistry Letters. 1998;8:1499–1502. doi: 10.1016/s0960-894x(98)00252-2. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K +-evoked depolarization. Brain Research. 1989;482:164–168. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: A dose-response relationship. Brain Research. 1987;424:199–203. doi: 10.1016/0006-8993(87)91211-x. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: Regulation of retinal melatonin rhythms by light and D2 dopamine receptors. Journal of Neuroscience. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford JJ, Waddington JL. Topographically based search for an “Ethogram” among a series of novel D(4) dopamine receptor agonists and antagonists. Neuropsychopharmacology. 2000;22:538–544. doi: 10.1016/S0893-133X(99)00141-4. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O'Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Experimental Eye Research. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Experimental Eye Research. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Asan E. The dopamine D2 receptor subfamily in rat retina: Ultrastructural immunogold and in situ hybridization studies. European Journal of Neuroscience. 1999;11:1391–1402. doi: 10.1046/j.1460-9568.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X. Inhibition of experimental myopia by a dopamine agonist: Different effectiveness between form deprivation and hyperopic defocus in Guinea pigs. Molecular Vision. 2011;17:2824–2834. [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. Synaptic mechanisms and chemistry. In: Dowling JE, editor. The Retina. Cambridge, MA: The Belknap Press of Harvard University Press; 2012. pp. 146–190. [Google Scholar]
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and cebus monkey retinas. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Fan SF, Yazulla S. Modulation of voltage-dependent K + currents [IK(V)] in retinal bipolar cells by ascorbate is mediated by dopamine D1 receptors. Visual Neuroscience. 1999a;16:923–931. doi: 10.1017/s095252389916512x. [DOI] [PubMed] [Google Scholar]
- Fan SF, Yazulla S. Suppression of voltage-dependent K + currents in retinal bipolar cells by ascorbate. Visual Neuroscience. 1999b;16:141–148. doi: 10.1017/s0952523899161091. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Visual Neuroscience. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Molecular Vision. 2011;17:903–919. [PMC free article] [PubMed] [Google Scholar]
- George A, Schmid KL, Pow DV. Retinal serotonin, eye growth and myopia development in chick. Experimental Eye Research. 2005;81:616–625. doi: 10.1016/j.exer.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annual Review of Neuroscience. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD. Substituted [(4-phenylpiperazinyl)-methyl]benzamides: Selective dopamine D4 agonists. Journal of Medicinal Chemistry. 1997;40:1771–1772. doi: 10.1021/jm970021c. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Yuen EY, Yan Z. Activation of dopamine D4 receptors induces synaptic translocation of Ca 2+/calmodulin-dependent protein kinase II in cultured prefrontal cortical neurons. Molecular Pharmacology. 2006;69:813–822. doi: 10.1124/mol.105.018853. [DOI] [PubMed] [Google Scholar]
- Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Investigative Ophthalmology & Visual Science. 2013;54:6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Molecular Vision. 2014;20:1643–1659. [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Experimental Eye Research. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, Smith W, Rose K, Morgan IG. Effect of time spent outdoors at school on the development of myopia among children in China: A randomized clinical trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacological Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Huang F, Yan T, Shi F, An J, Xie R, Zheng F, Li Y, Chen J, Qu J, Zhou X. Activation of dopamine D2 receptor is critical for the development of form deprivation myopia in the C57BL/6 mouse. Investigative Ophthalmology & Visual Science. 2014;55:5537–5544. doi: 10.1167/iovs.13-13211. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Investigative Ophthalmology & Visual Science. 1991;32:1674–1677. [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Hwang CK, Iuvone PM. Dopamine D(4) receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. European Journal of Neuroscience. 2011;34:57–64. doi: 10.1111/j.1460-9568.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Long K, Schaeffel F, Zhou X, Zheng Y, Ying H, Lu F, Stell WK, Qu J. Effects of dopaminergic agents on progression of naturally occurring myopia in albino Guinea pigs (Cavia porcellus) Investigative Ophthalmology & Visual Science. 2014;94:24–32. doi: 10.1167/iovs.14-14294. [DOI] [PubMed] [Google Scholar]
- Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Investigative Ophthalmology & Visual Science. 2015;56:299–309. doi: 10.1167/iovs.14-15499. [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., II Temporal constraints on experimental emmetropization in infant monkeys. Investigative Ophthalmology & Visual Science. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O'Brien J. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. Journal of Neuroscience. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett WP. Comparative Biology and Evolutionary Relationships of Tree Shrews. New York: Plenum Press; 1980. [Google Scholar]
- Maguire GW, Smith EL., II Cat retinal ganglion cell receptive-field alterations after 6-hydroxydopamine induced dopaminergic amacrine cell lesions. Journal of Neurophysiology. 1985;53:1431–1443. doi: 10.1152/jn.1985.53.6.1431. [DOI] [PubMed] [Google Scholar]
- Mariani AP, Hokoc JN. Two types of tyrosine hydroxylase-immunoreactive amacrine cell in the rhesus monkey retina. Journal of Comparative Neurology. 1988;276:81–91. doi: 10.1002/cne.902760106. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investigative Ophthalmology & Visual Science. 2001;42:2179–2187. [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Research. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan IG. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Experimental Eye Research. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Documenta Ophthalmologica Proceedings Series. 1981;28:187–192. [Google Scholar]
- Mijnster MJ, Isovich E, Flugge G, Fuchs E. Localization of dopamine receptors in the tree shrew brain using [3H]-SCH23390 and [125I]-epidepride. Brain Research. 1999;841:101–113. doi: 10.1016/s0006-8993(99)01795-3. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Boelen MK. A retinal dark-light switch: A review of the evidence. Visual Neuroscience. 1996;13:399–409. doi: 10.1017/s0952523800008087. [DOI] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Investigative Ophthalmology & Visual Science. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Peichl L. Morphology and distribution of cate-cholaminergic amacrine cells in the cone-dominated tree shrew retina. Journal of Comparative Neurology. 1991;308:91–102. doi: 10.1002/cne.903080109. [DOI] [PubMed] [Google Scholar]
- Muresan Z, Besharse JC. D2-like dopamine receptors in amphibian retina: Localization with fluorescent ligands. Journal of Comparative Neurology. 1993;331:149–160. doi: 10.1002/cne.903310202. [DOI] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Research. 1995;35:1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The effect of an interrupted daily period of normal visual stimulation on form deprivation myopia in chicks. Vision Research. 1997;37:1557–1564. doi: 10.1016/s0042-6989(96)00269-6. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caille I, Bloch B. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Visual Neuroscience. 1997a;14:545–552. doi: 10.1017/s0952523800012207. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Savy C. Dopaminergic and GABAergic retinal cell populations in mammals. Microscopy Research and Technique. 1997b;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Lee L, Totonelly K. Nitric oxide synthase inhibitors prevent the growth-inhibiting effects of quinpirole. Optometry and Vision Science. 2013;90:1167–1175. doi: 10.1097/OPX.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Experimental Eye Research. 2011;93:782–785. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Experimental Eye Research. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. Institute for Laboratory Animal Research Journal. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Visual Neuroscience. 1994;11:143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Research. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT., Jr Light levels, refractive development, and myopia—A speculative review. Experimental Eye Research. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology & Visual Science. 2006;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Investigative Ophthalmology & Visual Science. 2000;41 ARVO Abstract S-563. [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optometry and Vision Science. 2003;80:623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. European Journal of Neuroscience. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. Journal of Physiology. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D(2)-receptor mechanism acting in retina or pigmented epithelium. Visual Neuroscience. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-β) act as stop and go signals to modulate postnatal ocular growth in the chick. Experimental Eye Research. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Stell WK. Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain Research. 1995;695:110–116. doi: 10.1016/0006-8993(95)00700-z. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Research. 1995;35:1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Research. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Strang NC, Wildsoet CF. Imposed retinal image size changes–Do they provide a cue to the sign of lens-induced defocus in chick? Optometry and Vision Science. 1999;76:320–325. doi: 10.1097/00006324-199905000-00021. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Strasberg G, Rayner CL, Hartfield PJ. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Experimental Eye Research. 2013;110:88–95. doi: 10.1016/j.exer.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optometry and Vision Science. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends in Pharmacological Sciences. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optometry and Vision Science. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Research. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Laboratory Animal Science. 1994;44:292–294. [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Research. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology & Visual Science. 2002;43:291–299. [PubMed] [Google Scholar]
- Smith EL, Hung LF, Arumugam B, Huang J. Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Investigative Ophthalmology & Visual Science. 2013;54:2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Visual Science. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH. Research update: Report from a workshop on cell biology of retinal detachment. Experimental Eye Research. 1986;43:695–706. doi: 10.1016/s0014-4835(86)80001-x. [DOI] [PubMed] [Google Scholar]
- Stone RA, Laties AM, Raviola E, Wiesel TN. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Iuvone PM, Laties AM. Postnatal control of ocular growth: Dopaminergic mechanisms. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Chichester: John Wiley & Sons; 1990. pp. 45–57. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LC, Morris PE, Jr, Spollen JJ, Ashe SC. Stereospecific effects of ascorbic acid and analogues on D1 and D2 agonist binding. Life Sciences. 1992;51:921–930. doi: 10.1016/0024-3205(92)90400-j. [DOI] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Current Eye Research. 1987;6:993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Research. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Gibert JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neuroscience Letters. 1997;237:9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Wassle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. European Journal of Neuroscience. 1996;8:2286–2297. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: A role for glucagonergic regulation of emmetropization? Investigative Ophthalmology & Visual Science. 2005;46:3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HJ, Luo BG, Ariano MA, Sibley DR, Stell WK. Localization of D2 dopamine receptors in vertebrate retinae with anti-peptide antibodies. Journal of Comparative Neurology. 1993;331:469–481. doi: 10.1002/cne.903310404. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: Choroidal modulation of refractive state. Vision Research. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT, Jr, Frost MR, Norton TT. The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews. Experimental Eye Research. 2016;145:289–296. doi: 10.1016/j.exer.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Pettigrew JD. Experimental myopia and anomalous eye growth patterns unaffected by optic nerve section in chickens: Evidence for local control of eye growth. Clinical Vision Science. 1988;3:99–107. [Google Scholar]
- Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Research. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Documenta Ophthalmologica. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1adrenoceptor subtypes. Japanese Journal of Pharmacology. 2001;86:189–195. doi: 10.1254/jjp.86.189. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]


