Abstract
To accurately measure menthol levels in human urine, we developed a method using gas chromatography/electron ionization mass spectrometry with menthol-d4 stable isotope internal standardization. We used solid phase microextraction (SPME) headspace sampling for collection, preconcentration and automation. Conjugated forms of menthol were released using β-glucuronidase/sulfatase to allow for measuring total menthol. Additionally, we processed the specimens without using β-glucuronidase/sulfatase to quantify the levels of unconjugated (free) menthol in urine. This method was developed to verify mentholated cigarette smoking status to study the influence of menthol on smoking behaviour and exposure. This objective was accomplished with this method, which has no carryover or memory from the SPME fiber assembly, a method detection limit of 0.0017 μg/mL, a broad linear range of 0.002–0.5 μg/mL for free menthol and 0.01 – 10 μg/mL for total menthol, a 7.6% precision and 88.5% accuracy, and an analysis runtime of 17 min. We applied this method in analysis of urine specimens collected from cigarette smokers who smoke either mentholated or non-mentholated cigarettes. Among these smokers, the average total urinary menthol levels was three-fold higher (p <0.001) among mentholated cigarette smokers compared with non-mentholated cigarette smokers.
Keywords: menthol, GC/MS, SPME, urine, mentholated cigarette, tobacco
1. Introduction
Menthol is a naturally occurring compound with topical cooling and anesthetic properties used in a wide range of products including common cold medications, toothpastes, confectionery, pesticides, and cigarettes. Relevant to this study is its use as a flavor additive in milligram quantities (typically as L-menthol) in mentholated cigarettes. Although menthol is not considered a carcinogen, it may increase carcinogen uptake by numbing the respiratory tract so that smoke is inhaled deeper and held longer. Indeed, the high prevalence of mentholated cigarette use among African Americans has been hypothesized to explain this population’s disproportionately higher lung cancer risk per cigarette smoked (menthol cigarettes smoked by >88% vs. 26% for whites).[1]
Results from studies evaluating the association between mentholated cigarette usage and increased lung cancer risk have been varied. [2–6] For example, a Kaiser Permanente study conducted between 1979 and 1986 found a statistically significant increase in risk for menthol cigarette smokers.[2] In males, the relative risk for menthol smokers was 1.45 (95% confidence interval 1.03–2.02). In females, the relative risk was 0.75 (95% confidence interval 0.52–1.11). On the contrary, the Southern Community Cohort Study, found a statistically significant reduced risk of lung cancer in non-mentholated smokers vs. mentholated cigarette smokers.[7] To better understand conflicting studies and discern the influences of menthol on smoking behavior and associated health outcomes, a highly accurate and precise analytical method to verify biomarkers of menthol exposure is necessary.
Several researchers have investigated menthol analysis methods for biological specimens. Although high pressure liquid chromatography/mass spectrometry (HPLC/MS) has been demonstrated,[8] most method development has involved gas chromatography (GC) separation, because menthol is still within the realm of a volatile organic compound, with a boiling point of 212 °C and thermal stability.[9,10] As a result, GC methods have yielded better sensitivity than LC methods by at least two orders of magnitude, especially when combined with mass spectrometric detection. In 2004, Spichiger et al. incorporated solid phase microextraction (SPME) as a means to preconcentrate menthol collected in the headspace (HS) over urine and serum specimens before and after hydrolysis of menthol glucuronide adducts.[11] This SPME method experienced persistent background levels, especially for urine specimens, possibly from menthol penetration within the SPME fiber assembly caused by a high (80 °C) collection temperature and a relatively long (20 min) collection time. Schulz et al. used a similar method to measure menthol and three other compounds in serum specimens lowering the collection temperature to 50 °C over a 30 min collection time. They reported no carryover or memory, but a similar limit of detection (LOD) of 0.0046 μg/mL for the analysis of serum specimens.[12] However, analysis of menthol in urine is advangateous to analysis in serum because urine collection is non-invasive and menthol is readily partitioned from urine, which is a more polar matrix than serum. Moreover, menthol persists longer in urine than in blood[13] with an α-phase half-life of 56.2 min for blood vs. 74.9 min for urine.
Here we present an improved GC/MS method for analysis of free menthol and total menthol in urine specimens using a HS/SPME based method that eliminates carryover from the SPME fiber assembly. Our improved GC/MS method is validated with internal quality control, performance testing, and subsequent method verification involving analysis of menthol levels in urine from non-mentholated and mentholated cigarette smokers.
2. Experimental
2.1. Chemicals
Native L(−)-menthol (5-methyl-2-[1-methylethyl]cyclohexanol, 99.7%), mentholglucuronic acid ammonium salt, Type H-1 β–D-glucuronidase/sulfatase (Type H-1, from Helix pomatia), trisodium citrate dihydrate (SigmaUltra grade), and citric acid monohydrate (ACS reagent grade) were purchased from Sigma Chemical Company (St. Louis, MO, USA). (−)-Menthol-1,2,6,6-d4 (98%) was purchased from Cambridge Isotope Lab (Andover, MA, USA). Water (HPLC grade) was purchased from J.T. Baker (Phillipsburg, NJ, USA), and methanol (GC2 Capillary GC/GC-MS grade) was purchased from Burdick and Jackson (Muskegon, MI, USA). Helium gas for GC/MS (ultra high-purity grade) was purchased from Airgas Inc. (Jacksonville, FL, USA).
2.2. Urine Specimen Collection
The method was applied to urine collected from established smokers (i.e., individuals who smoked at least 6 cigarettes per day for at least the past three years). Pregnant participants, participants arriving intoxicated to any visit, and participants with self-reported smoking-related diseases were excluded. Participants signed informed consent documents and subsequently provided urine specimens. The study protocol was approved by CDC’s institutional review board. Urine from 95 smokers was used to verify this method. Smokers consisted of 26 non-mentholated and 68 mentholated cigarettes smokers.
This study was approved by the Battelle Centers for Public Health and Research Evaluation (CPHRE) Institutional Review Board (IRB# FG465925-04) to ensure the protection of participants’ safety, rights, and welfare. The Center for Disease Control and Prevention’s (CDC) role was limited to analysis of coded specimens and was determined to not constitute engagement in human subjects research.
2.3. Urine Specimen Preparation
Unknown specimens were prepared in 10 mL headspace SPME vials obtained from MicroLiter Analytical Supplies Inc. (Suwanee, GA, USA). For free menthol measurement, 100 μL of urine, 100 μL of 0.1 M trisodium citrate dihydrate buffer (pH 5.0), and 50 μL of 5 μg/mL menthol-d4 internal standard solution were added to the vial. The total liquid volume was 0.25 mL. For total menthol measurements, the buffer was replaced with the same volume of an enzyme solution that was made by adding β-D-glucuronidase into the buffer at a concentration of 3 mg/mL. The SPME vial was then sealed with a 1-mm thick, 20-mm PTFE/silicone septum (Supelco, St. Louis, MO, USA) and capped using a M-10 flat washer spacer (Hillman, Cincinnati, OH, USA) and a 20-mm open-center steel seal (Supelco, St. Louis, MO, USA). Specimens prepared for free menthol measurement were ready to analyze immediately. Specimens prepared for total menthol measurement were put into an oven and incubated at 37 °C for 24 hr to ensure complete deconjugation of the menthol glucuronide. Once samples are ready for analysis they equilibrate on the PAL autosampler in queue for approximately 5 hrs as instrument and fiber blanks, standards and QCs are run.
2.4. Standards Preparation
All standards were prepared identically to unknown specimens with the exception of replacing 100 μL urine with 100 μL synthetic urine (CTSI, Great Neck, NY, USA). For total menthol measurement, the calibration curve consisted of 6 points (0.01, 0.1, 0.5, 2, 5, and 10 μg/mL). For free menthol measurement, the calibration curve consisted of 8 points (0.001, 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, and 0.5 μg/mL).
2.5 Characterization of Quality Control (QC) Samples
Total and free quality control pool samples were characterized prior to any urine specimen analysis by spiking menthol or menthol glucuronide into an anonymously donated and homogenized urine pool. Total menthol QC pools were prepared at two different levels by adding an aqueous solution of mentholglucuronic acid ammonium salt into the urine pools to achieve the desired levels. These total menthol QC samples were characterized from 20 independent batches within a 5-month period, using the same deconjugation method as for the specimens. This determination yielded mean concentrations of 1.43 ± 0.08 μg/mL (N = 20) for the total QC low (TQCL) pool and 8.77 ± 0.50 μg/mL (N=20) for the total QC high (TQCH) pool.
Free menthol QC pools were prepared at two different levels by adding menthol solution to urine pools to achieve characterized levels of 0.039 ± 0.004 μg/mL (N = 13) for the free QC low (FQCL) pool and 0.267 ± 0.033 μg/mL (N=14) for the free QC high (FQCH) pool. The concentrations for both the total menthol and free menthol QC pools reflect the sum of the background levels in the anonymous urine and the spiked amount.
A typical run included at least four QC samples where a QC low and QC high bracketed the unknown specimens. The two samples from the same QC pool were averaged. All measurements within a run batch were considered invalid if: (1) the difference between the two averaged QCs was within a factor of 4 of the SD of the independent characterization QCs, (2) the level of QC low or QC high was more than 3 SDs of the characterized mean, (3) both QC low and QC high concentrations were outside 2 SD limits, and (4) the previous 9 consecutive QC results fall on the same side of the mean for either QC low or QC high.
2.6. HS-SPME Extraction
Headspace SPME extraction was performed automatically using a PAL auto-sampler (CTC Analytics AG, Zwingen, Switzerland) and a 65-μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber (Supelco Inc., St. Louis, MO, USA). For total menthol measurement, the fiber was exposed to the headspace for 1 min at 30 °C. For free menthol measurement, the fiber was exposed to the headspace for 3 min at 50 °C. In both cases, the sample was agitated at 500 rpm by the agitator tray during the entire extraction time. After headspace extraction the fiber was immediately transferred to the GC injection port where analytes were thermally desorbed. To minimize fiber contamination with volatiles in laboratory air, the fiber remained in the GC inlet for the remainder of the GC run.
2.7. GC-MS Conditions
Analyses were performed on an Agilent 6890 gas chromatograph coupled with an Agilent 5973 mass selective detector. The GC was equipped with a 2-mm inner diameter (ID) liner at the inlet and a Hewlett Packard HP-5 trace analysis column (30 m × 0.25 mm ID × 1.0 μm film thickness). The injection port and transfer line were both maintained at 250 °C throughout the run. The oven temperature started at 50 °C and immediately ramped up to 135 °C at 10 °C/min, followed by 15 °C/min to 260 °C. Total runtime was 17 min. Helium was used as carrier gas at a flow rate of 1.0 mL/min. The MS system was operated using electron impact ionization and in selected ion monitoring (SIM) mode. Ion m/z 138 was used to quantify native menthol, m/z 142 was used to quantify internal standard, and m/z 123 was used as the confirmation ion.
2.8. Quantification
Original chromatograms were analyzed by ChemStation software. Manual integration of native menthol (m/z 138) and internal standard (m/z 142) peaks was often needed. Data was then transferred to Xcalibur Quan Browser software (ThermoFinnigan, San Jose, CA, USA) where the native-to-internal standard ion ratio, in relation to the calibration curve acquired the same day, was used to quantitatively determine urinary menthol concentrations. Final data was transferred to an in-house laboratory information system, ATLIS (Microsoft Access-based database), using a visual basic module to import the Excel (Microsoft, Seattle, WA, USA) report generated by Quan Browser into the database.
In this study creatinine was measured in each urine specimen photometrically using the Boehringer Mannheim/Hitachi 912 analyzer (Roche Diagnostics Corporation, Indianapolis, IN, USA). The creatinine-corrected urinary menthol level was calculated as the ratio of the measured menthol to the measured creatinine concentration.
2.9. Statistical Analysis and Calculations
Descriptive statistical analyses were performed using JMP (Version 8.0, SAS, Cary, NC, USA).
3. Results and Discussion
This automated method was developed to accurately quantify a broad range of free and total menthol levels in urine to support large exposure assessment studies. As such, this method achieves high throughput, minimizes sample handling, and eliminates carryover from the SPME fiber assembly. The current approach involves homogenizing an aliquot of urine with buffer and an isotopically labelled analog ISTD in a headspace vial, which is sampled by equilibrium headspace SPME and then analyzed by GC/MS. Automation of the SPME sampling is accomplished on a CTC platform.
In SPME analysis of high boiling point VOCs such as menthol, absorption of VOCs into the sealing septum of the SPME fiber assembly can result in carryover and raise the LOD. To minimize carryover, we lowered the collection efficiency by using both short collection times and low equilibration temperatures. For the analysis of free menthol, we used a 3-min collection time with a 50 °C equilibration temperature and for total menthol we used a 1-min collection time and a 30 °C equilibration temperature. Because quantification is based on the use of an internal standard, higher concentrations can be quantified at lower extraction efficiencies and is advantageous in analyzing unknowns where concentration from one sample to the next can vary over two orders of magnitude. This approach resulted in optimal analytical figures of merit as described below.
3.1. Instrument Calibration Characteristics
Using this SPME GS/MS method, the instrument response to menthol remained linear up to 10 μg/mL with linear coefficients of determination (R2) often greater than 0.9996. On average (N = 32) the linear least squares prediction equation determined from calibrator responses was y = −0.0043 (± 0.0116) + 1.359 (± 0.130) x, where y was the area ratio of the primary quantification ion (m/z 138) to the ISTD ion (m/z 142) and × was the calibration standard concentration in μg/mL The y-intercept near 0 indicates minimal background contamination or spectral interference.
3.2. Selectivity
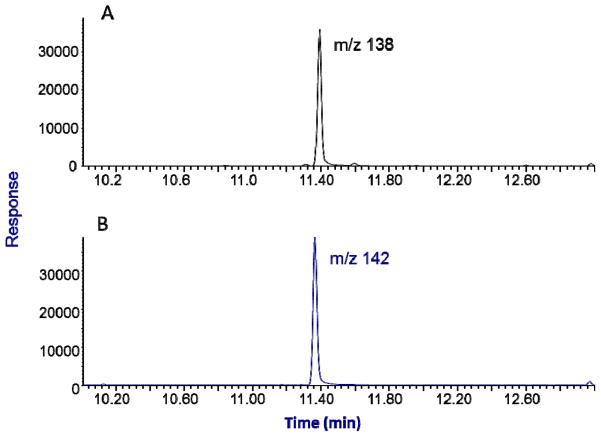
For this SPME GC/MS method the native menthol peak elutes at 11.65 min and the d4-labelled internal standard elutes at 11.62 min. Example selected ion chromatograms at m/z 138 (native) and m/z 142 (labeled ISTD) for an unknown urine specimen with a concentration measured at 0.86 μg/mL is shown for in Fig. 1. No interfering peaks were observed in urine specimen throughout the method development and specimen analyses.
Figure 1.
Typical selected ion chromatograms of total menthol measurement in a urine specimen: panel A is a mass chromatogram for m/z 138 produced by 0.86 μg/mL menthol; panel B is the mass chromatogram for m/z 142 produced by the internal standard d4-menthol.
3.3. Method Detection Limit (MDL)
The MDL for this method was calculated to be 0.0017 μg/mL based on three times the standard deviation (SD) at zero concentration (3S0) in an synthetic urine matrix.[14] The S0 value was deduced by extrapolating the linear regression equation for the corresponding SD versus concentrations (at least three) nearest the detection limit. If calculated MDL was lower than the lowest calibration standard, the lowest calibration standard was used as lowest reportable limit, which was 0.01 μg/mL for total menthol, and 0.002 μg/mL for free menthol.
3.4. SPME Fiber Selection
Because bipolar compounds, such as menthol, are often most efficiently extracted with fiber coatings containing both a polar and nonpolar phase,[15] three different two-phase SPME fibers were evaluated for headspace extraction in urinary menthol measurement (Table 1). Both 65 μm PDMS/DVB and 65 μm carbowax/divinylbenzene (CW/DVB) showed comparable extraction efficiency and low carryover effect (e.g., < 0.02%), Even though Carboxen and DVB have roughly the same surface area, Carboxen/PDMS had extraction efficiencies that were 1.7–2.8 times lower than the PDMS/DVB. Because the Carboxen/PDMS is more polar than either PDMS/DVB or CW/DVB, lower efficiency may be attributed to increased competition effects or poorer desorption efficiency of menthol from Carboxen. We suspect poorer desorption efficiency had greater influence, because menthol is small enough (at approximately 4 Å in diameter) to diffuse into the micropores (7–10 Å in diameter) of the Carboxen, yet has a polarity high enough to hinder rapid desorption. This explanation is supported by substantially higher carryover seen for the Carboxen/PDMS. Although the performance of the PDMS/DVB and CW/DVB were comparable, the 65 μm PDMS/DVB was selected to prepare all samples and standards because it proved to be the more rugged of the two coatings.
Table 1.
SPME fiber selection
| a. relative extraction efficiency (normalized to the response from a 20 μg/mL menthol aqueous solution sampled with a 75 μm Carboxen/PDMS SPME fiber) | |||
|---|---|---|---|
|
| |||
| Fiber ID | Matrix | ||
|
| |||
| water (20 μg/mL) | synthetic urine (20 μg/mL) | urine (~7 μg/mL) | |
| 65 μm PDMS/DVB | 3.0 | 2.0 | 1.7 |
| 75 μm Carboxen/PDMS | 1 | 1 | 1 |
| 65 μm CW/DVB | 2.8 | 1.7 | 2.1 |
| b. Percent carryover in μg/mL. | |||
|---|---|---|---|
|
| |||
| Fiber ID | Matrix | ||
|
| |||
| water (20 μg/mL) | synthetic urine (20 μg/mL) | urine (~7 μg/mL) | |
| 65 μm PDMS/DVB | 0.02 | 0.03 | 0.02 |
| 75 μm Carboxen/PDMS | 0.18 | 0.25 | 0.14 |
| 65 μm CW/DVB | 0.005 | 0.004 | N/A |
No significant carryover effect was observed throughout this study. To ensure that any possible analyte residue was removed from the SPME fiber and assembly between analyses, the SPME needle was intentionally kept in the GC inlet (250 °C) for the entire run. In addition, a blank run was always carried out immediately after the highest level of calibration standard (10 μg/mL), and the resulting chromatograms showed non-detectable menthol signals.
3.5. Precision and Accuracy
Long-term assay precision and instrument stability were determined from QC sample data including the QC characterization batches. Table 2 shows the QC data over a 7-month period. With every sample run, a QC low and QC high sample pair bracketed the unknown specimens, for a total of 4 QCs per run. Long-term variability achieved over this period for the total menthol measurement was 5.5 % relative standard deviation (RSD) for the TQCL and 7.6 % RSD for TQCH. Free menthol measurement, results had a long-term variability of 14.2 % RSD for the FQCL and 14.4 % RSD for the FQCH.
Table 2.
Comparison of all QCs evaluated (characterization and evaluation) over a 7-month period for free menthol and total menthol analysis
| Sample | N | Average (μg/mL) | Standard Deviation | %RSD | |
|---|---|---|---|---|---|
| Free menthol | QC Low | 22 | 0.037 | 0.005 | 14.2% |
| QC High | 23 | 0.297 | 0.043 | 14.4% | |
| Total menthol | QC Low | 32 | 1.44 | 0.08 | 5.5% |
| QC High | 32 | 8.81 | 0.67 | 7.6% | |
RSD = relative standard deviation
Assay accuracy was evaluated by measuring proficiency testing (PT) samples, consisting of 1 mL synthetic urine samples spiked with menthol to four gravimetrically determined concentrations of 1.0, 3.0, 6.0, and 9.0 μg/mL. This PT concentration range corresponded to 15 percentile to 80 percentile of the study population used for method verification in Section 3.8. Samples were blind coded and randomized by a quality control officer and stored at −70 °C prior to analysis. At the start of the study and at six month intervals thereafter, five blind coded PT samples were analyzed and reported to the external quality control officer for evaluation. These PT sample results are summarized in Table 3 and are expressed in terms of percent difference. The lowest PT level had the high percentage error of −11.50% (accuracy of 88.5%), which is attributed to experimental error associated with handling losses. Percentage error for the 3.0 and 6.0 μg/mL were lower at −2.08 and 0.58, respectively. At the highest PT concentration, greater imprecision of −10.22% is attributed to variability of the least squares curve fit at the highest concentrations caused by weighting the data 1/x.
Table 3.
Method accuracy and precision based on three proficiency tests
| formulated conc. (μg/mL) | calculated mean conc. (μg/mL) | % RSD | % Error | % Accuracy |
|---|---|---|---|---|
| 1.00 | 0.89 | 2.69 | −11.5 | 88.5 |
| 3.00 | 2.94 | 1.53 | −2.08 | 97.9 |
| 6.00 | 6.04 | 2.27 | 0.58 | 99.4 |
| 9.00 | 8.08 | 9.81 | −10.2 | 89.8 |
RSD = relative standard deviation
3.6. Specimen storage stability
To evaluate the long-term stability of specimens stored at −70 °C, 8 specimens were selected randomly and total menthol was measured again after a period of 4 months. Concentrations differed by +5.4% to −7.5% with an average of −1.3%. These differences were within the range of method accuracy demonstrated for the QC samples, and no changes that significantly affected the menthol level in the specimens were observed during storage duration.
3.7. Recovery of menthol glucuronide
To ensure complete menthol glucuronide recovery, a synthetic urine solution of menthol glucuronic acid ammonium salt ( was made at 22.4 μg/mL (equivalent to 10 μg/mL menthol glucuronide) and included in 12 different specimen runs over a 7-month period. Menthol levels for these recovery samples averaged 10.6 μg/mL (6.4 % RSD), which was within the precision seen for the TQCH characterized at 8.81 μg/mL (7.6 % RSD).
3.8. Method verification
To verify the method, we analyzed free menthol and total menthol concentrations in urine specimens from 27 non-mentholated cigarette and 68 mentholated cigarette smokers. Urinary free menthol levels were lower among non-mentholated cigarette smokers; 63% of specimens from non-mentholated cigarette smokers had free menthol levels below the MDL of 0.002 μg/mL compared with 13% of specimens from mentholated cigarette smokers. However, all participants total menthol levels above the MDL. Results below the MDL were imputed as MDL/√2.[16] Creatinine-adjusted urinary menthol level was calculated as the ratio of the measured menthol to the measured creatinine concentration to adjust for varying degrees of hydration and to enable comparison of our results with those from other studies.[17,18] The creatinine adjusted free menthol levels in these specimens were log normally distributed with a geometric mean of 0.001 mg/g and 0.002 mg/g. These low adjusted free menthol levels suggest that the UDP-glucuronosyltransferases process effectively metabolizes free menthol among smokers of non-mentholated and mentholated cigarettes.
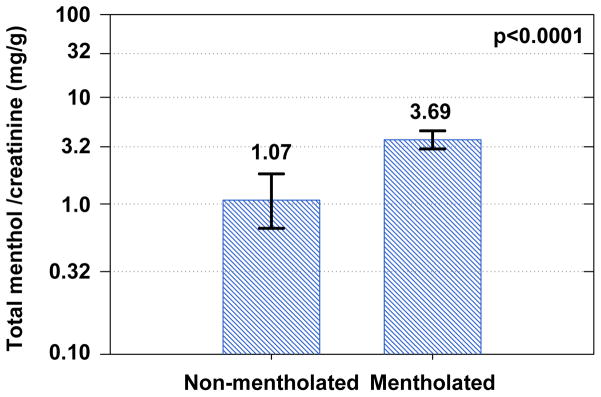
Shown in Fig. 2 is a comparison of creatinine-adjusted total menthol levels between non-mentholated cigarette smokers and mentholated cigarette smokers. All specimens from these two groups had total menthol levels higher than the MDL. Values ranged between 0.041 and 9.70 μg/mL among non-mentholated cigarette smokers and 0.330 and 38.0 μg/mL among mentholated cigarettes smokers. Distributions were log normal with a geometric mean of 1.07 mg/g creatinine for non-mentholated cigarette smokers and 3.69 mg/g for mentholated cigarette smokers, with means significantly different (Wilcoxon / Kruskal-Wallis test, p < 0.0001). The majority of urinary menthol was conjugated in all urines tested, and percent conjugation was not related to total menthol level. This finding is significant because it does not support the hypothesis that menthol contributes to smoking-related disease by saturating the UDP-glucuronosyltransferases process. [19]
Figure 2.
Comparison of creatinine adjusted total menthol concentration among non-mentholated cigarette smokers (N=27) and mentholated cigarette smokers (N= 68). Geometric mean and 95% confidence intervals are plotted in log scale. The threshold p-value corresponds to a Wilcoxon / Kruskal-Wallis test comparing the population means.
4. Summary and Conclusions
Although this quantitative method for analysis of free and total menthol in human urine provides high sample throughput similar to other methods, its sample preconcentration minimizes carryover from the SPME fiber assembly, thus broadening the dynamic range to 5 orders of magnitude and lowering the MDL to single unit ng/mL range. In addition, assay precision better than 7.6% and accuracy better than 88.5% is typically achieved.
This method was verified by comparing urinary menthol levels from 27 smokers of non-mentholated cigarettes with 68 smokers of mentholated cigarettes. Urinary menthol in both groups was generally more than 99% conjugated. On average, urinary menthol levels were three times higher for mentholated cigarette smokers than for non-mentholated cigarette smoker, confirming that smoking mentholated cigarettes increases menthol exposure levels. Moreover, there was no indication of saturation of the UDP-glucuronosyltransferases process among the mentholated cigarette smokers. This method will be used for further investigations of menthol as an independent factor in affecting racial/ethnic preference, nicotine dependence, smoking topography, and carcinogen exposure.
Acknowledgments
The authors wish to thank Christina Watson for providing survey data. John C. Morrow for database management and, Pam Olive for creatinine measurements. We also thank the Battelle Institute for collecting all specimens used in this study.
Footnotes
6. Disclaimers
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.”
Reference List
- 1.Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cummings KM. Nicotine Tob Res. 2004;6:S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- 2.Sidney S, Tekawa IS, Friedman GD, Sadler MC, Tashkin DP. Arch Intern Med. 1995;155:727–732. [PubMed] [Google Scholar]
- 3.Brooks DR, Palmer JR, Strom BL, Rosenberg L. Am J Epidemiol. 2003;158:609–616. doi: 10.1093/aje/kwg182. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Ann Epidemiol. 1999;9:114–120. doi: 10.1016/s1047-2797(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 5.Stellman SD, Chen Y, Muscat JE, Djordjevic MV, Richie JP, Lazarus P, Thompson S, Altorki N, Berwick M, Citron ML, Harlap S, Kaur TB, Neugut AI, Olson S, Travaline JM, Witorsch P, Zhang ZF. Ann Epidemiol. 2003;13:294–302. doi: 10.1016/s1047-2797(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 6.Kabat GC, Hebert JR. Cancer Res. 1991;51:6510–6513. [PubMed] [Google Scholar]
- 7.Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. J Natl Cancer Inst. 2011;103:810–816. doi: 10.1093/jnci/djr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., III Cancer Epidemiol Biomarkers Prev. 2010;19:3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaffenberger RM, Doyle MJ. J Chromatogr. 1990;527:59–66. doi: 10.1016/s0378-4347(00)82083-6. [DOI] [PubMed] [Google Scholar]
- 10.Valdez JS, Martin DK, Mayersohn M. J Chromatogr B Biomed Sci Appl. 1999;729:163–171. doi: 10.1016/s0378-4347(99)00161-9. [DOI] [PubMed] [Google Scholar]
- 11.Spichiger M, Muhlbauer RC, Brenneisen R. J Chromatogr B. 2004;799:111–117. doi: 10.1016/j.jchromb.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Schulz K, Bertau M, Schlenz K, Malt S, Dressler J, Lachenmeier DW. Anal Chim Acta. 2009;646:128–140. doi: 10.1016/j.aca.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Gelal A, Jacob P, Yu L, Benowitz NL. Clin Pharmacol Ther. 1999;66:128–135. doi: 10.1053/cp.1999.v66.100455001. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JK. Quality Assurance of Chemical Measurements. Lewis Publishers; New York: 1987. pp. 79–83. [Google Scholar]
- 15.Balasubramanian S, Panigrahi S. Food Bioprocess Tech. 2011;4:1–26. [Google Scholar]
- 16.Croghan CW, Egeghy PP. Methods of Dealing with Values Below the Limit of Detection using SAS. Presented at Sourthern SAS User Group; 9-22-2003; St. Petersburg, FL, USA. [Accessed December 2, 2016]. http://analytics.ncsu.edu/sesug/2003/SD08-Croghan.pdf. [Google Scholar]
- 17.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeniger MF, Lowry LK, Rosenberg J. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 19.Muscat JE, Djordjevic MV, Colosimo S, Stellman SD, Richie JP., Jr Cancer. 2005;103:1420–1426. doi: 10.1002/cncr.20953. [DOI] [PubMed] [Google Scholar]