Abstract
Background
Pulmonary hypertension has been associated with early allograft dysfunction and increased mortality following renal transplantation however this relationship has not been extensively studied.
Methods
We performed a retrospective cohort study of adult patients who underwent their first renal transplantation from 2003–2009 and had pre-transplantation echocardiograms. Pulmonary hypertension was defined as a right ventricular systolic pressure ≥ 40 mmHg in the absence of left-sided valvular disease and/or left ventricular ejection fraction ≤ 50%. The relationship between pulmonary hypertension and death-censored allograft failure (hemodialysis dependence or re-transplantation) and serum creatinine was assessed using Cox hazard regression and generalized mixed models. Eighty-two of 205 (40%) patients met inclusion criteria.
Results
The presence of pulmonary hypertension was associated with a 3.00 fold increase in the risk of death-censored allograft failure (95% confidence interval 1.20 – 7.32, p=0.02). Failure rates were 19% at 24 months and 51% at 96 months for those with pulmonary hypertension versus 7% at 24 months and 20% at 86 months for those without pulmonary hypertension (p=0.01). Among those without graft failure, there was an increase in creatinine levels after transplant (p=0.01). Effect estimates were unchanged by adjustment for multiple covariates and when pulmonary hypertension was defined as a right ventricular systolic pressure ≥ 36 mmHg.
Conclusions
Pulmonary hypertension prior to renal transplantation carries a 3.00-fold increased risk of death-censored allograft failure. The relationship between the pulmonary circulation and renal allograft failure warrants further study.
Introduction
Pulmonary hypertension (PH) is a disease state characterized by progressive dyspnea and exercise intolerance which can lead to right ventricular failure and death. A number of systemic diseases have been linked to pulmonary vascular disease, including end-stage renal disease (ESRD). PH in the setting of ESRD is currently classified as World Health Organization (WHO) Group 5 PH1, which by definition indicates there is a poorly understood or multifactorial explanation for this association. PH has been estimated to affect between 17–56% of ESRD patients and is associated with increased morbidity and mortality in this population2–12.
Preoperative PH has been associated with poor outcomes in general surgery13–15 and other solid organ transplantation16–20. A single retrospective study of 215 patients demonstrated increased mortality following renal transplantation in patients with PH observed on echocardiogram prior to surgery21, but the etiology of these deaths is not well characterized. Whether PH associated with ESRD has an effect on renal allograft function following transplantation, when many of the speculated causative factors (e.g., volume overload and fluid shifts, arterio-venous shunts, renin-angiotensin activation in ESRD8–10,12,22), have been removed or temporized is not known. Further, demonstration of an association between preoperative PH and post-transplant allograft function may have larger implications for organ allocation and recipient risk stratification.
Factors that directly regulate pulmonary arteriolar vasoconstriction and remodeling in pulmonary arterial hypertension (PAH), including nitric oxide, endothelin-1 (ET-1), and thromboxane A2, also have a role in renal disease6,7,23 and may contribute to pulmonary-renal vascular crosstalk and ESRD-PH phenotypes. Ongoing alterations in these important signaling pathways and/or accompanying vascular changes with lasting hemodynamic effects accrued in the pre-transplant period may impact post-transplant allograft function. Our objective was to determine whether ESRD patients with evidence of PH (defined by an estimated right ventricular systolic pressure [RVSP] ≥ 40 mmHg on transthoracic echocardiogram) have an increased risk of death-censored allograft failure. We hypothesized that preexisting PH would increase the risk of dialysis after transplantation and the need for retransplantation. We also examined the relationship between preoperative PH and post-transplant serum creatinine values in those who did not experience allograft failure.
Methods and Materials
Study Design
We conducted a retrospective cohort study of renal transplant recipients included in the Rhode Island Hospital’s Transplant Center Database (TCDB) from January 1, 2003 – December 31, 2009. The study was approved by the Rhode Island Hospital Institutional Review Board (IRB #401303).
Echocardiograms
Patients were included if they had available transthoracic echocardiograms and if these echocardiograms were performed prior to their first renal transplantation. Estimated RVSP was obtained using the modified Bernoulli equation and was recorded as a continuous variable; PH was defined as a RVSP ≥ 40 mmHg3,10,24–26. Sensitivity analyses were conducted in which PH was defined as a RVSP ≥ 36 mmHg and RVSP was treated as a continuous predictor of allograft failure24. Patients were excluded if they had a left ventricular ejection fraction (LVEF) ≤ 50% or had ≥ moderate aortic or mitral valve disease or ≥ moderate diastolic dysfunction.
Variables of Interest
Data was extracted from the TCDB and by medical record review. Patient characteristics including age, sex, race/ethnicity, body mass index (BMI), and factors previously reported to impact renal transplantation outcome including cytomegalovirus (CMV) status and transplantation type (live versus deceased donor) were collected from the TCDB27–30. Additional risk factors for PH, including systemic hypertension, smoking history, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and connective tissue disease (CTD) were collected from the medical record. Allograft failure date (defined as progression to hemodialysis or retransplantation), serial values of post-transplant serum creatinine and date of death were also recorded. Baseline covariates were assessed as close as possible to the time of echocardiogram.
Statistical Analysis
Continuous variables were expressed as median (interquartile range) and categorical variables were expressed as percentages. Independent sample t-tests were used to compare continuous variables and chi-square or Fisher’s exact tests were used to compare categorical variables, as appropriate. Cox hazard regression was used to model the relationship between predictors (PH as a dichotomous outcome and RVSP as a continuous measure) with death-censored allograft failure. Kaplan-Meier estimation was used to estimate the median time until failure. Generalized mixed models with sandwich estimation were used to examine the relationship between PH and creatinine values at 90 days, one year, and three years post transplantation. Interactions and confounding with covariates of interest (age, sex, race, BMI, systemic hypertension, transplant type, left atrial size, COPD, and CMV status) were assessed for all models (p < 0.2). Models were assessed for goodness of fit using Akaike information criterion (AIC) and examined for multicollinearity. Data were collected using Excel 2007 (Microsoft, Redmond, WA) and analyses were performed using STATA 10.0 (StataCorp, College Station, TX) and SAS (SAS institute Inc., Cary, NC). Statistical significance was established at the 0.05 level and all interval estimates were calculated for 95% confidence.
Results
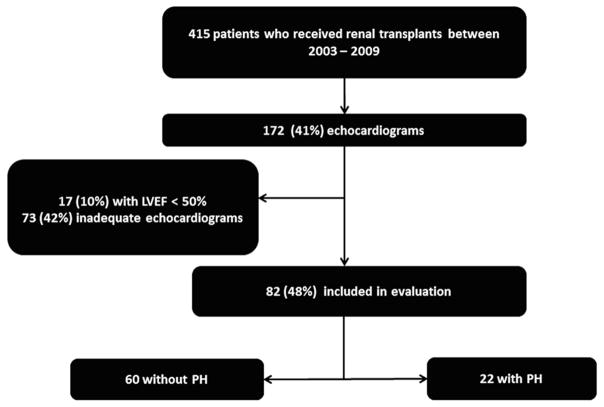
A total of 415 patients received renal transplants between January 1, 2003 and December 31, 2009. During the seven year period, 172 patients (41%) had echocardiograms completed prior to transplantation. Of those with echocardiograms 73 (42%) patients were excluded due to no recorded RVSP, 17 (10%) patients had LVEF ≤ 50%, and 30 (17%) had previously had a transplant (Figure 1). Of the 82 patients included in analysis 22 (27%) patients had echocardiographic evidence of PH.
Figure 1.
Study flow
The median (interquartile range) age was 49 (17 – 80), 48 (42%) were women and 45 (55%) were white. The majority of patients were never smokers (55 [67%]) and had a history of systemic hypertension (66 [80%]). Among those with documentation of the mode of dialysis, 44 (72%) had fistulas for chronic dialysis prior to transplantation. Characteristics of those with and without PH are shown in Table 1. Demographics were similar between the two groups, except a greater proportion of those with PH had a deceased donor transplant (18/22 [82%] vs. 32/60 [53%], p=0.02). The median (interquartile range) RVSP for patients with PH was 45 mmHg (40 – 54 mmHg) and 32 mmHg (20 – 39 mmHg) for those without PH.
Table 1.
Patient characteristics at time of transplantation
| Variables | No PH | PH | P value |
|---|---|---|---|
| Number | 60 (73) | 22 (27) | |
| RVSP, mmHg | 32 (20–39) | 48 (40–74) | |
| Male sex, n (%) | 37 (62) | 11 (50) | 0.34 |
| Age (yrs) | 48 (40–61) | 50 (47–55) | 0.51 |
| BMI | 26 (22–30) | 26 (23–28) | 0.72 |
| Race/ethnicity, n (%) | 0.06 | ||
| White | 37 (62) | 8 (36) | |
| Black | 16 (27) | 8 (36) | |
| Hispanic | 5 (8) | 6 (27) | |
| Asian | 2 (3) | 0 (0) | |
| Systemic HTN, n (%) | 48 (80) | 18 (82) | 0.85 |
| Diabetes mellitus, n (%) | 15 (25) | 8 (36) | 0.31 |
| Hyperlipidemia, n (%) | 17 (28) | 4 (18) | 0.41 |
| COPD, n (%) | 4 (7) | 3 (14) | 0.38 |
| CTD, n (%) | 4 (7) | 1 (5) | 0.99 |
| Left atrial enlargement*, n (%) | 22 (37) | 14 (64) | 0.05 |
| Time echo to transplant, yr | 1.3 (1.3) | 1.8 (1.6) | 0.12 |
| CMV status, n (%) | 0.99 | ||
| donor (−)/recipient (−) | 4 (67) | 1 (5) | |
| donor (− or +)/recipient (+) | 38 (63) | 16 (73) | |
| donor (+)/recipient (−) | 6 (10) | 3 (14) | |
| Transplant type, n (%) | 0.02 | ||
| live donor | 28 (47) | 4 (18) | |
| deceased donor | 32 (53) | 18 (82) | |
| Allograft failure, n (%) | 11 (18) | 9 (41) | 0.02 |
| Time to allograft failure, yr | 2.9 (0.8) | 3.3 (0.8) | 0.75 |
| Death, n (%) | 2 (3) | 1 (5) | 0.80 |
Data are shown as median (interquartile range) or absolute number (%).
≥ mild left atrial enlargement on an ordinal scale. BMI=body mass index, HTN=hypertension, COPD=chronic obstructive pulmonary disease, CTD=connective tissue disease, RVSP=right ventricular systolic pressure, CMV=cytomegalovirus
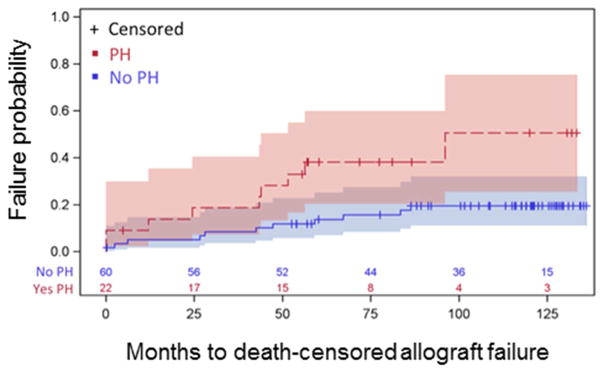
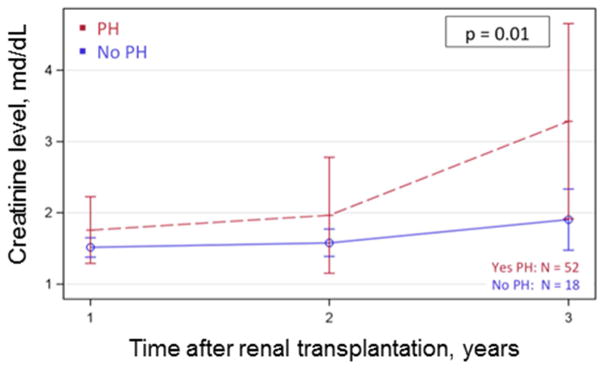
Those with PH prior to renal transplantation had a 3.00 fold (95% confidence interval [CI] 1.20 – 7.32, p=0.02) increased risk of experiencing death-censored allograft failure. Kaplan-Meier estimates showed renal transplant failure rates to be 19% at 24 months and 51% at 96 months for those with PH versus 7% at 24 months and 20% at 86 months for those without PH (p=0.01) (Figure 2). In individuals who did not meet the allograft failure end point, there was a trend towards higher creatinine values at three years in those with PH (3.3 mg/dL, 95% CI 1.9 – 4.7 mg/dL) as compared to those patients without PH (1.9 mg/dL, 95% CI 1.5 – 2.3 mg/dL), a difference (−1.4 mg/dL, 95% CI −3.5 – 0.79 mg/dL) which approached significance (p=0.06) at year 3 and was significant for the overall follow-up time (p = 0.01) (Figure 3).
Figure 2.
Kaplan Meier curves demonstrating death-censored allograft failure, 19% at 24 months, 51% at 96 months for those with PH vs. 7% at 24 months, 20% at 86 months for those without PH (p=0.01). PH=pulmonary hypertension.
Figure 3.
Mixed modeling displayed as least squares means showing creatinine levels at 90 days, 1 year, and 3 years after transplantation. P-value refers to overall F-test for total follow-up time. PH=pulmonary hypertension.
We also assessed RVSP as a continuous predictor. For every 1-mmHg increase in RVSP there was a 2% increase in the risk of death-censored allograft failure however this did not reach statistical significance (p=0.50). Hazard rate was unchanged when PH was defined as RVSP ≥ 36 mm Hg (HR 2.2), however as expected there was a decrease in precision due to greater “noise” (95% CI 0.9 – 5.5, p=0.08) 24.
Discussion
The results from this study support our hypothesis and suggest that echocardiographic PH prior to renal transplantation is associated with a three-fold increase in the risk of death-censored allograft failure and predicts declining renal function in those allografts that do not fail. To our knowledge, this is the first study to demonstrate a relationship between PH and the need for post-transplant hemodialysis or re-transplant and suggests that pre-existing PH may be more than a “secondary” phenomenon due to transient fluid shifts, volume overload or mode of dialysis, but rather indicative of a true pulmonary vascular-renal vascular connection.
A previous study of 215 patients demonstrated increased mortality after renal transplantation in recipients with echocardiographic evidence of PH21. This study did not exclude patients with reduced LVEF and in fact effects were attenuated when LV dysfunction was adjusted for, such that PH no longer had a significant relationship with death; the relationship between PH and graft function was not explored. In our study, we excluded those with significant left-sided disease and other potentially important confounders did not impact the odds of graft failure in those with PH. The 2% increase in the odds of failure with each unit increase in RVSP (while not statistically significant perhaps due to sample size) and increasing creatinine over time even among those who avoided dialysis or retransplantation lends further support to our observations. Worsening creatinine after transplantation among those with PH is concerning as renal dysfunction is an independent risk factor for cardiovascular death and is the leading cause of mortality in renal transplantation patients31,32.
Numerous studies have previously documented an association between echocardiographic PH (and a few have confirmed PAH by invasive hemodynamics) and chronic kidney disease2–10,12,23,31–34. It remains an elusive phenotype with multiple potential endotypes, however, and is therefore classified as WHO Group 5 PH. Certain obvious culprits, such as volume overload, may lead to pulmonary venous hypertension and transient pulmonary pressure elevation which are resolved by a return to euvolemia with HD or transplantation. Shared pulmonary-renal vascular and metabolic signaling pathways suggest that a “feed forward” cycle may also lead to long-lasting effects after transplant, and explain our observations. For example, the addition of the human ET-1 transgene leads to increased ET-1 activity in the brain, lung, and kidney and results in reduced glomerular filtration rate and fatal renal fibrosis in mice35. Given the shared importance of such pathways in PAH and ESRD, altered ET-1, thromboxane A2, nitric oxide, or parathyroid signaling36–40 in patients who develop renal failure may lead to pulmonary vascular changes that eventually cause renal allograft dysfunction post-transplant41–46.
Our study has limitations, including all that apply to a retrospective study. The study population was derived from a single transplant center. The results may not be generalizable to an unselected population of renal transplant recipients, but rather applied to those who have echocardiograms as part of their evaluation. Further, we had to exclude a number of patients due to inadequate echocardiograms, which may have created additional sampling bias. Baseline characteristics of those excluded were similar to those included however (data not shown). We attempted to collect tricuspid annular plane systolic excursion values however this was not routinely included during standard echocardiograms at the time the studies were completed. As our institution is a referral transplant center we were unable to obtain the cause of initial renal failure or concurrent medication use for most patients. Pulmonary vascular disease would have ideally been characterized by hemodynamics using right heart catheterization, especially to distinguish pulmonary arterial from pulmonary venous hypertension and high output states in this population. While hemodynamic data is not available for this cohort, we did carefully consider covariates of interest (e.g., left atrial size and systemic hypertension) and excluded those with overt left-sided abnormalities in an effort to address the potential for confounding. We were able to detect a signal (and in fact a large effect estimate which persisted when we re-categorized PH as RVSP ≥ 36 mm Hg) in spite of this but we cannot exclude residual confounding and our results need to be validated in a population with hemodynamically defined PAH. There was a low event rate for our outcomes of interest and the sample size was small, limiting our power; models were assessed for goodness of fit and multicollinearity and effect estimates did not change with covariate adjustment.
We have for the first time documented a link between pre-transplant echocardiographic PH and death-censored allograft failure. Our findings should be validated prospectively (in both PH and PAH) and further mechanistic work should delineate the pulmonary-renal vascular connection.
Research Highlights.
Pulmonary hypertension increases the risk of renal allograft failure
Pulmonary hypertension may be a risk factor for renal allograft dysfunction
The relationship between the pulmonary and renal vasculature should be further elucidated
Acknowledgments
Funding:
This work was supported by the American Heart Association 11FTF7400032 and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103652.
Footnotes
Disclosures:
CEV has served as a consultant for Bayer Pharmaceuticals and United Therapeutics; her institution has received research grants from Actelion.
Authorship:
A.E.F., A.B.L., P.E.M., R.Y.G., A.P., J.R.K., and C.E.V. participated in study concept and research design. A.E.F., G.L.B., A.B.L., P.E.M., R.Y.G., A.P., J.R.K., and C.E.V. participated in the writing of the manuscript. A.E.F., G.L.B., A.B.L., P.E.M., R.Y.G., A.P., J.R.K., and C.E.V. data collection. A.E.F., G.L.B., and C.E.V. participated in data analyses. All authors revised and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohe C, Nickenig G, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS One. 2012;7(4):e35310. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med. 2010;3:279–286. doi: 10.2147/IJGM.S12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75(9):969–975. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 5.Bozbas SS, Akcay S, Altin C, Bozbas H, Karacaglar E, Kanyilmaz S, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc. 2009;41(7):2753–2756. doi: 10.1016/j.transproceed.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28(6):990–997. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- 7.Kumbar L, Fein PA, Rafiq MA, Borawski C, Chattopadhyay J, Avram MM. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial. 2007;23:127–131. [PubMed] [Google Scholar]
- 8.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74(5):503–510. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- 9.Yigla M, Abassi Z, Reisner SA, Nakhoul F. Pulmonary hypertension in hemodialysis patients: an unrecognized threat. Semin Dial. 2006;19(5):353–357. doi: 10.1111/j.1525-139X.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 10.Tarrass F, Benjelloun M, Medkouri G, Hachim K, Benghanem MG, Ramdani B. Doppler echocardiograph evaluation of pulmonary hypertension in patients undergoing hemodialysis. Hemodial Int. 2006;10(4):356–359. doi: 10.1111/j.1542-4758.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 11.Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, et al. Canadian society of transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173(10):1181–1184. doi: 10.1503/cmaj.051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123(5):1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45(10):1691–1699. doi: 10.1016/j.jacc.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 14.Lafranca JA, Ijzermans JN, Betjes MG, Dor FJ. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13:111. doi: 10.1186/s12916-015-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA. Pulmonary hypertension: an important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med. 2011;105(4):619–624. doi: 10.1016/j.rmed.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494–500. doi: 10.1002/lt.500030503. [DOI] [PubMed] [Google Scholar]
- 17.Starkel P, Vera A, Gunson B, Mutimer D. Outcome of liver transplantation for patients with pulmonary hypertension. Liver Transpl. 2002;8(4):382–388. doi: 10.1053/jlts.2002.31343. [DOI] [PubMed] [Google Scholar]
- 18.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8(11):2445–2453. doi: 10.1111/j.1600-6143.2008.02384.x. [DOI] [PubMed] [Google Scholar]
- 19.Vakil K, Duval S, Sharma A, Adabag S, Abidi KS, Taimeh Z, Colvin-Adams M. Impact of pre-transplant pulmonary hypertension on survival after heart transplantation: a UNOS registry analysis. Int J Cardiol. 2014;176(3):595–599. doi: 10.1016/j.ijcard.2014.08.072. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, King CS, Puri N, Shlobin OA, Brown AW, Ahmad S, et al. Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J. 2016 doi: 10.1183/13993003.01532-2015. [DOI] [PubMed] [Google Scholar]
- 21.Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation. 2008;86(10):1384–1388. doi: 10.1097/TP.0b013e318188d640. [DOI] [PubMed] [Google Scholar]
- 22.Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20(8):1686–1692. doi: 10.1093/ndt/gfh840. [DOI] [PubMed] [Google Scholar]
- 23.Amin M, Fawzy A, Abdel-Hamid M, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124(6):2093–2097. doi: 10.1378/chest.124.6.2093. [DOI] [PubMed] [Google Scholar]
- 24.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Bunnapradist S, Danovitch GM. Evaluation of adult kidney transplant candidates. Am J Kidney Dis. 2007;50(5):890–898. doi: 10.1053/j.ajkd.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Marangoni S, Quadri A, Dotti A, Scalvini S, Volterrani M, Schena M, et al. Noninvasive assessment of pulmonary hypertension: a simultaneous echo-Doppler hemodynamic study. Cardiology. 1988;75(6):401–408. doi: 10.1159/000174410. [DOI] [PubMed] [Google Scholar]
- 27.Lafranca JA, Jermans JN, Betjes MG, Dor FJ. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13:111. doi: 10.1186/s12916-015-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Kim J, Shin M, Kim E, Moon J, Jung G, et al. Comparison of outcomes of living and deceased donor kidney grafts surviving longer than 5 years in Korea. Transplant Proc. 2010;42(3):775–777. doi: 10.1016/j.transproceed.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Linares L, Sanclemente G, Cervera C, Hoyo I, Cofan F, Ricart MJ, et al. Influence of cytomegalovirus disease in outcome of solid organ transplant patients. Transplant Proc. 2011;43(6):2145–2148. doi: 10.1016/j.transproceed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Hassan-Walker AF, Kidd IM, Sabin C, Sweny P, Griffiths PD, Emery VC. Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG) J Med Virol. 1999;58:182–187. [PubMed] [Google Scholar]
- 31.Bolignano D, Lennartz S, Leonardis D, D’Arrigo G, Tripepi R, Emrich IE, Mallamaci F, Fliser D, Heine G, Zoccali C. High estimated pulmonary artery systolic pressure predicts adverse cardiovascular outcomes in stage 2–4 chronic kidney disease. Kidney Int. 2015;88(1):130–136. doi: 10.1038/ki.2015.27. [DOI] [PubMed] [Google Scholar]
- 32.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 33.Navaneethan SD, Dweik RA. Elevated pulmonary pressure: A novel risk marker in kidney disease? Kidney Int. 2015;88(1):7–9. doi: 10.1038/ki.2015.111. [DOI] [PubMed] [Google Scholar]
- 34.Poggio ED, Batty DS, Flechner SM. Evaluation of renal function in transplantation. Transplantation. 2007;84(2):131–136. doi: 10.1097/01.tp.0000269108.59275.dc. [DOI] [PubMed] [Google Scholar]
- 35.Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99(6):1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 37.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB Journal. 2004;14:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 38.Sim JY. Nitric oxide and pulmonary hypertension. Korean J Anesthesiol. 2010;58(1):4–14. doi: 10.4097/kjae.2010.58.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: Pathobiology and implications for therapy. Pulm Circ. 2013;3(1):20–30. doi: 10.4103/2045-8932.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63(6):504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Lariviere R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol. 2003;81(6):607–621. doi: 10.1139/y03-012. [DOI] [PubMed] [Google Scholar]
- 42.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 43.Richter CM. Role of endothelin in chronic renal failure--developments in renal involvement. Rheumatology. 2006;45(Suppl 3):36–38. doi: 10.1093/rheumatology/kel278. [DOI] [PubMed] [Google Scholar]
- 44.Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens. 2001;14:83s–89s. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- 45.Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001;16(Suppl 1):60–62. doi: 10.1093/ndt/16.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- 46.Boffa JJ, Just A, Coffman TM, Arendshorst WJ. Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol. 2004;15(9):2358–2365. doi: 10.1097/01.ASN.0000136300.72480.86. [DOI] [PubMed] [Google Scholar]