Abstract
Problem
Free-roaming unowned stray and feral cats exist throughout the world, creating concerns regarding their welfare as well as their impact on the environment on public health. Millions of healthy cats are culled each year in an attempt to control their numbers. Surgical sterilization followed by return to the environment is an effective nonlethal population control method but is limited in scope due to expense and logistical impediments. Immunocontraception has the potential to be a more practical and cost-effective method of control.
Method of study
This is a review of current research in immunocontraception in domestic cats. Functional characteristics of an ideal immunocontraceptive for community cats would include a wide margin of safety for target animals and the environment, rapid onset and long duration of activity following a single treatment in males and females of all ages, and sex hormone inhibition. In addition, product characteristics should include stability and ease of use under field conditions, efficient manufacturing process, and low cost to the user. Two reproductive antigens, zona pellucida and GnRH, have been identified as possible targets for fertility control in cats.
Results
Zona pellucida, which is used successfully in multiple wildlife species, has achieved little success in cats. In contrast, immunization against GnRH has resulted in long-term contraception in both male and female cats following a single dose. GnRH is an ideal contraceptive target because it regulates pituitary and gonadal hormone responses in both males and females, thus suppressing nuisance behaviors associated with sex hormones in addition to preventing pregnancy.
Conclusion
The responsiveness of cats to fertility control via GnRH suppression should encourage researchers and cat control stakeholders to continue efforts to optimize vaccines that induce multi-year contraception following a single dose in a high proportion of treated cats.
Keywords: GnRH, immunocontraception, population control, zona pellucida
Introduction
Unowned, free-roaming cat populations exist throughout the world. Collectively referred to as community cats, some are well-socialized to people and pet-like in personality and others are elusive feral cats that must be trapped for handling. In many communities, cat populations are subsidized by sympathetic residents who provide food and shelter.1, 2 The number of community cats in the United States is unknown, but is suspected to rival that of pet cats (greater than 90 million) and to contribute substantially to cat overpopulation.
A reproductive strategy including early sexual maturity, seasonally polyestrous cycling, and multiparous pregnancies contribute to the prolific nature of Felis domesticus.3, 4 Highly adaptable, the species thrives in environments ranging from uninhabited subantarctic islands to metropolitan urban cities. Community cats may pose risks to human and animal health as a reservoir for pathogens. Under some conditions, they may also have a negative impact on the environment and native species via competition, predation, and infectious diseases.5 Of primary concern is the welfare of the cats themselves, particularly of kittens, which suffer >50% mortality prior to maturity.6
The most common control method for unwanted cats in the United States is impoundment in animal shelters, where several million cats are culled by lethal injection or carbon monoxide gas each year.7 Poisoned baits, trapping, and hunting are used in many other parts of the world.8, 9 Regardless of the method used, control programs must accommodate considerations for environmental safety of non-target animals and humans, be affordable for municipal agencies or charitable organizations, include plans to mitigate ongoing cat immigration and reproduction, and be aesthetically acceptable to the public.10
Trap-Neuter-Return (TNR) programs are increasingly popular as an alternative to lethal control and have been shown to decrease colony size over time.11 However, capturing free-roaming cats, transporting them to a central facility for sterilization, and returning them to the trapping site are resource-intensive activities. In addition, veterinarians with adequate facilities for aseptic procedures must be available to perform the surgeries. Although TNR programs have many benefits including improving the health of the free-roaming cats12 and controlling cat populations on a local basis, it is challenging to sustain surgical TNR programs on a larger scale. This is particularly true in regions of the world where veterinary care for cats is not widely available.
Contraceptive vaccines have been used successfully for fertility control in many overabundant wildlife species and have the potential for humane control of companion animal species as well.13–19 Compared to surgical sterilization, immunocontraception offers a less costly, technical, and invasive alternative for nonlethal cat management. In contrast to owned pet cats, for which owners expect a contraceptive technique that is 100% reliable with a predictable duration of effect in each individual animal, community cat control is treated as a population management issue.20, 21 Thus, a contraceptive treatment need not be effective in each animal, but a predictable effect at the population level is required. Most cat control models suggest that approximately 70–80% of female cats in a population must be rendered infertile to induce a decline in population size.22–25
As in wildlife species, free-roaming cat control is challenging because most cats can be captured and treated only once in their lives. This means that an ideal feline immunocontraceptive would induce long-term or permanent contraception following a single treatment. Although the life-span of cats likely varies in different regions,26, 27 one model suggests that a contraceptive with a three-year duration of effect may be successful in controlling cat populations.22 From a population biology perspective, controlling reproduction in females is the most critical factor in regulating populations; whereas an equivalent level of infertility in males has little effect.22 However, in addition to controlling reproduction, a secondary goal of management is to reduce adverse health impacts and objectionable behaviors associated with sex hormones in both sexes. Functional characteristics of an ideal immunocontraceptive would include a wide margin of safety for target animals and the environment, rapid onset and long duration of activity following a single treatment in males and females of all ages, and sex hormone inhibition. In addition, product characteristics should include stability and ease of use under field conditions, efficient manufacturing process, and low cost to the user.10, 21
Discussion
Zona pellucida
A number of proteins in the hypothalamic-pituitary-gonadal axis have been targeted for immunocontraception in other species. Zona pellucida (ZP), the glycoprotein matrix surrounding the mammalian egg, regulates sperm-egg interactions during fertilization and is subsequently removed prior to implantation.28–30 The relatively high amino acid sequence homology across species of some portions of the protein suggests that immunocontraceptive vaccines based on ZP proteins of one species may induce adequate cross-reactivity to immunize other species.31, 32 Indeed, native porcine ZP (pZP) isolated from pig ovaries collected at abattoirs has been used for successful long-term contraception in horses, deer, rabbits, elephants, seals, and other species.28 19, 33 In most reports, successful ZP immunocontraception is not associated with ovarian disruption leading to suppression of estrous cycling, which could be considered a weakness of this approach. Cats are seasonally polyestrous, and failure to conceive after breeding triggers a period of pseudopregnancy followed by return to estrus. Long-term, this may lead to an increase in complaints about nuisance behaviors associated with the cat breeding season and uterine or mammary diseases in vaccinated cats.
In the first report investigating pZP for immunocontraception in cats, a vaccine containing pZP and Freunds adjuvant [Freunds complete adjuvant with killed mycobacteria in oil (FCA) for the first vaccine dose and incomplete adjuvant lacking mycobacteria (FIA) for subsequent doses] was administered subcutaneously to eight adult cats five times over a period of 92–150 days.34 Serum ZP antibodies reacted more strongly against pig oocytes than against cat oocytes. In a subsequent 3-month breeding trial in five of the cats, only one became pregnant, suggesting that pZP may have immunocontraceptive potential in female cats. A subsequent study evaluating the binding activity of rabbit anti-pZP antibodies in immunohistochemical (IHC) evaluation of cat ovaries identified cross-reactivity with cat oocytes, further supporting this potential.35 In contrast, another study failed to confirm cross-reactivity of rabbit anti-pZP antibodies with feline ZP (fZP).36 Antibodies against pZP failed to block sperm binding or in vitro fertilization of cat oocytes, whereas antibodies raised in rabbits against fZP did inhibit fertilization. IHC studies demonstrated that pZP antibodies did not bind to cat oocytes, but fZP antibodies did. In a third study, antibodies raised in rats against a portion of the fZP protein containing an epitope recognized by rabbit anti-fZP serum (ZPB amino acid residue 130– 149) reduced in vitro sperm binding and fertilization of feline oocytes.37 These studies are contradictory, clouding the issue of whether ZP has contraceptive potential in cats. In addition, findings in all three studies were based on antibodies produced in rabbits or rats, not in cats, and none was confirmed in breeding trials. A fourth study comparing the effect of vaccines based on subunits of pZP confirmed that three monthly doses of pZPB+C emulsified in FCA (first vaccine) or FIA (second and third vaccines) and injected in multiple locations subcutaneously induced antibodies in 2–3 year old cats that failed to bind to cat oocytes during IHC or to prevent pregnancy.29 The investigators also tested DNA vaccines containing sequences coding for fZPA or fZPB+C in which three doses were administered intramuscularly at monthly intervals. In a breeding trial spanning a single estrous cycle, a lower proportion of cats receiving the DNA vaccines became pregnant than controls. However, a low conception rate in the controls coupled with a low number of cats that attempted to breed rendered the differences statistically insignificant. Histologic findings suggestive of possible underlying reproductive pathology in some cats contributed to further uncertainty regarding the effect of fZP vaccination on fertility of cats. Contradictory or ambiguous findings may be explained by a lack of standardization among studies, including differences in the source and preparation of ZP used for immunization, use of different adjuvants, variable vaccination schedules and procedures, and use of surrogate outcome markers in place of breeding trials in the target species.
In the first study in cats using a pZP vaccine with a history of multi-year immunocontraception in other species, three groups of 10 8–12 week old kittens were vaccinated once intramuscularly with one of two vaccine formulations or with a sham vaccine lacking pZP.30 The vaccine, SpayVac™, was composed of soluble pZP isolated from pig ovaries collected at abattoirs encapsulated in multi-lamellar liposomes for slow release and emulsified with one of two adjuvants, FCA or alum with oil and mannide oleate. Cats responded with the rapid induction of anti-pZP antibodies, but similar to previous studies IHC revealed binding only to pig oocytes and not cat oocytes. All cats had maintained normal estrous cycling and became pregnant. There were no differences in time to pregnancy or litter size among the three treatment groups. Ovaries removed from cats after parturition failed to show any binding of antibodies to oocytes in treated cats. This study confirmed earlier concerns questioning the degree of cross-reactivity between pZP and fZP.
It remained to be determined whether the ZP of species other than the pig would share more effective antigenic determinants with feline ZP versus whether the cat was unique in its failure to respond immunologically to this self antigen. It has been shown that homology in ZP sequences is greatest within the same mammalian class, suggesting that ZP from species more closely related to the cat may be more suitable candidates.31, 32 In addition, the previous finding that antibodies raised in rabbits against fZP bound to cat oocytes suggested that ZP isolated from cat ovaries might be capable of stimulating the production of antibodies that recognize fZP. To explore the effect of alternative sources of ZP, native soluble ZP isolated from the ovaries of ferrets, mink, dogs, cows, and cats were used in SpayVac™ formulated with FCA.38 Groups of three 15–20 week old kittens were vaccinated once intramuscularly with SpayVac™ containing one of the five species ZP. As previously shown for pZP, anti-ZP antibody titers were generally higher against the species ZP contained in the vaccine than against fZP. None of the sera reacted with cat oocytes, and all cats became pregnant. Interestingly, the three cats vaccinated against fZP had litter sizes of one, one, and five, respectively. This suggested that immunization with fZP may reduce reproductive success. To further evaluate this observation, these three cats were boostered with fZP emulsified in FIA 32 weeks after the first dose. A second round of breeding beginning five weeks after the booster resulted in litter sizes of four, six, and four (stillborn), respectively, reducing enthusiasm for the potential of fZP as a contraceptive antigen in cats.
Gondadotropin releasing hormone
The hypothalamic decapeptide gondadotropin releasing hormone (GnRH) controls pituitary release of LH and FSH, which in turn control testosterone and spermatogenesis in the male and estrogen and follicular development in the female.39, 40 Since this master reproductive hormone controls fertility and behavioral responses in both sexes, it is an ideal target antigen for immunocontraception.21, 33 GnRH is widely conserved across all mammalian species. This is both an opportunity to develop immunocontraceptives with activity in multiple overabundant species, and a threat in regards to safeguarding non-target species and personnel who must handle the products. As a small self-antigen, GnRH is a poor immunogen. Immune recognition is enhanced by conjugating GnRH to large foreign proteins and administration in the presence of immune-stimulating adjuvants.
In the first report in which the hypothalamic-pituitary-gonadal axis was targeted for fertility control in the cats, synthetic GnRH was conjugated to tetanus toxoid, emulsified, and mixed with n-acytyl-nor-muramyl-l-d-isoglutamine as an adjuvant.41 Six cats vaccinated three times at weeks 1, 2, and 4 experienced only modest and transient decreases in serum testosterone, whereas dogs vaccinated in a similar protocol experienced several months of testosterone levels consistent with immunocastration before recovering fertility. In a second study, an attempt to block the downstream effects of GnRH stimulation by prevention of LH binding utilized vaccination against LH receptors. Bovine LH receptors emulsified in N-acetylglucosaminyl-(β1–4) Nacetylmuramyl-L-alanyl-D-isoglutamine adjuvant prepared from mycobacterium cell walls was packaged into silastic implants. Implants were placed via surgical incision in the intrascapular space in seven anesthetized 8-month-old female cats.42 Cats were also vaccinated intramuscularly with the same preparation on days 98, 139, 160, and 193. Cats produced antibodies against LH, resulting in suppression of serum progesterone for more than a year. On day 345, cats were stimulated with GnRH, resulting in a rise in serum LH indicating that pituitary function remained intact. Breeding studies were not performed. In a third study, a recombinant fusion protein containing multiple copies of GnRH fused to a carrier protein fragment of leukotoxin A was emulsified in an oil-in-water adjuvant containing the immunostimulant dimethyl dioctadecyl ammonium bromide and administered subcutaneously at eight and twelve weeks of age to 10 female and four male kittens.43 Tissue reactions were palpable at the injection site in all cats, but resolved in most cats within 28 days. Immunocontraceptive GnRH antibody titers persisted past 20 months in 14 of 15 cats. In males, this was associated with undetectable serum testosterone concentrations, whereas in females it was reflected in low progesterone concentrations and failure to conceive in a breeding trial. Booster vaccines given on day 643 resulted in even higher GnRH antibody titers.
The first description of successful immunization against a self-antigen in cats following a single vaccination occurred when 9–12 month old male cats were vaccinated intramuscularly with GonaCon™.44 The GonaCon™ vaccine is constructed with multiple copies of synthetic GnRH coupled to keyhole limpet hemocyanin carrier protein and emulsified in an oil and water adjuvant containing killed mycobacterium (AdjuVac™). Six of nine vaccinated cats responded with persistently high GnRH antibody titers, which correlated with undetectable serum testosterone concentrations by three months post-injection. Immunocastration was associated with progressive decrease in scrotal volume and regression of penile spines (Fig. 1). Semen analysis performed six months post-vaccination demonstrated absence of motile sperm in the responding cats. Testicular weights in those cats were only one quarter that of the sham-treated cats. Three cats with poor response to GnRH vaccination as defined by low GnRH antibody titers had testosterone concentrations, sperm counts, and scrotal volumes that were intermediate between the responding cats and the sham-treated cats.
Fig. 1.
Phenotypic responses of cats six months after receiving a single sham treatment (left) or GonaCon™, a vaccine containing synthetic GnRH coupled to keyhole limpet hemocyanin and emulsified in oil and killed mycobacterium (box). Testes size six months after vaccination was corrected to GnRH antibody titer and inversely correlated with serum testosterone concentration. Three cats receiving a sham-treatment had normal-sized testes (a), whereas cats vaccinated against GnRH had variable testicular atrophy depending on the GnRH titer and resulting suppression of testosterone (b). Penile spines remained prominent in sham-treated cats (c) and regressed in cats that responded to GnRH vaccination (d).
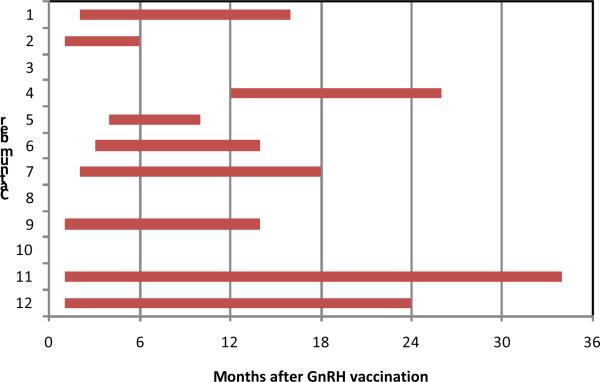
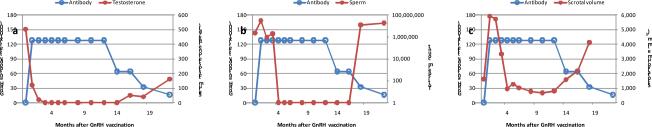
Successful immunocastration of male cats with a single GnRH vaccination in the short-term study was followed by a multi-year study in which fertility of GonaCon™-vaccinated male cats was tested in a breeding trial.45 For the nine of 12 vaccinated cats that responded to vaccination with high GnRH antibody titers, the median onset of testosterone becoming undetectable was 2 months (range 1–12 months) and the median duration of effect was 14 months (range 5–33 months) (Fig 2). One cat still had undetectable testosterone at the end of the observation period 34 months after treatment. Loss of detectable testosterone was generally followed in 1–2 months by azoospermia, and restoration of normal sperm counts lagged behind recovery of testosterone by 2 months (Fig. 3). Semen characteristics, including morphology and viability, were similar in cats prior to treatment and following recovery of fertility. Three treated cats that failed to respond with high GnRH antibody titers had minimal to no suppression of testosterone. The average time from introduction of the female cats to successful breeding was 12 months (range 3–12 months) for the responding cats, five months (range 5–6 months) for the poorly responding cats, and three months (range 0–9 months) for the sham-treated cats. In one extreme case, GnRH antibody titer did not begin to increase until 6 months post vaccination, testosterone was not suppressed until 12 months, and azoospermia did not occur until 14 months. In this cat, the contraceptive effect lasted 14 months, after which GnRH antibody titer waned, normal testosterone concentration and semen characteristics recovered, and the cat sired a litter.46 The mechanism for this delayed response is unknown. A second late peak of response to GonaCon™ after initial decline in GnRH antibodies has been reported in rats eight months after vaccination47 and in a dog six months after vaccination,48 indicating persistence of antigen for prolonged periods of time in some individuals. Median litter size sired by the cats was not significantly different between the groups. In this study GnRH immunocontraception showed efficacy in 75% of male cats following a single treatment, but response was highly variable and all but one cat recovered fertility by three years post-treatment.
Fig. 2.
Intervals during which testosterone was undetectable in individual male cats following a single dose of the GnRH vaccine GonaCon™. Onset and duration of immunocastration varied among cats and three cats with a poor antibody response to vaccination never had more than one month without detectable serum testosterone.
Fig. 3.
GnRH antibody titer correlation with testosterone (a), total sperm count (b), and scrotal volume (c) in a representative cat following a single dose of the GnRH vaccine GonaCon™. The GnRH antibody titer reached a contraceptive level within one month of vaccination after which testosterone and sperm count decreased to undetectable levels and scrotal volume decreased. GnRH antibody titer waned 12 months after vaccination, which was associated with recovery of testosterone production, normal sperm counts, and increased scrotal volume.
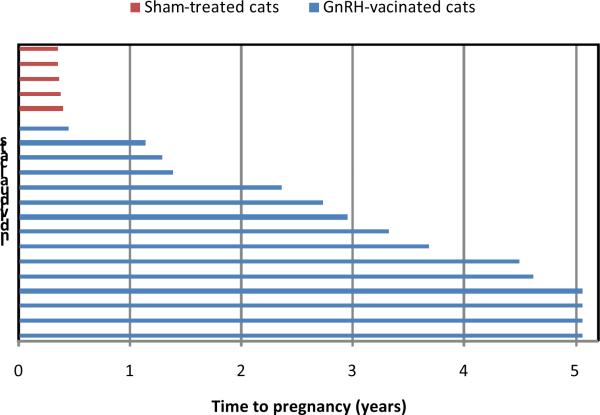
Although suppression of sex hormones in male cats is valuable due to a reduction in nuisance behavior, population control is primarily dependent on fertility control in females. A breeding trial in female cats demonstrated multi-year immunocontraception in 11 of 15 8–14 month old female cats vaccinated intramuscularly with a single dose of GonaCon™, including four cats still infertile five years after vaccination (Fig. 4).49 Similar to male cats, four females (27%) failed to produce high GnRH antibody titers and became pregnant less than two years after vaccination. Altogether, vaccinated cats had a significantly longer time to conception (median 39.7 mo) compared to sham-treated cats (4.4 mo). Late-onset injection site reactions developed in five of the cats. Histology revealed granulomatous inflammation containing inactivated mycobacteria from the adjuvant, suggesting that continued inflammation stimulated by the vaccine may be responsible for the durable immune response observed in a high proportion of vaccinated female cats. The duration of response to GonaCon™ was longer in female cats than in male cats. Less predictable immunocontraception of males compared to females has been in observed in other species as well, possibly related to more difficulty in blocking continuously secreted GnRH in males compared to episodic secretion in females.50, 51
Fig. 4.
Results of a breeding trial in female cats following a single sham treatment (n=5 cats) or the GnRH vaccine GonaCon™ (n=15 cats). Cats were group-housed with fertile males beginning 4 months after GnRH vaccination and remained until they were successfully bred. Four cats maintained high GnRH antibody titers and remained infertile throughout the study until it was completed five years following a single GnRH vaccination.
Summary
GnRH has many of the attributes sought in an ideal contraceptive target, including activity in both males and females, suppression of sex hormones in addition to fertility, and a scalable manufacturing process based on mass production of synthetic or recombinant peptides. Aside from differences in immunocontraceptive efficacy, GnRH is functionally superior to zona pellucida because animals rendered infertile with zona pellucida continue to undergo estrous cycling. GonaCon™, which was approved in 2009 for control of white-tail deer, was capable of inducing multi-year fertility control in cats following a single dose. However, not all cats responded to vaccination and response by individual cats was unpredictable, especially in males.
Safety for the target species is another prerequisite for the development of an acceptable immunocontraceptive. Granulomatous reactions observed in cats receiving adjuvants containing oil and mycobacteria have been observed following both zona pellucida and GnRH vaccinantion.49, 52 The persistence of antigen and adjuvant at the injection site may serve to prolong the immune response, but also has the potential to lead to systemic inflammation,52 hypercalcemia,52 painful draining wounds,48 or neoplastic transformation.53 Balancing the need for durable immunity in a high proportion of vaccinated cats with the need for a risk profile meeting or exceeding that of surgical sterilization is the greatest challenge in the development of a single-dose immunocontraceptive for community cats. Alternate adjuvants or delivery systems such as viral vectors or slow-release implants may improve the safety profile and duration of activity.
Long-lasting single-dose contraceptives suitable for administration in the field by trained technicians would be a powerful tool in the control of overabundant species such as community cats. Immunocontraceptive vaccines can be administered to cats confined in humane traps without sedation, eliminating the need for skilled veterinary participation in control programs. In addition, it should be possible to vaccinate cats simultaneously against rabies and other feline or zoonotic infections, resulting in a population that is safer, healthier, and better controlled.54, 55
Acknowledgements
Our studies in feline immunocontraception have been made possible by support from Morris Animal Foundation, PetSmart Charities, the Kenneth A. Scott Charitable Trust, the Geraldine R. Dodge Foundation, the National Institutes of Health RR-00124, Humane Friends of Georgia, Humane Services of Metro Atlanta, Eugene L Kirshbaum Foundation for Animals, and private donors.
Caption for Corresponding author's photo
Julie K. Levy Maddie's Shelter Medicine Program, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA

References
- 1.Levy JK, Woods JE, Turick SL, Etheridge DL. Number of unowned free-roaming cats in a college community in the southern United States and characteristics of community residents who feed them. J Am Vet Med Assoc. 2003;223:202–205. doi: 10.2460/javma.2003.223.202. [DOI] [PubMed] [Google Scholar]
- 2.Centonze LA, Levy JK. Characteristics of free-roaming cats and their caretakers. J Am Vet Med Assoc. 2002;220:1627–1633. doi: 10.2460/javma.2002.220.1627. [DOI] [PubMed] [Google Scholar]
- 3.Kutzler MA. Estrus induction and synchronization in canids and felids. Theriogenology. 2007;68:354–374. doi: 10.1016/j.theriogenology.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Griffin B. Prolific Cats: The impact of their fertility on the welfare of the species. Compend Contin Educ Pract Vet. 2001;23:1058–1069. [Google Scholar]
- 5.Jessup DA. The welfare of feral cats and wildlife. J Am Vet Med Assoc. 2004;225:1377–1383. doi: 10.2460/javma.2004.225.1377. [DOI] [PubMed] [Google Scholar]
- 6.Nutter FB, Levine JF, Stoskopf MK. Reproductive capacity of free-roaming domestic cats and kitten survival rate. J Am Vet Med Assoc. 2004;225:1399–1402. doi: 10.2460/javma.2004.225.1399. [DOI] [PubMed] [Google Scholar]
- 7.Humane Society of the United States Common questions about animal shelters. [Accessed February 13, 2011]. www.hsus.org.
- 8.Nogales M, Martin A, Tershy BR, Donlan CJ, Veitch D, Puerta N, Wood B, Alonso J. A review of feral cat eradication on islands. Conserv Biol. 2004;18:310–319. [Google Scholar]
- 9.Short J, Turner B, Risbey D, Carnamah R. Control of feral cats for nature conservation. II. Population reduction by poisoning. Wildlife Res. 1997;24:703–714. [Google Scholar]
- 10.Rhodes L, Briggs J. Non-surgical sterilization: Priorities and challenges. Proc 4th Int Symp Non-Surgical Contraceptive Methods Pet Population Control. 2010:80–81. [Google Scholar]
- 11.Levy JK, Gale DW, Gale LA. Evaluation of the effect of a long-term trap-neuter-return and adoption program on a free-roaming cat population. J Am Vet Med Assoc. 2003;222:42–46. doi: 10.2460/javma.2003.222.42. [DOI] [PubMed] [Google Scholar]
- 12.Scott KC, Levy JK, Gorman SP, Newell SM. Body condition of feral cats and the effect of neutering. J Appl Anim Welf Sci. 2002;5:203–213. doi: 10.1207/S15327604JAWS0503_04. [DOI] [PubMed] [Google Scholar]
- 13.Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar AG. Recent advances in contraceptive vaccine development: a mini-review. Hum Reprod. 2005;20:3271–3283. doi: 10.1093/humrep/dei256. [DOI] [PubMed] [Google Scholar]
- 14.Rutberg A, editor. Humane Wildlife Solutions: The role of immunocontraception. Humane Society Press; Washington, DC: 2005. pp. 63–92. [Google Scholar]
- 15.Hardy CM, Braid AL. Vaccines for immunological control of fertility in animals. Rev Sci Tech. 2007;26:461–470. [PubMed] [Google Scholar]
- 16.Purswell BJ, Kolster KA. Immunocontraception in companion animals. Theriogenology. 2006;66:510–513. doi: 10.1016/j.theriogenology.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Cooper DW, Larsen E. Immunocontraception of mammalian wildlife: ecological and immunogenetic issues. Reproduction. 2006;132:821–828. doi: 10.1530/REP-06-0037. [DOI] [PubMed] [Google Scholar]
- 18.Jewgenow K, Dehnhard M, Hildebrandt TB, Goritz F. Contraception for population control in exotic carnivores. Theriogenology. 2006;66:1525–1529. doi: 10.1016/j.theriogenology.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Fayrer-Hosken R. Controlling animal populations using anti-fertility vaccines. Reprod Domest Anim. 2008;43(Suppl):179–185. doi: 10.1111/j.1439-0531.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 20.Fagerstone KA. Mechanism of GnRH contraceptive vaccine-mediated infertility and its applications. Proc 3rd Int Symp Non-Surgical Contraceptive Methods Pet Population Control. 2006:200–218. [Google Scholar]
- 21.Alliance for Contraception in Cats and Dogs Immunocontraceptive approaches for sterilization of dogs and cats. Proc 4th Int SymNon-Surgical Methods Pet Population Control. 2010:106–113. [Google Scholar]
- 22.Budke CM, Slater MR. Utilization of matrix population models to assess a 3-year single treatment nonsurgical contraception program versus surgical sterilization in feral cat populations. J Appl Anim Welf Sci. 2009;12:277–292. doi: 10.1080/10888700903163419. [DOI] [PubMed] [Google Scholar]
- 23.Foley P, Foley JE, Levy JK, Paik T. Analysis of the impact of trap-neuter-return programs on populations of feral cats. J Am Vet Med Assoc. 2005;227:1775–1781. doi: 10.2460/javma.2005.227.1775. [DOI] [PubMed] [Google Scholar]
- 24.Andersen MC, Martin BJ, Roemer GW. Use of matrix population models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats. J Am Vet Med Assoc. 2004;225:1871–1876. doi: 10.2460/javma.2004.225.1871. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt PM, Swannack TM, Lopez RR, Slater MR. Evaluation of euthanasia and trap- neuter-return (TNR) programs in managing free-roaming cat populations. Wildlife Res. 2009;36:117–125. [Google Scholar]
- 26.Schmidt PM, Lopez RR, Collier BA. Survival, fecundity, and movements of free-roaming cats. J Wildlife Management. 2007;71:915–919. [Google Scholar]
- 27.Nutter FB. Evaluation of a Trap-Neuter-Return Management Program for Feral Cat Colonies: Population Dynamics, Home Ranges, and Potentially Zoonotic Diseases [Dissertation] North Carolina State University; Raleigh: 2005. [Google Scholar]
- 28.Kirkpatrick JF, Rowan A, Lamberski N, Wallace R, Frank K, Lyda R. The practical side of immunocontraception: zona proteins and wildlife. J Reprod Immunol. 2009;83:151–157. doi: 10.1016/j.jri.2009.06.257. [DOI] [PubMed] [Google Scholar]
- 29.Eade JA, Roberston ID, James CM. Contraceptive potential of porcine and feline zona pellucida A, B and C subunits in domestic cats. Reproduction. 2009;137:913–922. doi: 10.1530/REP-08-0471. [DOI] [PubMed] [Google Scholar]
- 30.Gorman SP, Levy JK, Hampton AL, Collante WR, Harris AL, Brown RG. Evaluation of a porcine zona pellucida vaccine for the immunocontraception of domestic kittens (Felis catus) Theriogenology. 2002;58:135–149. doi: 10.1016/s0093-691x(02)00904-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Naz RK. Comparison of ZP3 protein sequences among vertebrate species: to obtain a consensus sequence for immunocontraception. Front Biosci. 1999;4:D212–215. doi: 10.2741/zhu. [DOI] [PubMed] [Google Scholar]
- 32.Harris JD, Hibler DW, Fontenot GK, Hsu KT, Yurewicz EC, Sacco AG. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: the ZPA, ZPB and ZPC gene families. DNA Seq. 1994;4:361–393. doi: 10.3109/10425179409010186. [DOI] [PubMed] [Google Scholar]
- 33.Delves PJ, Roitt IM. Vaccines for the control of reproduction-status in mammals, and aspects of comparative interest. Dev Biol (Basel) 2005;121:265–273. [PubMed] [Google Scholar]
- 34.Ivanova M, Petrov M, Klissourska D, Mollova M. Contraceptive potential of porcine zona pellucida in cats. Theriogenology. 1995;43:969–981. doi: 10.1016/0093-691x(95)00046-b. [DOI] [PubMed] [Google Scholar]
- 35.Barber MR, Lee SM, Steffens WL, Ard M, Fayrer-Hosken RA. Immunolocalization of zona pellucida antigens in the ovarian follicle of dogs, cats, horses and elephants. Theriogenology. 2001;55:1705–1717. doi: 10.1016/s0093-691x(01)00514-3. [DOI] [PubMed] [Google Scholar]
- 36.Jewgenow K, Rohleder M, Wegner I. Differences between antigenic determinants of pig and cat zona pellucida proteins. J Reprod Fertil. 2000;119:15–23. [PubMed] [Google Scholar]
- 37.Ringleb J, Rohleder M, Jewgenow K. Impact of feline zona pellucida glycoprotein B-derived synthetic peptides on in vitro fertilization of cat oocytes. Reproduction. 2004;127:179–186. doi: 10.1530/rep.1.00076. [DOI] [PubMed] [Google Scholar]
- 38.Levy JK, Mansour M, Crawford PC, Pohajdak B, Brown RG. Survey of zona pellucida antigens for immunocontraception of cats. Theriogenology. 2005;63:1334–1341. doi: 10.1016/j.theriogenology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Herbert CA, Trigg TE. Applications of GnRH in the control and management of fertility in female animals. Anim Reprod Sci. 2005;88:141–153. doi: 10.1016/j.anireprosci.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Kutzler M, Wood A. Non-surgical methods of contraception and sterilization. Theriogenology. 2006;66:514–525. doi: 10.1016/j.theriogenology.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Ladd A, Tsong YY, Walfield AM, Thau R. Development of an antifertility vaccine for pets based on active immunization against luteinizing hormone-releasing hormone. Biol Reprod. 1994;51:1076–1083. doi: 10.1095/biolreprod51.6.1076. [DOI] [PubMed] [Google Scholar]
- 42.Saxena BB, Clavio A, Singh M, Rathnam P, Bukharovich EY, Reimers TJ, Jr., Saxena A, Perkins S. Effect of immunization with bovine luteinizing hormone receptor on ovarian function in cats. Am J Vet Res. 2003;64:292–298. doi: 10.2460/ajvr.2003.64.292. [DOI] [PubMed] [Google Scholar]
- 43.Robbins SC, Jelinski MD, Stotish RL. Assessment of the immunological and biological efficacy of two different doses of a recombinant GnRH vaccine in domestic male and female cats (Felis catus) J Reprod Immunol. 2004;64:107–119. doi: 10.1016/j.jri.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Levy JK, Miller LA, Crawford PC, Ritchey JW, Ross MK, Fagerstone KA. GnRH immunocontraception of male cats. Theriogenology. 2004;62:1116–1130. doi: 10.1016/j.theriogenology.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Friary J, Levy J, Miller L, Fagerstone K. Efficacy and duration of immunity for single-dose GnRH immunocontraception in male cats. Proc 3rd Int Symp Non-Surgical Methods Pet Population Control. 2006:577. [Google Scholar]
- 46.Levy JK. Multi-year studies of single-dose GnRH immunocontraception in cats. Proc 3rd Int Symp Non-Surgical Methods Pet Population Control. 2006:219–237. [Google Scholar]
- 47.Miller LA, Johns BE, Elias DJ, Crane KA. Comparative efficacy of two immunocontraceptive vaccines. Vaccine. 1997;15:1858–1862. doi: 10.1016/s0264-410x(97)00141-2. [DOI] [PubMed] [Google Scholar]
- 48.Griffin B, Baker H, Welles E, Miller L, Fagerstone K. Response of dogs to a GnRH-KLH conjugate contraceptive vaccine adjuvanted with Adjuvac®. Proc 2nd Int Symp Non-Surgical Contraceptive Methods Pet Population Control. 2004:189–190. [Google Scholar]
- 49.Levy JK, Friary JA, Miller LA, Tucker SJ, Fagerstone KA. Long-term fertility control in female cats with GonaCon, a GnRH immunocontraceptive. 2011 doi: 10.1016/j.theriogenology.2011.06.022. in press. [DOI] [PubMed] [Google Scholar]
- 50.Killian G, Miller L, Rhyan J, Doten H. Immunocontraception of Florida feral swine with a single-dose GnRH vaccine. Am J Reprod Immunol. 2006;55:378–384. doi: 10.1111/j.1600-0897.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 51.Killian G, Wagner D, Miller L. Observations on the use of the GnRH vaccine GonaCon in male white-tailed deer. Proc Wildlife Damage Management Conf. 2005;11:256–263. [Google Scholar]
- 52.Munson L, Harrenstien LA, Acton AE, Graham PA, Chassy LM, Kirkpatrick JF. Immunologic responses and adverse reactions to Freund's-adjuvanted porcine zona pellucida immuno-contraceptives in domestic cats. Vaccine. 2005;23:5646–5654. doi: 10.1016/j.vaccine.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 53.Hauck M. Feline injection site sarcomas. Vet Clin North Am Small Anim Pract. 2003;33:553–557. doi: 10.1016/s0195-5616(03)00006-8. [DOI] [PubMed] [Google Scholar]
- 54.Wu X, Franka R, Svoboda P, Pohl J, Rupprecht CE. Development of combined vaccines for rabies and immunocontraception. Vaccine. 2009;27:7202–7209. doi: 10.1016/j.vaccine.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Bender SC, Bergman DL, Wenning KM, Miller LA, Slate D, Jackson FR, Rupprecht CE. No adverse effects of simultaneous vaccination with the immunocontraceptive GonaCon and a commercial rabies vaccine on rabies virus neutralizing antibody production in dogs. Vaccine. 2009;27:7210–7213. doi: 10.1016/j.vaccine.2009.09.026. [DOI] [PubMed] [Google Scholar]