Abstract
Background
Early life BPA exposure could affect neurobehavior, but few studies have investigated whether there are developmental periods when the fetus or child is more vulnerable to these potential effects.
Objectives
We explored windows of vulnerability to BPA exposure in a multiethnic cohort of 228 mothers and their children from Cincinnati, Ohio.
Methods
We measured urinary BPA concentrations at up to two prenatal and six postnatal time points from the 2nd trimester of pregnancy until the child was age 8 years. At age 8 years, we administered the Behavioral Assessment System for Children-2 (BASC-2), Behavior Rating Inventory of Executive Function, and Wechsler Intelligence Scale for Children-IV. We estimated covariate-adjusted differences in composite scores from each instrument using a multiple informant model designed to identify heightened windows of vulnerability.
Results
Among all children, there was not strong evidence that the associations between BPA and neurobehavior varied by the timing of exposure (Visit × BPA p-values=0.16). However, child sex modified the associations of repeated BPA measures with BASC-2 scores (Visit × Sex × BPA p-values=0.02–0.23). For example, each 10-fold increase in prenatal BPA was associated with more externalizing behaviors in girls (β=6.2, 95% CI: 0.8, 11.6), but not boys (β=−0.8, 95% CI: −5.0, 3.4). In contrast, a 10-fold increase in 8-year BPA was associated with more externalizing behaviors in boys (β=3.9, 95% CI: 0.6, 7.2), but not girls (β=0.3, 95% CI: −3.5, 4.1).
Conclusions
We found that sex-dependent associations between BPA and child neurobehavior may depend on the timing of BPA exposure.
Keywords: Bisphenol A, children, neurodevelopment, epidemiology
Introduction
Bisphenol A (BPA) is a weak estrogenic monomer widely used in the manufacture of plastics and resins found in a variety of consumer products, including some plastic food and beverage storage containers, food can linings, thermal paper receipts, medical equipment, dental sealants, and children’s toys (Chapin et al. 2008). Exposure to BPA is almost universal in the United States; over 90% of the U.S. population has detectable levels of urinary BPA (Calafat et al. 2008). Diet is the primary route of exposure, although exposure may also occur via inhalation and dermal absorption, particularly in occupational settings (Ehrlich et al. 2014; Hehn 2016; Li et al. 2010; Hines et al. 2017; Thayer et al. 2015b; Von Goetz et al. 2010; Zalko et al. 2011). BPA is capable of binding to the membrane and nuclear estrogen receptors and can affect gonadal hormone signaling, which play critical roles in the developing brain, influencing pathways that lead to sex-specific development and behavior (Henrichs et al. 2013; Schug et al. 2015).
The fetus, infant, and child may be particularly vulnerable to the endocrine disrupting effects of BPA because exposures that occur during specific periods of development can have a lifelong impact on health (Barker 2007; Rice and Barone 2000). A number of epidemiologic studies have reported associations between urinary BPA concentrations at individual time points during gestation or childhood and maladaptive behaviors, such as hyperactivity and aggression, in children (Evans et al. 2014; Harley et al. 2013; Hong et al. 2013; Perera et al. 2012; Perez-Lobato et al. 2016; Roen et al. 2015). However, only a few of these studies have investigated whether there are windows of heightened vulnerability to BPA exposure, and none have employed statistical methods to formally identify these windows. Previously, we found that prenatal urinary BPA concentrations were associated with externalizing behaviors in 2-year-old children and with behavioral and emotional regulation in 3-year-olds, particularly among girls (Braun et al. 2009; Braun et al. 2011). The primary objective of the present study was to extend these prior findings and identify potential windows of vulnerability to the neurotoxic effects of BPA using repeated measures of prenatal and postnatal BPA exposures and neurobehavioral assessments at age 8 years.
Methods
Study Participants
We used data collected from mothers and their children who were participating in the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort in the Cincinnati, Ohio, metropolitan area designed to study the health effects of environmental exposures. We previously described eligibility criteria and participant recruitment and follow-up (Braun et al. 2016). In summary, eligibility requirements included that women were ≥ 18 years of age, at 13–19 weeks of gestation, living in the Cincinnati, Ohio area in a home built before 1978, not on medications for thyroid disorders or seizures, planning to continue prenatal care and deliver at the collaborating clinics and hospitals, planning to live in the Cincinnati area for the next year, fluent in English, and had no diagnosis of diabetes, bipolar disorder, schizophrenia, HIV infection, or cancer that resulted in radiation treatment or chemotherapy. The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved this study. The Centers for Disease Control and Prevention (CDC) IRB relied on the determination made by the CCHMC IRB. All mothers provided written informed consent for themselves and their children.
Urinary BPA Concentrations
We collected up to 2 urine samples from mothers at 16- and 26-weeks of pregnancy between March 2003 and January 2006 and averaged BPA concentrations from these two measurements to obtain an estimate of prenatal BPA exposure for the present study. We collected up to 6 urine samples from children between 2004 and 2014 at annual clinic visits from 1–5 years of age and again at 7.5–10 years of age (the “8-year” visit). We collected urine onto Kendall abdominal pads placed inside the diaper for non-toilet trained children, a training potty lined with inserts for children who were being toilet trained, or directly into polypropylene specimen cups for children who were toilet trained and adults. BPA was not detected in the inserts prior to sample collection. Urine samples were stored at −20°C until shipment to the CDC Division of Laboratory Sciences, where they were stored at or below −20°C until analysis. We followed all provisions described in Ye et al. (2013) to minimize external contamination during sample storage and analysis.
The concentrations of total (free plus conjugated) BPA were measured at the CDC using analytical chemistry methods described previously (Ye et al. 2005). The limit of detection (LOD) was 0.4 ng/mL for samples collected at the prenatal and 1–5 year visits and 0.1 ng/mL for the 8-year visit; concentrations below the LOD were assigned a value of LOD/√2 (Hornung and Reed 1990). More than 90% of women and children had detectable urinary BPA concentrations at our time points of interest (Braun et al. 2011, Stacy et al. 2016). Each analytic batch included low and high concentration quality control (QC) samples, and the coefficients of variation of repeated QC samples were less than 10%. We measured free BPA to evaluate potential external contamination in the first batch of samples, but free BPA concentrations were either undetectable or very close to the LOD. For subsequent analytic batches, we repeated total extractions only to check higher than expected results (Sathyanarayana et al. 2011), which never suggested contamination. We measured urinary creatinine concentrations using a previously described assay (Larsen 1972). Urinary BPA concentrations were divided by creatinine concentrations to account for urine dilution and log10-transformed for statistical analysis.
8-Year Neurobehavioral Outcomes
We assessed children’s behavior, executive function, and cognitive abilities at 7.5 to 10 years of age. Detailed descriptions of the scales and traits measured on each neurobehavioral assessment are available in the supplement (Table S1). Previous studies have shown that the traits measured using these assessments, briefly described below, are associated with prenatal exposure to environmental chemicals, including BPA (Braun et al. 2011; Dietrich et al. 2005; Harley et al. 2013). Some of these tests assess omnibus features of child cognitive function, such as IQ, while others measure specific features of behavioral disorders like Attention Deficit Hyperactivity Disorder (ADHD).
We evaluated children’s behavior using the Behavioral Assessment for Children-2 (BASC-2), a 160-item, valid, reliable, parent-report assessment of a child’s adaptive and problem behaviors in community and home settings (Reynolds and Kamphaus 2004). We analyzed three composite scales from the BASC-2: Externalizing Problems, which reflects disruptive behavior problems; Internalizing Problems, such as depression and anxiety; and the Behavioral Symptoms Index, a measure of a child’s overall level of problem behaviors. To assess executive function, we used the Behavior Rating Inventory of Executive Function (BRIEF), an 86-item, parent-report inventory that includes 8 clinical scales and 3 composite scales (Strauss et al. 2006). We analyzed the 3 composite scores from the BRIEF: the Behavioral Regulation Index, Metacognition Index, and Global Executive Composite, a summary score for the former two composites. Finally, we administered the Wechsler Intelligence Scale for Children-IV (WISC-IV) to evaluate children’s overall intellectual ability (Full-Scale IQ), speed of mental and graphomotor processing (Processing Speed Index), perceptual reasoning and organization skills (Perceptual Reasoning Index), and verbal abilities (Verbal Comprehension Index). All three assessments are age-standardized by the developers and the BASC-2 is additionally sex-standardized. Lower scores on the WISC-IV indicate lower cognitive abilities and higher scores on the BASC-2 and BRIEF indicate more behavior problems.
Covariates
We considered adjusting for variables associated with BPA exposure and child neurobehavior in previous studies or believed to confound other environmental toxicant-neurodevelopment relationships (Bellinger 2004a; Stacy et al. 2016). Child’s sex was retrieved from hospital medical charts, while the remaining sociodemographic covariates (child’s race, mother’s education, household income, marital status, and prenatal vitamins) were obtained by trained interviewers. To assess gestational exposure to tobacco smoke, we calculated the mean concentration of cotinine (a metabolite of nicotine) from maternal serum samples collected during pregnancy or at birth. We administered the Home Observation for Measurement of the Environment during the 1-year home visit to assess the quality and quantity of the caregiving environment (Caldwell and Bradley 2003). We also considered measures of maternal depression, ADHD, and IQ. Maternal depressive symptoms were measured at 20 weeks of gestation using the Beck Depression Inventory-II (Beck et al. 1997). We administered the Conners’ Adult ADHD Rating Scale to mothers at the 8-year visit to assess her ADHD behaviors. Additionally, we administered the Wechsler Abbreviated Scale of Intelligence to each mother once at any of the eight visits to obtain her full-scale IQ. We considered baseline (prenatal) measurements of other sociodemographic covariates.
Statistical Analysis
We used a multiple informant model to investigate associations between repeated prenatal and postnatal urinary BPA concentrations and the 8-year neurobehavioral outcomes (Sanchez et al. 2011). This approach is applied when information gathered from multiple individuals or sources is used to measure the same construct and can be used when there are repeated and sparsely sampled environmental exposure measures at different time points (or windows) that serve as informants. Using linear regression, this method jointly estimated the difference in neurobehavioral score for a 10-fold increase in urinary BPA concentration at each time point (average prenatal and ages 1, 2, 3, 4, 5, and 8 years). The multiple informant model also provides a 6 degree-of-freedom heterogeneity p-value that assesses whether the timing of exposure modifies the BPA-outcome association. Lower heterogeneity p-values suggest that at least one of the exposure-outcome associations differs from the others (i.e., the association depends on the timing of exposure).
Using this approach, we examined associations of urinary BPA concentrations at each of our seven time points with BASC-2, BRIEF, and WISC-IV composite scores. We used the p-value of the BPA × visit interaction term (the heterogeneity p-value) to determine whether BPA-outcome associations differed across visits and considered those with p-values <0.2 to indicate that the association depended on timing (Rothman and Greenland 1998). All outcomes were analyzed as continuous variables, while each window was treated categorically. We adjusted all models for child’s sex, child’s race, mother’s education, household income, caregiving environment, marital status, maternal serum cotinine, and prenatal vitamin use. For the BASC-2 and BRIEF models, we also adjusted for maternal depression and ADHD scores, while WISC-IV models also included maternal IQ. Since we previously observed that child sex modified the association between BPA concentrations and neurobehavior in this cohort (Braun et al. 2009; Braun et al. 2011), we repeated the multiple informant analysis including all 2- and 3-way interaction terms between urinary BPA concentrations, child sex, and visit.
Secondary Analyses
We conducted several sensitivity analyses for the multiple informant model. To compare different methods of accounting for urine dilution, we adjusted all models for log10-transformed urinary creatinine as a covariate, with unstandardized log10-transformed urinary BPA as the exposure variable. We also repeated the analysis by 1) jointly adjusting for prenatal and 8-year BPA in the same model and 2) further adjusting for year of birth, since BPA exposure may have been decreasing over the period of the study due to changes in manufacturing practices and personal behaviors. Given the speed of fetal and placental growth and development during the prenatal period, we also repeated the analysis considering urinary BPA concentrations separately at 16- and 26-weeks of gestation.
It is possible children with certain behavioral problems may have higher urinary BPA concentrations because they are more likely to engage in behaviors (e.g., eating specific foods) that increase their BPA exposure. To address this possibility of reverse causality in our cross-sectional analysis of data at age 8 years, we conducted a sensitivity analysis adjusting for a summary variable of potential BPA exposures in the past 24 hours, which included consuming canned food, canned beverages, and beverages in carton or pouch, and receipt handling, variables previously found to be associated with higher child urinary BPA concentrations in the HOME Study (Stacy et al. 2016). Finally, we also adjusted the 8-year analysis for the number of cans of canned vegetables consumed in the last 24 hours (0, >0 to <0.5, ≥0.5) and the prenatal analysis for frequency of maternal canned vegetable consumption (≤1–3 times per month, 1–3 times per week, ≥4–6 times per week).
Results
Descriptive Statistics
Out of 1,263 eligible women, 468 enrolled in our study (37%) between March 2003 and January 2006. Of these, 67 dropped out before delivery, and we excluded 9 twins, 3 stillbirths, and 2 infants with congenital or genetic anomalies. Distributions of maternal and child urinary BPA concentrations are available in the Supplemental Material (Table S2), and we previously found BPA concentrations in HOME Study children decreased as they got older, with children’s median BPA concentrations at 8 years of age being similar to median BPA concentrations among the mothers during pregnancy (Braun et al. 2011; Stacy et al. 2016).
Among the remaining 387 singleton children and their mothers, 228 (59%) parents completed the BASC-2 and BRIEF and 220 (57%) children completed the WISC-IV at 7.5 to 10 years of age. Of the 228 children in our sample, 40% (n=91) had all 7 BPA measures, 23% (n=53) had 6 measures, 14% (n=31) had 5 measures, 14% (n=31) had 4 measures, 6% (n=14) had 3 measures, and 3% (n=8) had 2 measures. The median 8-year urinary BPA concentration (25th, 75th percentile) was 1.6 (1.0, 3.6) ng/mL. Their mothers had a median prenatal BPA concentration of 2.1 (1.0, 3.5) ng/mL (Table 1). Urinary BPA concentrations during pregnancy and childhood had a 1.8 to 3.2 order of magnitude range. The demographic composition of the participants who completed follow-up at age 8 years was similar to the overall cohort (Braun et al. 2016).
Table 1.
Prenatal maternal and child 8-year urinary BPA concentrations and 8-year Behavioral Symptom Index (BSI), Global Executive Composite (GEC), and Full-Scale IQ (FSIQ) scores according to sociodemographic, caregiving, maternal, and behavioral factors.
| Covariate | Prenatal BPA (ng/mL) | 8-Year BPA (ng/mL) | BSI Score | GEC Score | Full-Scale IQ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (25th, 75th) | N | Median (25th, 75th) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Overall | 228 | 2.1 (1.0, 3.5) | 222 | 1.6 (1.0, 3.6) | 228 | 50 (9) | 228 | 48 (11) | 220 | 102 (16) |
|
| ||||||||||
| Child Sex | ||||||||||
|
| ||||||||||
| Female | 127 | 2.2 (0.9, 3.6) | 122 | 1.6 (1.0, 3.9) | 127 | 49 (9) | 127 | 49 (10) | 122 | 102 (16) |
| Male | 101 | 2.0 (1.1, 3.1) | 100 | 1.7 (1.0, 3.3) | 101 | 51 (9) | 101 | 48 (11) | 98 | 101 (15) |
|
| ||||||||||
| Child Race | ||||||||||
|
| ||||||||||
| White | 128 | 1.5 (0.7, 2.5) | 126 | 1.5 (0.8, 3.1) | 128 | 49 (9) | 128 | 47 (10) | 124 | 108 (12) |
| Black | 80 | 3.3 (2.4, 5.2) | 78 | 2.3 (1.3, 4.8) | 80 | 51 (10) | 80 | 51 (11) | 78 | 91 (15) |
| Other | 15 | 1.4 (1.1, 1.9) | 13 | 1.5 (1.0, 1.9) | 15 | 49 (9) | 15 | 50 (13) | 13 | 110 (16) |
|
| ||||||||||
| Maternal Education | ||||||||||
|
| ||||||||||
| College graduate Tech school/Some | 106 | 1.4 (0.7, 2.2) | 102 | 1.5 (1.0, 2.9) | 106 | 49 (9) | 106 | 46(10) | 101 | 109 (12) |
| College | 63 | 2.6 (1.4, 4.0) | 63 | 2.1 (1.1, 4.1) | 63 | 51 (10) | 63 | 50 (11) | 62 | 100 (14) |
| High school graduate | 35 | 3.1 (2.1, 5.3) | 33 | 1.6 (1.1, 3.4) | 35 | 49 (8) | 35 | 49 (10) | 33 | 90 (18) |
| Less than grade 12 | 24 | 3.2 (2.2, 5.1) | 24 | 2.4 (1.2, 3.4) | 24 | 52 (10) | 24 | 52 (9) | 24 | 90 (13) |
|
| ||||||||||
| Household Income | ||||||||||
|
| ||||||||||
| >$80K | 58 | 1.5 (0.8, 2.8) | 57 | 1.5 (0.9, 3.5) | 58 | 49 (8) | 58 | 46 (10) | 56 | 112 (12) |
| $40–80K | 75 | 1.5 (0.7, 2.7) | 71 | 1.5 (1.0, 3.0) | 75 | 49 (9) | 75 | 47 (11) | 71 | 104 (14) |
| $20–40K | 34 | 2.6 (1.6, 3.8) | 34 | 1.5 (0.7, 3.7) | 34 | 49 (8) | 34 | 50 (11) | 34 | 101 (12) |
| <$20K | 61 | 3.4 (2.2, 5.3) | 60 | 2.3 (1.4, 4.0) | 61 | 52 (10) | 61 | 51 (10) | 59 | 89 (15) |
|
| ||||||||||
| Marital Status | ||||||||||
|
| ||||||||||
| Married | 141 | 1.6 (0.8, 2.7) | 136 | 1.5 (0.9, 3.0) | 141 | 49 (9) | 141 | 47 (10) | 135 | 108 (13) |
| Unmarried, living with someone | 28 | 3.1 (2.1, 5.4) | 27 | 3.4 (1.8, 4.6) | 28 | 49 (7) | 28 | 49 (9) | 27 | 94 (17) |
| Unmarried, living alone | 59 | 3.1 (1.9, 5.0) | 59 | 1.8 (1.1, 4.0) | 59 | 51 (10) | 59 | 52 (11) | 58 | 91(15) |
|
| ||||||||||
| Caregiving Environment | ||||||||||
|
| ||||||||||
| <35 | 37 | 3.6 (2.7, 5.8) | 36 | 2.9 (1.3, 5.9) | 37 | 50 (8) | 37 | 50 (9) | 36 | 91(15) |
| 35–<40 | 42 | 2.5 (1.5, 4.0) | 42 | 1.7 (1.0, 4.0) | 42 | 52 (10) | 42 | 52 (11) | 42 | 96 (16) |
| 40+ | 133 | 1.6 (0.8, 2.7) | 128 | 1.5 (1.0, 3.0) | 133 | 49 (9) | 133 | 47 (11) | 126 | 108 (13) |
|
| ||||||||||
| Maternal BDI–II | ||||||||||
|
| ||||||||||
| Minimal: <14 | 176 | 1.8 (0.9, 3.3) | 172 | 1.6 (1.0, 3.4) | 176 | 49 (9) | 176 | 47 (11) | 170 | 104 (15) |
| Mild: 14–19 | 30 | 2.5 (1.8, 4.2) | 30 | 2.3 (1.3, 4.0) | 30 | 52 (10) | 30 | 52 (11) | 29 | 93 (16) |
| Moderate/Severe: >19 | 20 | 2.6 (1.3, 3.9) | 18 | 1.6 (1.0, 3.4) | 20 | 55 (9) | 20 | 55 (8) | 19 | 95 (15) |
|
| ||||||||||
| Maternal CAARS | ||||||||||
|
| ||||||||||
| <40 | 56 | 2.3 (1.4, 4.2) | 54 | 1.4 (0.9, 3.2) | 56 | 43 (8) | 56 | 41 (8) | 54 | 100 (17) |
| 40–45 | 62 | 2.2 (1.2, 3.5) | 60 | 1.8 (1.3, 3.7) | 62 | 50 (9) | 62 | 49 (10) | 61 | 101 (15) |
| 46–52 | 58 | 1.6 (0.7, 3.3) | 58 | 1.6 (1.0, 3.5) | 58 | 51 (7) | 58 | 49 (9) | 54 | 102 (15) |
| >52 | 52 | 2.0 (1.3, 2.8) | 50 | 1.8 (1.0, 3.9) | 52 | 55 (9) | 52 | 54 (11) | 51 | 104 (17) |
|
| ||||||||||
| Maternal FSIQ | ||||||||||
|
| ||||||||||
| <96 | 68 | 3.3 (2.3, 5.0) | 67 | 2.0 (1.3, 4.0) | 68 | 50 (10) | 68 | 50 (10) | 67 | 92 (14) |
| 96–109 | 46 | 1.8 (0.9, 2.9) | 45 | 1.6 (0.9, 4.0) | 46 | 48 (8) | 46 | 49 (11) | 44 | 98(17) |
| 109–117 | 49 | 1.8 (1.0, 3.2) | 48 | 1.6 (1.0, 3.3) | 49 | 49 (8) | 49 | 48 (11) | 47 | 108 (10) |
| >=117 | 65 | 1.3 (0.7, 2.3) | 62 | 1.5 (0.8, 2.5) | 65 | 50 (10) | 65 | 47 (11) | 62 | 110 (14) |
|
| ||||||||||
| Maternal Cotinine | ||||||||||
|
| ||||||||||
| <LOD | 78 | 1.3 (0.7, 2.2) | 75 | 1.5 (0.8, 3.0) | 78 | 49 (9) | 78 | 45 (9) | 74 | 107 (13) |
| LOD - 3 ng/mL | 123 | 2.5 (1.3, 4.0) | 121 | 1.7 (1.1, 3.7) | 123 | 50 (9) | 123 | 50 (11) | 121 | 100 (16) |
| >3 ng/mL | 27 | 2.7 (2.1, 5.3) | 26 | 2.8 (1.1, 4.1) | 27 | 51 (9) | 27 | 52 (11) | 25 | 94 (16) |
|
| ||||||||||
| Prenatal Vitamin | ||||||||||
|
| ||||||||||
| Rarely/Never | 34 | 2.7 (1.8, 4.2) | 32 | 1.9 (1.2, 3.7) | 34 | 52 (9) | 34 | 51 (10) | 32 | 91(17) |
| Weekly/Daily | 194 | 1.9 (0.9, 3.3) | 190 | 1.6 (1.0, 3.5) | 194 | 49 (9) | 194 | 48 (11) | 188 | 103 (15) |
Abbreviations: BSI=Behavioral Symptom Index, GEC=Global Executive Composite, FSIQ=Full-Scale IQ, BDI= Beck Depression Inventory, CAARS= Conners’ Adult ADHD Rating Scale
On average, children in the HOME Study had typical composite scores on the BASC-2, BRIEF, and WISC-IV tests (see Table 1). Mean composite scores from the BASC-2 and BRIEF at 8 years of age differed across sociodemographic factors and increased with worsening categories of maternal depression and ADHD scores (Table 1). Full-scale IQ scores were lowest among children that were black, from the lowest income families (<$20,000 per year), born to mothers with less educational attainment, or had mothers with lower IQ scores (Table 1).
Urinary BPA Concentrations and Neurobehavioral Outcomes
After adjustment for potential confounders, prenatal urinary BPA concentrations were generally associated with more maladaptive behaviors and impaired executive functioning, although the 95% CI of these point estimates included the null value (Figure 1 and Table S3). In contrast, prenatal BPA was not associated with WISC-IV scores. Childhood urinary BPA concentrations at 3, 4, and 8 years were positively associated with the behavioral symptoms index as well as several composite scores on the BRIEF, indicating more problematic behaviors and executive functioning (Figure 1). Children’s urinary BPA concentrations at most ages were not associated with worse WISC-IV scores, except at 8-years of age where BPA concentrations were inversely associated with overall cognition, verbal abilities, and speed of mental processing. BPA × visit interaction term p-values were <0.2 for the global executive composite and behavioral regulation index scores from the BRIEF (Table S4), suggesting that the associations between these scales and urinary BPA concentrations differed across windows of exposure.
Figure 1.
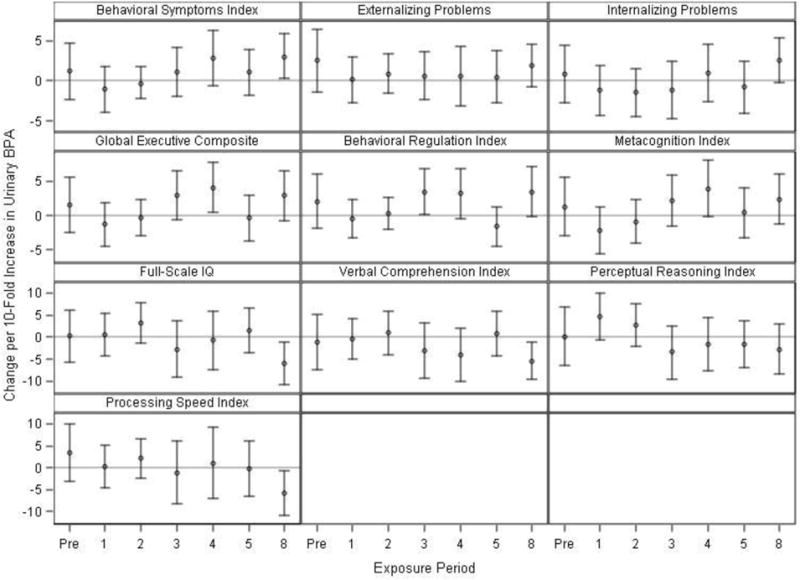
Adjusted differences in BASC-2 (row 1),1 BRIEF (row 2),1 and WISC-IV (rows 3–4)2 scores at 8 years of age with 10-fold increases in prenatal and childhood creatinine-standardized urinary BPA concentrations (Prenatal: N=202–210, Age 1: N=178–185, Age 2: N=159–165, Age 3: N=163–168, Age 4: N=131–132, Age 5: N=159–161, Age 8: N=200–204).
1Adjusted for: visit, BPA × visit, child’s sex, child’s race, mother’s education, household income, caregiving environment, marital status, prenatal serum cotinine concentrations, prenatal vitamins, mother’s BDI, and mother’s CAARS.
2Adjusted for: visit, BPA × visit, child’s sex, child’s race, mother’s education, household income, caregiving environment, marital status, prenatal serum cotinine concentrations, prenatal vitamins, and mother’s full-scale IQ.
BPA × visit interaction term p-values were 0.18 for the global executive composite and 0.16 for the behavioral regulation index, indicating that these associations depended on the timing of BPA exposure. Heterogeneity p-values were >0.2 for all other outcomes. Exact sample sizes at each visit are available in Supplemental Table S3.
Higher BASC-2 and BRIEF scores indicate worse behavior or executive function, while higher WISC-IV scores indicate better cognitive abilities.
Abbreviations: Pre=Prenatal, BASC-2=Behavioral Assessment for Children-2, BRIEF=Behavior Rating Inventory of Executive Function, WISC-IV=Wechsler Intelligence Scale for Children-IV, BDI=Beck Depression Inventory, CAARS= Conners’ Adult ADHD Rating Scale
Child’s sex modified the patterns of associations between repeated BPA measures and several of the outcomes, particularly the behavioral symptoms index and externalizing scales of the BASC-2 (BPA × sex × visit interaction terms≤0.05) and the BRIEF behavioral regulation index (BPA × sex × visit interaction term=0.17; Table S4). Prenatal urinary BPA concentrations were associated with more externalizing scores on the BASC-2 and poorer behavioral regulation scores on the BRIEF among girls (Figure 2 and Table S5). Eight-year BPA was associated with higher scores on all the BASC-2 and BRIEF scales and lower scores on all the WISC-IV scales among boys. Urinary BPA concentrations at ages 3 and 4 years were also modestly associated with higher composite and behavioral regulation BRIEF scores among boys.
Figure 2.
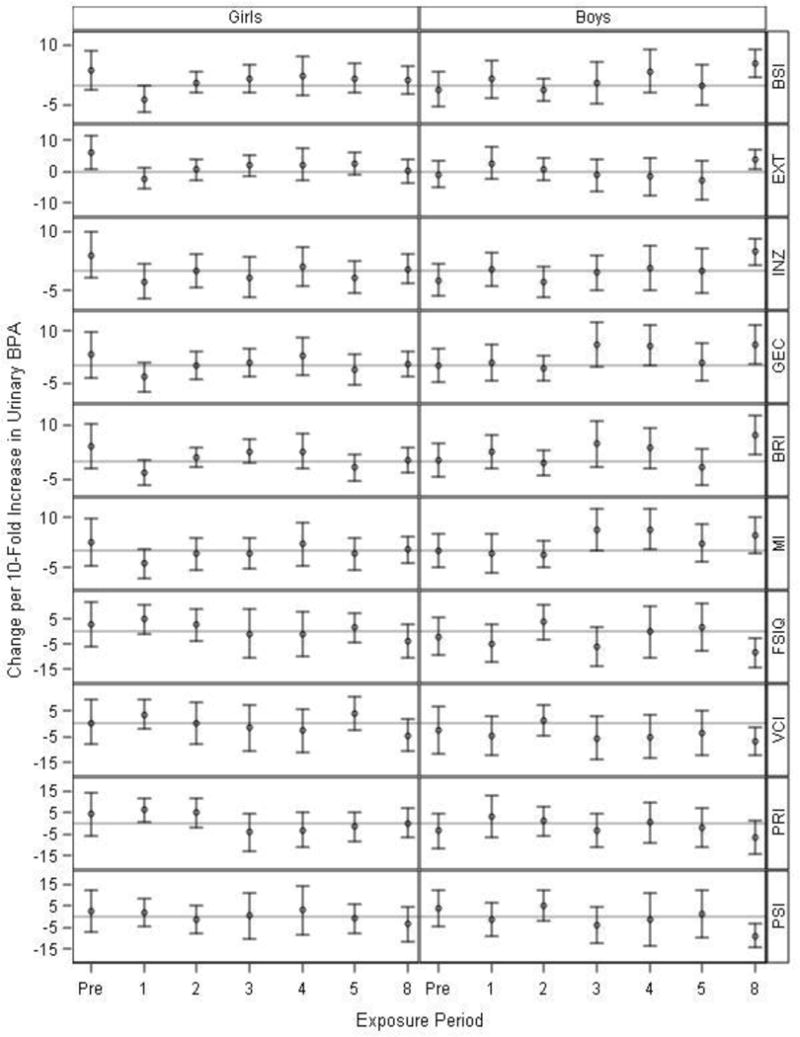
Adjusted1 difference in BASC-2,1 BRIEF,1 and WISC-IV2 IV scores at 8 years of age with 10-fold increases in prenatal and childhood creatinine-standardized urinary BPA concentrations, in girls (N=72–117) and boys (N=59–93).
1Adjusted for: visit, BPA × visit, child’s sex × visit, BPA × child’s sex, BPA × visit × child’s sex, child’s race, mother’s education, household income, caregiving environment, marital status, prenatal serum cotinine concentrations, prenatal vitamins, mother’s BDI, and mother’s CAARS.
2Adjusted for: visit, BPA × visit, child’s sex × visit, BPA × child’s sex, BPA × visit × child’s sex, child’s race, mother’s education, household income, caregiving environment, marital status, prenatal serum cotinine concentrations, prenatal vitamins, and mother’s full-scale IQ.
BPA × visit × sex interaction term p-values were 0.05, 0.02, 0.23, and 0.17 for the BSI, EXT, INZ, and BRI, respectively, indicating differences in time windows of BPA exposure between girls and boys. Heterogeneity p-values were >0.2 for all other outcomes. Exact sample sizes at each visit are available in Supplemental Table S5.
Higher BASC-2 and BRIEF scores indicate worse behavior or executive function, while higher WISC-IV scores indicate better cognitive abilities.
Abbreviations: Pre=Prenatal, BASC-2=Behavioral Assessment for Children-2, BRIEF=Behavior Rating Inventory of Executive Function, WISC-IV=Wechsler Intelligence Scale for Children-IV, BSI=Behavioral Symptom Index, EXT=Externalizing Problems, INZ=Internalizing Problems, GEC=Global Executive Composite, BRI=Behavioral Regulation Index, MI=Metacognition Index, FSIQ=Full-Scale IQ, VCI=Verbal Comprehension Index, PRI=Perceptual Reasoning Index, PSI=Processing Speed Index, BDI=Beck Depression Inventory, CAARS= Conners’ Adult ADHD Rating Scale
Secondary Analyses
Adjusting the multiple informant models for log10-creatinine to account for urine dilution did not substantially change the overall patterns of associations we observed for each outcome (Supplemental Table S6). Results using the multiple informant approach were similar after joint adjustment for prenatal and 8-year BPA in the same model and after additional adjustment for year of birth. For concision, we show results of these sensitivity analyses in Table S6 only for externalizing problems and prenatal (for girls) and 8-year BPA (for boys), since these associations were statistically significant in the primary analysis. When we investigated finer prenatal windows of vulnerability, we observed stronger associations of 16-week, as opposed to 26-week BPA, and several outcomes, including externalizing problems in girls (Table S7). The cross-sectional associations between 8-year urinary BPA concentrations and 8-year outcomes we observed among boys were similar when we further adjusted for BPA exposures in the past 24 hours (Supplemental Figure S1). Adjusting the prenatal and 8-year analyses for maternal and child canned vegetable consumption, respectively, did not appreciably change the results of these analyses (Table S6).
Discussion
Our results suggest that the sex-dependent associations of pre- and postnatal urinary BPA concentrations with child neurobehavior in this cohort may depend on the timing of exposure. Prenatal urinary BPA concentrations were associated with more maladaptive behaviors, specifically externalizing problems among girls. In boys, postnatal urinary BPA concentrations at 8 years of age were associated with more behavioral, executive function, and cognitive impairments.
The results of an increasing number of epidemiological studies suggest that BPA exposure during gestation and/or early childhood influences neurobehavioral outcomes in young children (Braun et al. 2009; Braun et al. 2011; Casas et al. 2015; Evans et al. 2014; Harley et al. 2013; Hong et al. 2013; Perera et al. 2012; Perez-Lobato et al. 2016; Roen et al. 2015). Our finding of an association between prenatal BPA exposures and worse externalizing problems at 8 years of age among girls agrees with previous findings in the HOME Study (Braun et al. 2009; Braun et al. 2011). Another study of prenatal BPA and early infant neurobehavior in the HOME cohort found a trend toward increased infant hypotonia with increasing prenatal BPA concentrations (Yolton et al. 2011). Other studies have reported associations between prenatal BPA exposure and increases in behavioral problems primarily in boys (Casas et al. 2015; Evans et al. 2014; Harley et al. 2013; Perera et al. 2012). In a cohort of 198 low income, African-American and Dominican women and their children, prenatal BPA exposure was associated with higher emotional reactive and aggressive behaviors in boys, but lower scores in girls (Perera et al. 2012). Similarly, other investigators reported that maternal urinary BPA concentrations were associated with increases in externalizing and internalizing behaviors in mainly Caucasian boys (Evans et al. 2014). Miodovnik et al. did not find an association between maternal urinary BPA concentrations and neurobehavior in 7 to 9-year-old inner-city minority children, although their focus was social impairment related to autism spectrum disorders (Miodovnik et al. 2011).
Previous epidemiologic studies have found associations between postnatal exposure to BPA and neurobehavior primarily among girls (Mustieles et al. 2015). In our study, urinary BPA concentrations at approximately 3, 4, and 8 years of age were associated with several measures of behavior and executive function among boys. Eight-year BPA was also associated with lower WISC-IV scores in boys. Associations between childhood BPA and WISC-IV scores were mostly null among girls, except for BPA concentrations at age 1 year being associated with improved perceptual reasoning index scores. Harley et al. found that urinary BPA concentrations in 5-year-old children were associated with increased internalizing behaviors, inattention, and hyperactivity in both boys and girls at 7 years of age and with externalizing behaviors in girls (Harley et al. 2013). In an inner-city population, Roen et al. found that high postnatal urinary BPA concentrations were associated with increased internalizing and externalizing scores among 7 to 9-year-old girls (Roen et al. 2015). In another investigation, childhood urinary BPA concentrations in approximately 300 school-aged Spanish children were associated with more internalizing symptoms and with thought and social problems using the Child Behavior Checklist, although the study was cross-sectional and only included boys (Perez-Lobato et al. 2016). Urinary BPA concentrations have also been positively associated with ADHD diagnosis and behaviors in a nationally representative sample of U.S. boys (Tewar et al. 2016). Some studies have not found a relationship between postnatal BPA exposure and some aspects of neurobehavior in children (Braun et al. 2011; Perera et al. 2012). Discrepancies in the literature could be partly due to differences in the characteristics of the study populations (e.g. race, socioeconomic status) or study designs, including differences in the timing of exposure or outcome assessment, types of neurobehavioral assessments, or BPA exposure misclassification.
Our findings are consistent with some experimental animal studies reporting increased hyperactivity, aggressive behaviors, and memory impairment in offspring following low-dose BPA exposure during gestation or lactation (Anderson et al. 2013; Gonçalves et al. 2010; Kundakovic et al. 2013; Poimenova et al. 2010; Wang et al. 2016). The doses evaluated in these animal studies ranged from 2 to over 500 μg/kg body weight/day, while estimated median intakes in the HOME Study and other human populations are in the 10s to 100s of ng/kg body weight/day range. For example, Kundakovic 2013 exposed pregnant mice to several doses of BPA, two of which (2 and 20 μg/kg bw/day) were less than the reference dose for BPA of 50 μg/kg bw/day (US EPA 2016), and found that low-dose prenatal BPA exposure induced enduring epigenetic disruption in the brain that might explain BPA’s effects on brain function and behavior in offspring. However, the doses these animals were exposed to are several orders of magnitude higher than the estimated median intakes in humans. A study of rats found that maternal BPA exposure led to impairment of object recognition memory in male offspring, which the authors attribute to inhibition of the hippocampal extracellular regulated kinase pathway, believed to play a critical role in synaptic plasticity, learning, and memory (Wang et al. 2016). A recent study of postnatal BPA exposure in male mice attributed the impairment of spatial memory and adverse effects on synaptic remodeling of hippocampal neurons to BPA’s anti-androgen effects (Fang et al. 2017).
BPA’s ability to disrupt gonadal hormone pathways may explain the sex-dependent results we observed in the present study as these hormones play pivotal roles in sexually dimorphic neurodevelopment (Cohen-Bendahan et al. 2005; Zhang et al., 2011). Experimental animal studies have also reported sex-specific effects following BPA exposure (Arambula et al. 2016), although it is unclear as to whether exposure to BPA and other endocrine disruptors reduces, eliminates, or widens sex differences in behaviors (Palanza et al. 2008). Disruption of thyroid hormone homeostasis may be another mechanism through which BPA affects neurodevelopment (Chevrier et al. 2013; Romano et al. 2015). Deficiencies in thyroid hormones can alter cell migration in the developing brain and affect neuronal cell differentiation (Henrichs et al. 2013). We speculate, but could not confirm with these data, that BPA may have sex-specific associations with neurobehavior due to its ability to affect sex-specific neurodevelopmental mechanisms. However, we previously observed that prenatal BPA concentrations were associated with reduced cord serum thyroid stimulating hormone concentrations among girls in this cohort (Romano et al. 2015).
To date, few studies have comprehensively and systematically evaluated pre- and postnatal windows of heightened vulnerability to BPA exposure and neurobehavior in children. Our longitudinal data enabled us to study associations at seven potentially sensitive time points from the 2nd trimester through age 8 years. We were able to apply a novel approach used in previous investigations of environmental exposures and health outcomes (Sánchez et al. 2011). This approach could be useful in future studies exploring windows of vulnerability to BPA exposure or other endocrine disruptors and health outcomes in children. Using the multiple informant model, we identified the prenatal period as an important window of vulnerability to the neurotoxic effects of BPA among girls and several relevant postnatal windows (ages 3, 4, and 8) among boys. Few prior studies have examined the relations between BPA exposure and child cognitive abilities, and we found an association between postnatal BPA exposure and reduced cognition among boys. Although further studies are needed to confirm these findings, identifying windows of vulnerability could inform population- or individual-level interventions to reduce BPA exposure during these time periods. Even the more subtle effects of exposure could have substantial population-level impacts by shifting the distribution of continuous neurobehavioral traits and thus increasing the risk for learning disabilities and other disorders (Bellinger 2004b, Bellinger 2012).
Additional strengths of our study include the use of valid and reliable tests to assess behavior, executive function, and cognition in children and the ability to account for many sociodemographic, caregiving, and maternal mental health factors related to BPA exposure or our outcomes of interest. As with any epidemiologic study, it is possible that the observed associations are due to confounding by other neurotoxic chemicals or to unknown or unmeasured confounders; however, these other factors would have to be correlated with BPA exposure and explain additional variation in our outcomes beyond the covariates we were able to adjust for in our models. The findings of our study are also limited to neurobehavioral assessments made at 8 years of age. It is possible that associations with urinary BPA could differ if the outcomes were measured at a later time point, such as adolescence. Further, we observed cross-sectional associations between 8-year urinary BPA concentrations and BASC-2, BRIEF, and WISC-IV scores. It is difficult to imagine how concurrent BPA exposure would result in neurobehavioral deficits. We tried to reduce the possibility of reverse causality by adjusting for some specific behaviors that might be associated BPA exposure (e.g., canned food consumption), and these cross-sectional associations remained after further adjustment for these behaviors.
Although we measured urinary BPA concentrations over several time points during pregnancy and childhood, BPA has a relatively short half-life in the body (<6 hours) (Thayer et al. 2015a), and urinary BPA concentrations can vary substantially within a day for an individual. We previously observed that HOME Study child urinary BPA concentrations, measured in six serial urine samples, have a low degree of reproducibility (ICC<0.2) (Stacy et al. 2016). One study performed a surrogate category analysis of over 2,000 urine specimens from 83 Utah couples and found that 6 or more samples were required to adequately predict categories of low, medium, and high BPA exposure (Cox et al. 2015). Thus, single spot urine measurements can result in misclassification of BPA exposure. To help reduce exposure variability, we averaged 16- and 26-week maternal urinary BPA concentrations to estimate prenatal exposure, but we only have one measurement at each postnatal visit to reflect approximately year-long exposures. Future studies might consider pooling multiple urine samples from each individual and analyzing the pooled sample to reduce the potential for exposure misclassification bias (Perrier et al. 2016). Our sample size was modest, which reduced our ability to precisely estimate associations. Some years also had lower sample sizes than others, further reducing our statistical power to detect associations at these time points. Finally, we made a large number of comparisons between multiple measures of BPA exposure and neurobehavioral domains. While there are potential concerns when examining multiple exposure-outcome relations, the consistency of the associations of prenatal and 8-year BPA concentrations with multiple neurobehavioral domains suggests that these individual results may not be spurious. Further, the associations for prenatal and 8-year BPA remained when we mutually adjusted for these windows in the same models.
In conclusion, urinary BPA concentrations during gestation and early childhood were associated with impairments in behavior and executive function in children at age 8 years in this cohort; the associations differed in boys and girls. Most notably, the strength of the association between BPA and children’s neurobehavior depended on the timing of BPA exposure in this cohort. Additional follow-up of this and other cohorts should determine if early life BPA exposure is associated with an increased risk of clinically significant behaviors or other sequelae related to the constellation of maladaptive behaviors associated with BPA exposure. Future studies to validate these findings in other cohorts would benefit from using the statistical approach we employed to identify windows of heightened vulnerability to BPA or other endocrine disruptors and from collecting serial urine samples to reduce BPA exposure misclassification.
Supplementary Material
Highlights.
We propose a new method for exploring windows of vulnerability to BPA exposure.
We examine BPA-neurobehavior associations across 7 windows of early life exposure.
These associations depend on timing of BPA exposure and differ in girls and boys.
Acknowledgments
We acknowledge the technical assistance of X. Ye, A. Bishop, X. Zhou, and T. Jia (Centers for Disease Control and Prevention, Atlanta, GA) for measuring the urinary concentrations of bisphenol A.
Financial Support: This work was supported by grants R00 ES020346, R01 ES024381, P01 ES11261, R01 ES014575, and R01 ES020349 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Conflicts of Interest: The authors have no actual or potential competing financial interests.
References
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. The FASEB Journal. 2013;27:1784–1792. doi: 10.1096/fj.12-223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study. Endocrinology. 2016 doi: 10.1210/en.2016-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for primary care. Behaviour Research and Therapy. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Assessing environmental neurotoxicant exposures and child neurobehavior: Confounded by confounding? Epidemiology. 2004a;15:383–384. doi: 10.1097/01.ede.0000129525.15064.a4. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environmental Research. 2004b;95:394–405. doi: 10.1016/j.envres.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environmental Health Perspectives. 2012;120(4):501–507. doi: 10.1289/ehp.1104170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environmental Health Perspectives. 2009:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental Health Perspectives. 2011;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. Cohort profile: The Health Outcomes and Measures of the Environment (HOME) study. International Journal of Epidemiology. 2016 doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives. 2008:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Inventory Administration Manual. Little Rock, AK: University of Arkansas at Little Rock; 2003. [Google Scholar]
- Casas M, Forns J, Martínez D, Avella-García C, Valvi D, Ballesteros-Gómez A, et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the Inma-Sabadell cohort. Environmental Research. 2015;142:671–679. doi: 10.1016/j.envres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, et al. Maternal urinary bisphenol A during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environmental Health Perspectives. 2013:121–138. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neuroscience and Biobehavioral Reviews. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cox KJ, Porucznik CA, Anderson DJ, Brozek EM, Szczotka KM, Bailey NM, et al. Exposure classification and temporal variability in urinary bisphenol-A concentrations among couples in Utah: The Hope Study. Environmental Health Perspectives. 2016;124(4):498–506. doi: 10.1289/ehp.1509752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, et al. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014;45:91–99. doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Zhu Q, Gu T, Shen X, Yang Y, Liang Y, et al. Anti-androgenic effects of bisphenol-A on spatial memory and synamptic plasticity of the hippocampus in mice. Horm Behav. 2017;93:151–158. doi: 10.1016/j.yhbeh.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Gonçalves CR, Cunha RW, Barros DM, Martínez PE. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environmental Toxicology and Pharmacology. 2010;30:195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental Research. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehn RS. NHANES data support link between handling of thermal paper receipts and increased urinary bisphenol A excretion. Environmental Science and Technology. 2016;50:397–404. doi: 10.1021/acs.est.5b04059. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: How and why? Clinical endocrinology. 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Jackson MV, Deddens JA, Clark JC, Ye X, Christianson AL, et al. Urinary Bisphenol A (BPA) Concentrations among Workers in Industries that Manufacture and Use BPA in the USA. Ann Work Expo Health. 2017;61(2):164–182. doi: 10.1093/annweh/wxw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Hong YC, Kim JW, Park EJ, Shin MS, Kim BN, et al. Bisphenol A in relation to behavior and learning of school-age children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54:890–899. doi: 10.1111/jcpp.12050. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay in the presence of protein with LKB 8600 reaction rate analyser. International Journal of Clinical Chemistry. 1972;38:475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25:519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V, Pérez-Lobato R, Olea N, Fernández MF. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental Research. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environmental Health Perspectives. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lobato R, Mustieles V, Calvente I, Jimenez-Diaz I, Ramos R, Caballero-Casero N, et al. Exposure to bisphenol A and behavior in school-age children. Neurotoxicology. 2016;53:12–19. doi: 10.1016/j.neuro.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology. 2016;27:378–388. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167:741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children Manual. 2nd. Bloomington, MN: Pearson; 2004. [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environmental Research. 2015;142:739–745. doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Webster GM, Vuong AM, Zoeller RT, Chen A, Hoofnagle AN, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME study. Environmental Research. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. 2nd. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 1998. [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environmental Health Perspectives. 2011;119:409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Braun JM, Yolton K, Liddy S, Lanphear BP. Case report: High prenatal bisphenol A exposure and infant neonatal neurobehavior. Environmental Health Perspectives. 2011;119:1170–1175. doi: 10.1289/ehp.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156:1941–1951. doi: 10.1210/en.2014-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Calafat AM, Chen A, Lanphear B, Hauser R, et al. Patterns, variability, and predictors of urinary bisphenol A concentrations during childhood. Environmental Science & Technology. 2016;50(11):5981–5990. doi: 10.1021/acs.est.6b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests. 3rd. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Tewar S, Auinger P, Braun JM, Lanphear B, Yolton K, Epstein JN, Ehrlich S, Froehlich TE. Association of Bisphenol A exposure and Attention-Deficity/Hyperactivity Disorder in a national sample of U.S. children. Environmental Research. 2016;150:112–118. doi: 10.1016/j.envres.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment International. 2015a;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, Schurman S, Kissling GE, Hunt D, et al. Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) in urine and blood of cashiers. Environmental Health Perspectives. 2015b;124(4):437–444. doi: 10.1289/ehp.1409427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency Integrated Risk Information System (IRIS) Bisphenol A. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=356 (accessed June 14, 2017)
- Von Goetz N, Wormuth M, Scheringer M, Hungerbühler K. Bisphenol A: How the most relevant exposure sources contribute to total consumer exposure. Risk Analysis. 2010;30:473–487. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Li Z, Han H, Luo G, Zhou B, Wang S, et al. Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of AKT and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology. 2016:341–343. 56–64. doi: 10.1016/j.tox.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical Chemistry. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environmental Health Perspectives. 2013:121–283. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicology and Teratology. 2011;33:558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424–430. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chang H, Wiseman S, He Y, Higley E, Jones P, et al. Bisphenol A disrupts steroidogenesis in human H295R cells. Toxicol Sciences. 2011 doi: 10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


