Abstract
Studies of major depressive disorder (MDD) in postmortem brain tissue report enhanced binding to inhibitory serotonin-1A autoreceptors in midbrain dorsal raphe and reductions in length of axons expressing the serotonin transporter (SERT) in dorsolateral prefrontal cortex. The length density of axons expressing SERT in the orbitofrontal cortex (OFC) was determined in 18 subjects with MDD and 17 age-matched control subjects. A monoclonal antibody was used to immunohistochemically label the SERT in fixed sections of OFC. The 3-dimensional length density of SERT-immunoreactive (ir) axons in layer VI of OFC was estimated. The age of subjects with MDD was negatively correlated with SERT axon length (r=−0.77, p<0.0005). The significant effect of age persisted when removing four depressed subjects with an antidepressant medication present at the time of death, or when removing nine depressed subjects that had a recent prescription for an antidepressant medication. Neither gender, tissue pH, postmortem interval, 5-HTTLPR genotype, time in fixative, nor death by suicide had a significant effect on axon length. The age-related decrease in SERT-ir axon length in MDD may reflect pathology of ascending axons passing through deep white matter hyperintensities. Greater length of axons expressing SERT in younger subjects with MDD may result in a significant deficit in serotonin availability in OFC.
Keywords: Serotonin Transporter, Major Depressive Disorder, Orbitofrontal Cortex, Age, Postmortem, Morphometry
Introduction
Clinical and basic research implicates the serotonin neurotransmitter system in the pathophysiology and treatment of major depressive disorder (MDD). Extracellular serotonin levels are regulated by the serotonin transporter (SERT), which is located either on the cell membranes presynaptically or along axons (Zhou et al., 1998). Although the most widely used treatments for MDD inhibit the SERT, there is no consistent evidence for altered radioligand binding to the SERT in cerebral cortex in MDD, as determined in postmortem brain tissue or with neuroimaging (see review by Stockmeier, 2003; and meta-analyses by Gryglewski et al., 2014; Kambeitz and Howes, 2015).
The recent meta-analyses of neuroimaging studies in MDD by Gryglewski et al., (2014) and Kambeitz and Howes (2015) establish consistent decreases in SERT binding in subcortical regions but not in the frontal cortex. However, other studies note greater SERT binding in prefrontal cortical and subcortical regions in a subgroup of depressed patients with highly negativistic dysfunctional attitudes (Meyer et al., 2004) or a greater seasonal increase in SERT binding in patients with seasonal affective disorder (Tyrer et al., 2016a). In a comprehensive review of studies of neuroimaging in humans, rodent models, and neuroimmunology, Savitz and Drevets (2013) found support for the hypothesis that function at the SERT is increased in depression. Further, in neuroimaging studies, it appears that alterations in SERT binding are found only in subgroups of depressed patients and in discrete brain regions.
In contrast to studies examining living subjects with depression, research in postmortem tissues from younger suicide victims with MDD that were antidepressant drug-free shows a significant decrease in radioligand binding to SERT across all cortical layers in the orbitofrontal cortex (OFC) vs. normal control subjects (Underwood et al., 2012) or OFC and dorsolateral prefrontal cortex in subjects with MDD where no monoamine-related antidepressant drug was present at death (Mann et al., 2000). In an immunohistochemical assessment of regional expression of SERT, there was a reduction in the overall length of axons immunoreactive for SERT in one layer only (VI) of the dorsolateral prefrontal cortex of suicide victims with MDD (Austin et al., 2002).
The present study was undertaken to test the hypothesis that the length of SERT immunoreactive (-ir) axons is lower in OFC in subjects with MDD, some of whom died by suicide. Orbitofrontal cortex was also selected as a region of interest in this study because of previous observations in OFC of reduced neuronal density and sizes in subjects with MDD (Rajkowska et al., 1999; Rajkowska et al., 2005; Underwood et al., 2012). In addition, there was a significant age-related decrease in neuronal density in OFC in subjects with MDD but not in control subjects (Rajkowska et al., 2005). Most subjects examined in the present study were the same as those studied in Rajkowska et al. (1999; 2005). The 5HTTLPR genotype was also assessed as there is evidence that the 5HTTLPR polymorphism affects SERT expression (Lesch et al., 1996). Older subjects with MDD have more frontal deep white matter hyperintensities than age- matched controls (Krishnan et al., 1988; Rabins et al., 1991; O’Brien et al., 1996; Thomas et al., 2002; Tupler et al., 2002; van Agtmaal et al., 2017). Increases with age in deep white matter hyperintensities in depression may induce pathology in ascending serotonergic axons from the midbrain raphe system projecting to the OFC.
Experimental Procedures
Human Subjects
The Declaration of Helsinki was adhered to for all experiments involving human subjects. The Institutional Review Boards of University Hospitals Case Medical Center, Cleveland, OH, and the University of Mississippi Medical Center approved the research protocol for recruitment of next-of-kin, collection of brain tissue, and informant-based interviews. The left orbitofrontal cortex was sampled from 17 psychiatrically-normal control subjects and 18 age-matched subjects that met clinical criteria for MDD at autopsy at the Cuyahoga County Medical Examiner’s Office (Cleveland, OH). Informed consent was acquired from all legally-defined next-of-kin to permit tissue collection and informant-based retrospective diagnostic interviews. The Diagnostic and Statistical Manual of Mental Disorders (4th ed.)(DSM-IV; APA, 1994) was administered regarding all subjects by a trained interviewer using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995), as described (Cobb et al., 2013). Consensus diagnosis with the aid of medical records was used to determine lifetime and recent Axis I psychopathology. Information on psychoactive substance use and history of medications was collected from informants and medical records. Head trauma, neurologic or neuropathological disease were exclusion criteria. Eighteen subjects met criteria for a lifetime diagnosis of MDD and 16 met criteria for a major depressive episode in the last month of life. Psychotic features were also present in two subjects with MDD. Four subjects with depression also experienced another Axis I disorder (Table 1). None of the control subjects ever met DSM-IV criteria for MDD or other disorders with the exception of the following: one subject was diagnosed with alcohol dependence 3 years prior to death and another was diagnosed with alcohol abuse at 30 years prior to death. These alcohol disorders were in full remission at the time of death. Suicide was ruled the cause of death by the medical examiner for 8 of the 18 subjects with MDD, and all causes of death are noted in Table 1. The medical examiner’s office evaluated urine and blood for the evidence of psychotropic medications or psychoactive substances. A prescription for an antidepressant medication was written by a physician within the last month of life for 9 of the 18 subjects with MDD, however, an antidepressant or antipsychotic medication was detected postmortem in only four subjects (Table 1). Control and MDD subjects were matched as closely as possible for age and postmortem interval. Table 1 also include data regarding gender, tissue pH, storage time in formalin (TF), and the time tissue sections were stored in ethanol. Laboratory personnel were blinded to diagnosis and age-matched pairs of coded control and depressed subjects were yoked for all subsequent assays and morphometric analyses.
Table 1.
Demographic and disease characteristics of control and MDD subjects.
| Parameter | Controls (n=17) | MDD (n=18) |
|---|---|---|
|
| ||
| Age (years) (range) | 55 ± 5 (27 – 86) | 57 ± 4 (30 – 87) |
|
| ||
| Gender (F:M) | 7:10 | 9:9 |
|
| ||
| PMI (hrs) (range) | 20 ± 1 (11 – 27) | 20 ± 1 (10 – 26) |
|
| ||
| Tissue pH (range) | 6.74 ± 0.05 (6.32 – 7.01) | 6.57 ± 0.05 (6.24– 6.97) |
|
| ||
| TF (months) (range) | 36 ± 7 (7 – 103) | 19 ± 2 (7 – 43) |
|
| ||
| Time in Ethanol (years) (range) | 11± 0.6 (7 – 14) | 13 ± 0.4 (10 – 16) |
|
| ||
| Cause of death | Cardiovascular disease n=13; asthma n=1; accidental electrocution n=1; homicide by firearm n=1; pulmonary thromboembolism n=1 | Suicide n=8 (firearm n=2; CO poisoning n=2; hanging n=2; drowning n=1; drug overdose n=1 ) |
| Other causes n=10 (cardiovascular disease n=7; pulmonary thromboembolism n=1; homicide by firearm n=1; undetermined n=1) | ||
|
| ||
| Psychiatric Diagnosis | None (n=15) | MDD (n=18) |
| Remote history of alcohol dependence (n=1) | MDD plus alcohol dependence (n=2) | |
| Remote history of alcohol abuse (n=1) | MDD plus polysubstance dependence (n=2) | |
|
| ||
| Duration of MDD (years) (range) | Not applicable | 16.8 ± 3.5 (0.17 – 50) |
|
| ||
| Antidepressant drug history | None | n=9 |
|
| ||
| Postmortem toxicology | None | n=4 (amitriptyline n=1; nortriptyline n=1; sertraline n=2; chlorpromazine n=1) |
Data represent the mean ± S.E.M. Abbreviations: CO - carbon monoxide; F - female; M - male; MDD - major depressive disorder; PMI - Postmortem interval; TF - Time in fixative;
Immunohistochemistry
The ventral portion of the left prefrontal cortex containing OFC (Uylings et al., 2010) was dissected at autopsy and fixed in 10% phosphate-buffered formalin. The blocks of tissue were then embedded in 12% celloidin as described (Rajkowska and Goldman-Rakic, 1995). Blocks were sectioned coronally at 40 µm, and sections of caudal OFC were stored in 70% ethanol. Three sections per subject located about 400 µm apart were selected for removal of celloidin and subsequent immunohistochemistry (Miguel-Hidalgo and Rajkowska, 1999). Three additional sections immediately adjacent to those selected for immunohistochemistry were stained for Nissl substance with cresyl violet acetate and used to delineate the borders of layer VI. Free floating sections were immunostained for axons expressing SERT using the SERT-51 monoclonal antibody (MAB Technologies, Stone Mountain, GA). Briefly, sections were incubated for 48 hours at 4°C with the primary SERT-51 anti-mouse monoclonal antibody at a dilution of 1:300. Binding of the primary antibody was detected with the biotinylated rabbit anti-mouse secondary antibody (dilution 1:200) according to the ABC method (Vectastain Universal Elite ABC kit-PK6200; Burlingame, CA) using 3′3′-diaminobenzidine enhanced with nickel ammonium sulfate. SERT-immunolabeled sections were mounted on glass slides and cover-slipped. To minimize inter-assay variability in the intensity of staining, each experiment included yoked sections from age-and gender-matched MDD and control subjects. The specificity of the anti-SERT antibody was tested by performing parallel immunohistochemical assays where either the primary antibody or the secondary antibody was omitted. In both cases, immunoreactivity was absent.
Morphometric Analysis
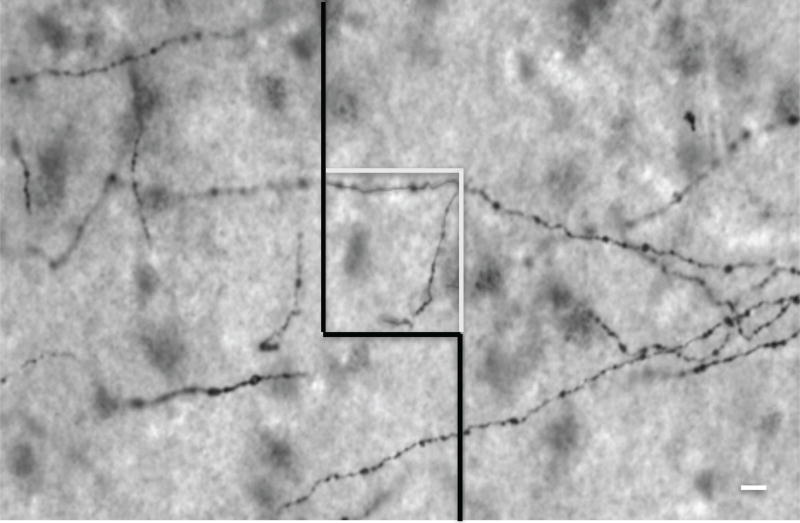
Contours outlining the lateral, caudal OFC and its layer VI were drawn on the printed images of every SERT-ir tissue section using adjacent Nissl-stained sections as a guide. Caudal OFC was distinguished from surrounding cortical areas based on cytoarchitectonic criteria of Brodmann area 47 located on the medial orbital gyrus (Uylings et al., 2010; see Figure 1A in Rajkowska et al., 2005). The thickness of the entire ORB gray matter and of layer VI was measured on SERT-ir sections along a line perpendicular to the cortical surface at three selected levels in each subject. Outlines of layer VI were then traced in every SERT-ir tissue section under the 20x objective of a Nikon microscope (E600) using StereoInvestigator software (version 11.03; MBF Bioscience, Williston, VT). The Optical Fractionator method of StereoInvestigator software was then used to estimate the length density of SERT-ir axons. Length density (mm/mm3) was determined under a 40X oil objective (numerical aperture 1.0) by measuring the 3-dimensional length of the axon through the depth of the section within 21 to 35 3-dimensional virtual counting boxes (50 × 50 × 15 µm) per section that were systematically and randomly superimposed on the contour of layer VI (Figure 1). Layer VI was chosen for analysis because Austin et al. (2002) showed a significant reduction in SERT-ir axon length in this particular layer of the prefrontal cortex in MDD. The length of axon segments from these sampling boxes was summated and expressed per total mm3 volume of tissue assessed, and then expressed as length density (mm axon length/mm3 of tissue). Values of average length density from three sections per brain were used for statistical analysis. The 3-dimensionally determined axon length density will be referred to as “axon length” for the remainder of this manuscript. The coefficient of error (mean ± S.D.) was 0.119 ± 0.001 for control subjects and 0.109 ± 0.001 for subjects with MDD. The mean coefficient of error represents the mean of 35–37 sampling frames per subject group.
Figure 1.
Photomicrograph of axons expressing the serotonin transporter (SERT) in layer VI of the orbitofrontal cortex as visible using the 40× objective of the microscope. The square located in the center of the image represents a 3-dimensional counting box. The 3-dimensional length density of all SERT-immunoreactive axons was determined within randomly initiated and systematically placed counting boxes. Scale bar = 10 µm.
5-HTTLPR genotyping
DNA was isolated from frozen right dorsolateral prefrontal cortex from the same subjects for genotyping using the QIAamp DNA mini kit (Qiagen, Hilden, Germany). Genotyping of 5-HTTLPR was done using PCRx enhancer solution, 0.8 mM MgSO4, amplification buffer, 200 µM dNTPs, 0.625U Platinum Taq PCRx polymerase (ThermoFisher Scientific, Waltham, MA) and 2 pmol of each respective PCR primers: forward, 5'-AAC GTG GGA GGC AGC AGA CAA CT-3' and reverse, 5'-GGG ATG CGG GGG AAT ACT GGT −3'. Cycling conditions were: 5 min at 94°C; 10 cycles of 94°C for 30 sec, 70°C – 0.5°C/cycle for 30 sec, 72°C for 30 sec; 30 cycles of 94°C for 30 sec, 65°C for 30 sec , 72°C for 30 sec; 5 min at 72°C. The expected PCR products are 515-bp and 472-bp for the long or short form, respectively.
Statistical Analysis
The main goal of this study was to compare SERT-ir axon length between MDD and control subjects across age. Subject characteristics between cohorts (MDD vs control) were compared using student t-tests for continuous variables and chi-square tests for categorical variables. To examine differential effects of the depression group across age on SERT-ir axon length, linear regression models were fit with main effects of cohort, age (in years), and an interaction term between cohort and age. Adjusted models included potential confounders of gender, postmortem interval, months in fixative, days in ethanol, tissue pH, and section thickness, and were compared to unadjusted estimates. We additionally examined MDD effects across death by suicide by using similar models with a three-level categorical cohort definition of control, MDD-nonsuicide, and MDD-suicide. A sensitivity analysis to potential effects of antidepressant medications was also conducted by removing the 4 subjects with MDD that had an antidepressant medication present in blood at the time of death. Lowess smoother diagnostics indicated no strong departure from linearity assumptions in the regression models. The thickness of gray matter, either all 6 layers or layer VI, was compared between the two cohorts using analysis of covariance (ANCOVA) with age as the covariate. D’Agostino; & Pearson omnibus normality test confirmed that all thickness- related values were normally distributed. Genotype frequencies for both cohorts were assessed using the Chi-squared test.
Results
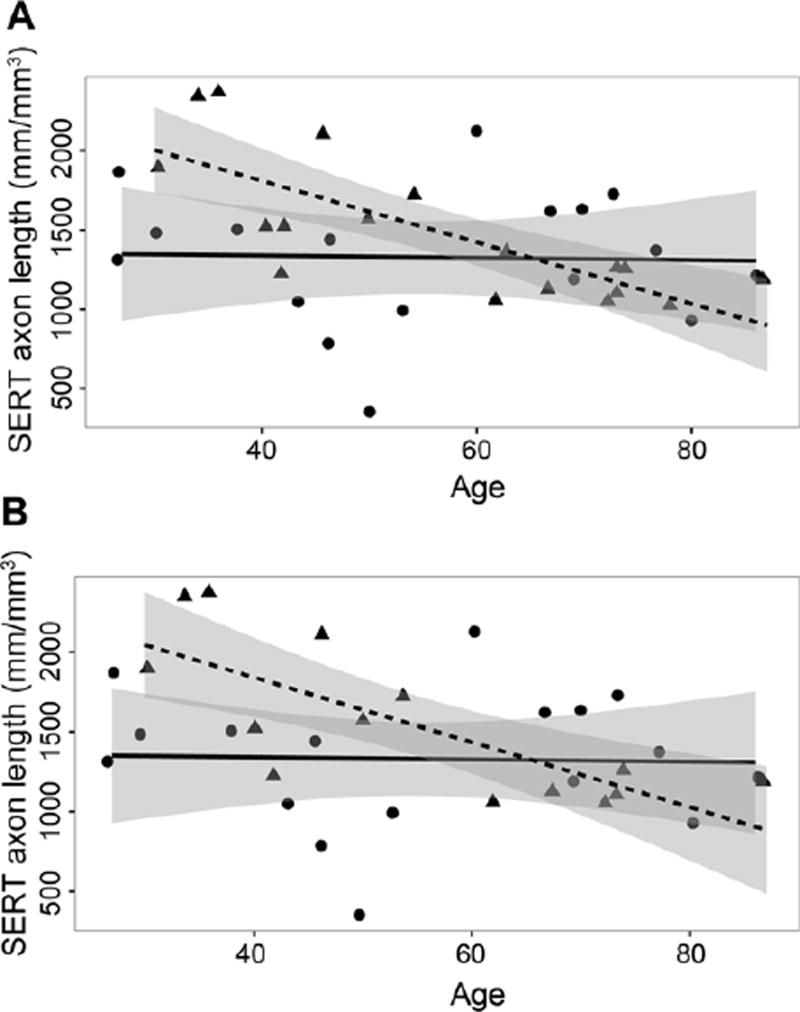
There were no significant differences between the MDD and control cohorts in age (t=0.231, df=33, p>0.05), postmortem interval (t=0.349, df=33, p>0.05), or gender (t=0.511, df=33, p>0.05) (Table 1). However, the two cohorts were significantly different regarding time in fixative (t=2.455, df=33, p<0.05), time that sections were stored in ethanol (t=2.792, df=33, p<0.01), and tissue pH (t=2.436, df=33, p<0.05) (Table 1). To determine whether these variables influenced the main outcome of this study, correlations between each of the variables and the length of SERT-ir axons were investigated (Table 2). For either cohort, or a combined value for both cohorts, there were no significant Pearson correlations between the length of SERT-ir axons and postmortem interval, time in fixative, time in ethanol, or tissue pH (Table 2). However, for all subjects there was a significant negative correlation between SERT-ir axon length and age (r= −0.38, p<0.05). This negative correlation was driven by the subjects with MDD (r= −0.77, p<0.001; Figure 2A, triangles) and not by control subjects (r= −0.03, p>0.05; Figure 2A, circles).
Table 2.
Correlations between demographic/storage characteristics and SERT-ir axon length.
| Age | PMI | Time in fixative |
Time in ethanol |
Tissue pH | |
|---|---|---|---|---|---|
| Axon length | |||||
| Combined | −0.38 | −0.26 | 0.08 | −0.004 | 0.047 |
| (0.01) | (0.131) | (0.644) | (0.982) | (0.788) | |
| Control | 0.03 | −0.4 | 0.25 | −0.144 | 0.35 |
| (0.905) | (0.111) | (0.329) | (0.58) | (0.157) | |
| MDD | −0.77 | −0.16 | 0.01 | −0.03 | −0.06 |
| (0.0002) | (0.533) | (0.958) | 0.916) | (0.828) |
MDD - major depressive disorder; PMI - postmortem interval (hrs). SERT-ir - serotonin transporter-immunoreactive. P values are located beneath the regression coefficients (r).
Figure 2.
Correlation between age and serotonin transporter (SERT)-immunoreactive (ir) axon length in layer VI of the orbitofrontal cortex for control and depressed subjects. A) all control subjects (circles, N=17) and all subjects with major depressive disorder (MDD, triangles, N=18), and B) all control subjects (circles, N=17) and subjects with MDD without an antidepressant drug in postmortem toxicology (triangles, N=14). For (A), there was a significant negative correlation (r= −0.77, p<0.0005) between SERT-ir axon length and age in MDD; there was no significant correlation (r= 0.03, p>0.05) between SERT-ir axon length density and age in control subjects. For (B), there was a significant negative correlation (r= −0.76, p<0.001) between SERT-ir axon length density and age in MDD; there was no significant correlation (r= 0.03, p>0.05) between SERT-ir axon length density and age in control subjects. Regression lines for control subjects are solid and those for MDD subjects are dashed. The 95 percent confidence intervals for the regression lines are shaded gray.
The effects of potentially confounding variables on the overall effect of age were also examined. The association of age and SERT-ir axon length remained statistically significant (Unadjusted model: β= −19.386, 95% confidence intervals: −29.958, −8.815, p<0.001; Adjusted model: β= −21.465, 95% confidence intervals: −32.722, −10.207, p<0.005) when including gender, postmortem interval, tissue pH, time in formalin, time in ethanol, and section thickness into the model. To account for effect sizes, we examined the association of SERT-ir axon and a 10-year natural progression in age. For each 10 years of age, SERT-ir axon length is expected to decrease by −193.864 mm/mm3 (95%Confidence Intervals: −299.5836, −88.14504; p<0.005) for subjects with MDD versus −7.112 mm/mm3 (95%Confidence Intervals: −106.6943, 92.47039; p>0.05) for control subjects.
Four depressed subjects had an antidepressant medication present in blood at the time of death. When these four subjects were removed from the overall statistical analysis, the results were highly comparable to the analysis including all subjects with MDD. There was a significant negative correlation between age and SERT-ir axon length that was driven by the subjects with MDD (r= −0.77, p<0.0005; Figure 2B, triangles) and not by control subjects (r= −0.03, p>0.05)(Figure 2B, circles). An analysis of covariance was also performed with age as the covariate, comparing nine depressed subjects with a recent antidepressant drug prescription vs. nine depressed subjects without a recent antidepressant drug prescription. SERT-ir axon length was not significantly different between these two groups (F (1,17) = 3.734, p>0.05).
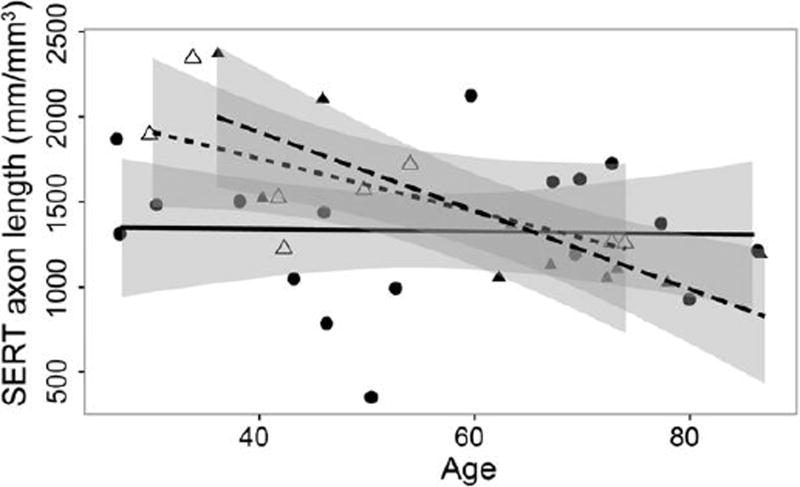
To determine whether the length of SERT-ir axons was different in those dying by suicide, cause of death was assessed in subjects with MDD. All values are mean ± S.E.M., unless otherwise noted. There was no significant difference (t=1.584, df=16, p>0.05) in the age of MDD dying by suicide (n=8; mean age was 49.9 ± 5.8) and MDD dying of other causes (n=10; 62.4 ± 5.3). There was no significant difference (t=1.001, df=16, p>0.05) in the mean length of SERT-ir axons between subjects with MDD dying by suicide and subjects with MDD dying by other causes (1,600 ± 136 vs. 1,392 ± 150 mm/mm3, respectively). There was a significant negative correlation between SERT-ir axon length and age in MDD-non-suicide (r= −0.813, p<0.005, Figure 3, closed triangles) but not in MDD-suicide (r= −0.668, p>0.05, Figure 3, open triangles). There was no significant correlation (r= 0.03, p>0.05) between SERT-ir axon length density and age in control subjects (Figure 3, circles). There was no difference between depressed subjects dying of suicide or dying of other causes in the significant association of age on SERT-ir axon length (β= −7.478, 95% confidence intervals: - 31.1142,16.15914, p>0.05).
Figure 3.
Influence of suicide on correlation between age and serotonin transporter (SERT)- immunoreactive (ir) axon length density in layer VI of the orbitofrontal cortex for control and depressed subjects. All control subjects (N=17) are represented by circles and solid line, subjects with major depressive disorder dying by suicide (MDD, N=8) are represented by open triangles and short-dashed line, and subjects with MDD dying by other causes (N=10) are represented by closed triangles and long-dashed line. There was a significant negative correlation between SERT-ir axon length density and age in MDD-non-suicide (r= −0.813 p<0.005) but not in MDD-suicide (r= −0.668 p>0.05). There was no significant correlation (r= 0.03, p>0.05) between SERT-ir axon length density and age in control subjects.
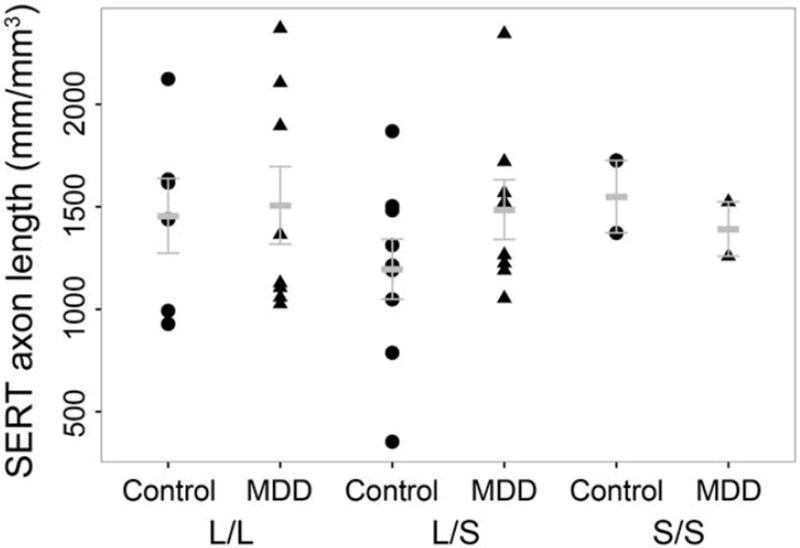
Frequencies for the 5HTTLPR genotypes for both groups were in Hardy-Weinberg equilibrium (controls, p>0.05; Chi-squared = 0.2484, p>0.05; MDD, p>0.05, Chi-squared = 0.0) and were not significantly different from each other (Chi-square = 0.3162, df=2; p>0.05). Analysis of SERT-ir axon length vs. number of 5-HTTLPR risk alleles revealed no significant correlation (r2<0.01; p>0.05) for either controls or MDD (Figure 4).
Figure 4.
Influence of 5-HTTLPR genotype on serotonin transporter (SERT)-immunoreactive (ir) axon length. Analysis of the effects of serotonin transporter genotypes (L/L, L/S, and S/S) on SERT-ir axon length density in layer VI of the orbitofrontal cortex of control subjects and subjects with major depressive disorder (MDD). Mean and S.E.M. values are presented.
There was no significant difference in thickness of gray matter of the OFC (layers I-VI) between control (1.891 ± 0.086 mm, N=17) and depressed (1.98 ± 0.09 mm, N=18) subjects (ANCOVA, F(1,32)=0.455, p>0.05). Likewise, the thickness of layer VI was not significantly different between control (0.391 ± 0.016 mm) and depressed (0.41 ± 0.019 mm) subjects (ANCOVA, F(1,32)=0.539, p>0.05). Finally, there was no significant correlation between overall thickness of gray matter and age, or thickness of layer VI and age in either cohort (data not shown).
Discussion
The length density of SERT-ir axons was examined in layer VI of the OFC in subjects with MDD and control subjects. There was a significant negative correlation between age and the length of SERT-ir axons in subjects with MDD but not in age-matched control subjects. The negative correlation between age and SERT-ir axon length persisted when removing the four depressed subjects in which a psychotropic medication was present at death, and for non-suicide victims with MDD.
The origin of serotonergic axonal fibers in rodent, non-human primate, and human frontal cortex have been identified in the dorsal raphe nucleus and median raphe nuclei (Azmitia and Gannon, 1986; Wilson et al., 1989; Mamounas et al., 1991; Wilson and Molliver, 1991a; Zhou et al., 1998). The laminar distribution of axonal fibers is distinct in non-human primate and human association cortex, with beaded axons, from the median raphe nucleus, predominantly located in layer I, and fine axons, from the dorsal raphe nucleus, predominantly located in layers II-VI (Wilson and Moliver, 1991b; Raghanti et al., 2008). These axons can be labeled in their entirety with an antibody or with a radioligand to the serotonin transporter (Zhou et al., 1998). Thus, in the current study, the axons of layer VI of the OFC labeled with an antibody to SERT likely originated in the dorsal raphe nucleus and are so-called fine axonal fibers.
In the present study, the greater length of likely fine SERT-ir axons in layer VI of OFC in younger subjects with MDD may be related to pathology at the cell bodies of origin in the brainstem. Underwood et al. (1999) noted a significantly greater number of serotonin neurons in the dorsal raphe nucleus in suicide victims, most of whom were younger and diagnosed with MDD. The greater number of serotonin neurons in younger depressed subjects might be responsible for the enhanced age-dependent length of SERT-ir axons in younger MDD noted in the present study. Greater SERT-ir axon length in younger subjects with MDD may provide more available serotonin transporters to remove serotonin from en passant and terminal bouton types of synapses and be a critical feature of the pathophysiology of MDD. The loss of serotonin or pathology in the serotonin-1B receptor can alter serotonergic innervation in various brain regions (Ase et al., 2001; Migliarini et al., 2013). There is a strong depression-linked phenotype in a mutant mouse model of serotonergic hypofunction induced by overexpression of SERT in prefrontal cortex (Mouri et al., 2012). Expression of SERT is regulated by microRNA 135 (miR135). Knockdown of miR135a in serotonin neurons in the mouse brainstem raphe results in overexpression of mRNA and protein for SERT and anxiety-like behavior (Issler et al., 2014). Interestingly, levels of miR135a are significantly decreased in the dorsal raphe of suicide victims with affective disorder (Issler et al., 2014), suggesting there may be an overexpression of SERT in axonal projection areas such as orbitofrontal cortex that might contribute to altered SERT-ir axon length.
Additional cell pathological mechanisms may underlie the age-dependent changes noted in SERT-ir axon length in MDD. Other evidence of pathology of dorsal raphe neurons in younger subjects with MDD is provided by studies showing greater serotonin-1A autoreceptor binding in postmortem midbrain from suicide victims with MDD, suggesting that these neurons are under enhanced inhibitory control which may result in diminished release of serotonin into prefrontal cortex (Stockmeier et al., 1998; Boldrini et al., 2008). Thus, the combination of additional length of SERT-ir axons and the potential for decreased firing of midbrain serotonin cell bodies in younger subjects with MDD may result in a significant deficit in serotonin availability in OFC. As the disease presents in older subjects with MDD, the decrease in the length of SERT-ir axons in comparison to younger subjects with MDD may reflect pathology in the ascending serotonin axonal input to the prefrontal cortex. These axons ascending from serotonin cell bodies in the brainstem may be damaged in older depressed subjects as they pass through the internal capsule and corona radiata where enhanced deep white matter hyperintensities are localized in older subjects with depression and less so in age-matched control subjects (Krishnan et al., 1988; Rabins et al., 1991; O’Brien et al., 1996; Thomas et al., 2002; Tupler et al., 2002; Hornung, 2003; van Agtmaal et al., 2017).
Mechanisms related to gene expression affecting the protein expression of SERT on axonal membranes in brain appear to be under the regulation of protein kinase C (Blakely et al., 1998). With the SERT, activation of PKC results in the phosphorylation and endocytotic internalization of activity-dependent SERT from the plasma membrane, leading to a reduction in uptake of serotonin (Qian et al., 1997; Bauman et al., 2000). Decreased expression of PKC genes and proteins reported in prefrontal cortex of young suicide victims or younger subjects with MDD (Pandey et al., 2004; Kang et al., 2007; Shelton et al., 2009) may diminish phosphorylation and sequestration of the SERT, increase expression of axonal SERT, and lead to enhanced expression of SERT-ir axons.
There is evidence that the 5-HTTLPR polymorphism affects SERT expression, as evidenced in lymphoblasts and cell lines (Lesch et al., 1996; Heils et al., 1996). Although our study was not powered to assess genetic influences on axon length, there was no difference between our two cohorts in frequency for the 5HTTLPR genotypes and there was no significant correlation between the number of risk alleles and SERT-ir axon length. While Du et al. (1999) noted a significantly higher frequency of the 5-HT transporter gene long (L) allele in depressed subjects dying by suicide, the data presented here confirm Mann et al. (2000), namely that the 5-HTTLPR genotypes do not contribute significantly to MDD status. Finally, although the sample size is insufficient to reliably assess genetic influences on axon length, the 5-HTTLPR genotypes do not significantly affect SERT-ir axon length in control or depressed subjects presented here.
In a previous study in the dorsolateral prefrontal cortex, Austin et al. (2002) noted a significant decrease in the length of SERT-ir axons in layer VI of suicide victims with a diagnosis of MDD, regardless of the presence of antidepressant medications at death. Differences between the Austin et al. (2002) study and the current study include the morphometric analysis (Austin et al.: two-dimensional assessment vs. current study: three-dimensional), examining a different prefrontal cortical region (Austin et al.: dorsolateral prefrontal cortex vs. current study: OFC), death by suicide (Austin et al.: 12 of 12 vs. current study: 8 of 18) and subtype of depression with psychotic features (Austin et al.: 5 of 12 vs. current study: 2 of 18). While no single difference may account for the varying results between the two studies, a combination of all differences may have affected the results.
The relationship between age and SERT has been examined in human brain. One other study examined SERT-ir axon length in depression and did not observe a significant effect of age in either control or depressed subjects, (Austin et al., 2002). Several other studies of postmortem brain tissues have examined age and radioligand binding to SERT. There was no significant correlation between age and radioligand binding to SERT in midbrain raphe in control subjects or depressed subjects dying by suicide (Bligh-Glover et al., 2000). Likewise, neither Arango et al. (1995) nor Mann et al. (2000) detected age-related changes in radioligand binding to SERT in ventrolateral prefrontal cortex in control or depressed subjects or suicide victims. In PET studies, there was no significant correlation between age and binding to the SERT in OFC or frontal cortex of normal control subjects (Bose et al., 2011; Selvaraj et al., 2011), while Cannon et al. (2007) noted no significant age by diagnosis correlation for SERT binding in the cingulate cortex for either normal controls or subjects with MDD. Thus, radioligand binding studies have not noted a significant correlation between SERT and age.
Chronic exposure to antidepressant medications may have a significant impact on markers for the serotonin transporter. In a wide-ranging study, Zhao et al. (2009) reported that following 14-day treatment with a variety of antidepressant drugs, including sertraline, protriptyline, reboxetine, venlafaxine, or phenelzine, only rats treated with sertraline experienced a reduction in SERT radioligand binding and protein expression in cerebral cortex. Thus, it is unlikely that the age-related decrease in SERT-ir axon length reported in this study was due to treatment with an SSRI, particularly since axon length was greater in younger depressives than in controls, and only two of our subjects were taking sertraline at the time of death.
A decrease in OFC gray matter thickness in depression could structurally be related to an increase in SERT-ir axon length. However, in the present study there was no significant difference between control and MDD subjects in the thickness of overall OFC or of its layer VI, and there were no significant correlations between any measure of thickness of gray matter and age in either cohort. The lack of a decrease in OFC gray matter thickness here in postmortem tissue is consistent with a recent meta-analysis of MRI studies on over 1,300 adults with MDD where no reductions were noted in gray matter in the left lateral orbitofrontal cortex (Schmaal et al., 2016).
It is not apparent why the present study noted a greater length of SERT-ir axons in OFC in younger subjects with MDD and decreased radioligand binding to the SERT is found in OFC in younger suicide victims with MDD (Underwood et al., 2012) or suicide victims without MDD (Mann et al., 2000). In contrast to studies of radioligand binding to SERT in postmortem OFC and other prefrontal cortex in depression, a meta-analysis of neuroimaging studies in living subjects with MDD reported no significant change in SERT binding in the frontal cortex (Gryglewski et al., 2014). However, intriguing PET studies note greater SERT binding in prefrontal cortical regions in a subgroup of depressed patients with more highly negativistic dysfunctional attitudes (Meyer et al., 2004) and a greater seasonal increase in SERT binding in patients with more severe symptoms of seasonal affective disorder which is reversible by light therapy (Tyrer et al., 2016a; b). However, a limitation of the present study is that negativistic dysfunctional attitudes cannot be extracted from the retrospective questionnaires used for psychopathology.
There are several other limitations to this study. Although the sample size is relatively small and only one brain region and one cortical layer was examined, the study was predicated upon a very similar study in dorsolateral prefrontal cortex (Austin et al., 2002). As a cross-sectional study, we are not able to definitively comment on disease progression or the effect of age on SERT-ir axon length data collected only at the time of death. However, the effect of age on axon length in MDD is present for all depressed subjects, and for depressed subjects with no psychotropic drug in blood at the time of death. Furthermore, covarying for age, there was no significant difference between depressed subjects with or without a recent antidepressant prescription. There were also no significant correlations between a proxy of ‘disease progression’ (i.e. age of onset of MDD, and the duration of MDD) and SERT-ir axon length. Another potential limitation is a lack of complete details on lifetime exposure to antidepressant medication. Such detailed medication histories and compliance records are rarely, if ever, available for studies of postmortem tissues in MDD. Another potential limitation includes the death by suicide of some but not all the subjects with MDD. Another potential drawback to the study is assessing psychiatric illness primarily by information collected from knowledgeable informants. Nevertheless, there is a high degree of agreement between diagnoses of MDD based on interviewing next-of-kin and diagnoses based on examination of living subjects (DeJong and Overholser, 2009). Finally, the use of fixed tissues to measure the length density of fibers expressing the SERT protein does not allow determination of the level of SERT protein per se.
Conclusions
There was a significant diagnosis-by-age effect on SERT-ir axon length in subjects retrospectively diagnosed with MDD. The age of subjects with MDD was negatively correlated with SERT-ir axon length. The effect of age on SERT-ir axon length in MDD was not due to gender, postmortem interval, processing of tissue, death by suicide, 5HTTLPR genotype, and does not appear to be due to treatment with an antidepressant medication. The inverse relationship between age and SERT-ir axon length in MDD may reflect age-dependent pathology of ascending axons passing to OFC through deep white matter hyperintensities. Greater length of axons expressing SERT in younger subjects with MDD, together with enhanced inhibitory tone on serotonin cell bodies in the dorsal raphe nucleus, may result in a significant deficit in serotonin availability in OFC in depression.
Axons expressing the serotonin transporter are examined in orbitofrontal cortex from subjects with major depressive disorder
There was a significant diagnosis by age effect on axon length in subjects diagnosed with major depressive disorder
Effect of age on axon length in MDD was not due to gender, death by suicide, or presence of antidepressant drug
Acknowledgments
The Authors acknowledge the assistance of the Cuyahoga County Medical Examiner’s Office and the families of the deceased. We also acknowledge the contributions of Drs. James Overholser and George Jurjus and of Lesa Dieter in the retrospective psychiatric assessments. Supported by MH54846 and the Imaging and Postmortem Brain Cores of the NIH/NIGMS COBRE Center for Psychiatric Neuroscience (P30 GM103328).
Abbreviations
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ir
immunoreactive
- MDD
Major Depressive Disorder
- OFC
Orbitofrontal Cortex
- PMI
Postmortem Interval
- SERT
Serotonin Transporter
- TF
storage Time in Formalin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution by Authors
Grazyna Rajkowska, Craig Stockmeier, Mark Austin and Jose Miguel-Hidalgo designed the study. Grazyna Rajkowska and Craig Stockmeier wrote the first draft of the manuscript, with assistance from Gouri Mahajan, Michael Griswold, Lavanya Challagundla, and Jose Miguel-Hidalgo. Beata Legutko assisted in designing the study and performed the immunohistochemistry. Gouri Mahajan supervised the immunohistochemistry and measured axon length density. Michael Griswold and Lavanya Challagundla performed the statistical analyses. Paul Albert and Mireille Daigle performed the genotyping. Grazyna Rajkowska and Craig Stockmeier, with assistance from David Steffens, Paul Albert, Randy Blakely, and Mark Austin, interpreted the data and wrote the manuscript. All authors contributed to the drafting of this manuscript and have approved its final form.
Disclosure
None of the authors have any actual, potential, or perceived financial, professional, or personal conflict of interest to declare. Financial support provided by the National Institutes of Health had no involvement in the study design, in the collection, analysis and interpretation of data, or in preparation of the manuscript.
References
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4. Washington: APA; 1994. [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Regional changes in density of serotonin transporter in the brain of 5- HT1A and 5-HT1B knockout mice, and of serotonin innervation in the 5- HT1B knockout. J Neurochem. 2001;78:619–630. doi: 10.1046/j.1471-4159.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114:807–815. doi: 10.1016/s0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Bligh-Glover W, Kolli TN, Shapiro-Kulnane L, Dilley GE, Friedman L, Balraj E, Rajkowska G, Stockmeier CA. The serotonin transporter in the midbrain of suicide victims with major depression. Biol Psychiatry. 2000;47:1015–1024. doi: 10.1016/s0006-3223(99)00313-3. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, Grasby PM, Turkheimer FE, Murthy V. Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology. 2011;36:2258–2265. doi: 10.1038/npp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan G, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2013;47:299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong TM, Overholser JC. Assessment of depression and suicidal actions: agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39:38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, Demeter E, Bakish D, Lapierre YD, So´tonyi P, Hrdina PD. Frequency of long allele in serotonin transporter gene is increased in depressed suicide victims. Biol Psychiatry. 1999;46:196–201. doi: 10.1016/s0006-3223(98)00376-x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for the DSM-IV Axis I disorders (SCID patient edition), version 2.0. New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- Gryglewski G, Lanzenberger R, Kranz GS, Cumming P. Meta-analysisof molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab. 2014;34:1096–1103. doi: 10.1038/jcbfm.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, Awatramani R, Binder EB, Deneris ES, Lowry CA, Chen A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Kambeitz JP, Howes OD. The serotonin transporter in depression:Meta- analysis of in vivo and postmortem findings and implications for understanding and treating depression. J Affect Disord. 2015;186:358–366. doi: 10.1016/j.jad.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, Overholser JC, Meltzer HY, Jurjus GJ, Konick LC, Newton SS, Duman RS. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, Goli V, Ellinwood EH, France RD, Blazer DG, Nemeroff CB. Leukoencephalopathy in patients diagnosed as major depressive. Biol Psychiatry. 1988;23:519–522. doi: 10.1016/0006-3223(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O'Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol. 1991;314:558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106–1118. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Mouri A, Sasaki A, Watanabe K, Sogawa C, Kitayama S, Mamiya T, Miyamoto Y, Yamada K, Noda Y, Nabeshima T. MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J Neurosci. 2012;32:4562–4580. doi: 10.1523/JNEUROSCI.6458-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. Br J Psychiatry. 1996;168:477–485. doi: 10.1192/bjp.168.4.477. Erratum in: Br J Psychiatry (1996) 168:792. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch Gen Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1991;148:617–620. doi: 10.1176/ajp.148.5.617. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol Dis. 2013;52:49–65. doi: 10.1016/j.nbd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TG, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bülow R, Selonke M, Völzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy-Duchesne B, Rentería ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya-Maldonado R, Gruber O, Krämer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx BW, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD, Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry Mol Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, Cowen PJ. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11 C]DASB. Psychopharmacology (Berl) 2011;213:555–562. doi: 10.1007/s00213-009-1660-y. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Hal Manier D, Lewis DA. Protein kinases A and C in post-mortem prefrontal cortex from persons with major depression and normal controls. Int J Neuropsychopharmacol. 2009;12:1223–1232. doi: 10.1017/S1461145709000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53:665–676. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Tyrer AE, Levitan RD, Houle S, Wilson AA, Nobrega JN, Meyer JH. Increased Seasonal Variation in Serotonin Transporter Binding in Seasonal Affective Disorder. Neuropsychopharmacology. 2016a;41:2447–2454. doi: 10.1038/npp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer AE, Levitan RD, Houle S, Wilson AA, Nobrega JN, Rusjan PM, Meyer JH. Serotonin transporter binding is reduced in seasonal affective disorder following light therapy. Acta Psychiatr Scand. 2016b;134:410–419. doi: 10.1111/acps.12632. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2012;15:435–447. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry May. 2017;31 doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience. 1991a;44:555–570. doi: 10.1016/0306-4522(91)90077-2. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: Regional distribution of axon terminals. Neuroscience. 1991b;44:537–553. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Ricaurte GA, Molliver ME. Distinct morphologic classes of serotonergic axons in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylenedioxymethamphetamine. Neuroscience. 1989;28:121–137. doi: 10.1016/0306-4522(89)90237-6. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang HT, Bootzin E, Millan MJ, O’Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2009;34:1467–1481. doi: 10.1038/npp.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]