Abstract
Background
In the Western World malignant mesothelioma (MM) is most prevalent in the pleura of older males professionally exposed to asbestos. Information about MM from rapidly industrializing countries, such as China, is minimal. There is concern that a proportion of MM diagnoses in China may be incorrect because most Chinese physicians do not have experience diagnosing this rare cancer. We recently reported an unusual high incidence of peritoneal MM among Eastern Chinese female patients. Here, we review the accuracy of MM diagnoses in China and provide suggestions to improve the accuracy of diagnosis.
Methods
We reviewed 92 pathological diagnosis of MM during 2002–2015 from two reference centers in the province of Zhejiang, Eastern China. We performed, a large set of immunohistochemical (IHC) analyses to increase the reliability of the diagnosis.
Results
We confirmed the MM diagnosis in 12/34 (35.3%) of the pleural tumors, in 38/56 (67.9%) of the peritoneal tumors, and in 2/2 (100%) of the MM of the tunica vaginalis. MM were characterized by tumor cells showing nuclear WT1 and calretinin staining and by strong membranous staining for cytokeratin (CK) CAM5.2. The epithelial markers, CEA, TTF1, MOC31, BerEP4, p63, p40, PAX8, ER and PR were negative. BAP1 nuclear staining was lost in percentages similar to what reported for samples from Western countries.
Conclusions
Our findings suggest that MM –especially in its pleural localization– is often misdiagnosed in Eastern China. Identifying pitfalls and possible solutions in the pathological diagnosis of MM will impact both standard of care and research in China.
Keywords: mesothelioma, IHC, China, BAP1
INTRODUCTION
Malignant mesothelioma (MM) is an aggressive cancer with dismal prognosis and limited therapeutic options, usually associated to exposure to asbestos and other carcinogenic mineral fibers.1 Because of this strong association with asbestos, in Western countries MM is most often diagnosed in the parietal pleura of older males professionally exposed to asbestos.1
There are very few reports in the English literature about MM in China.2–4 The MM incidence in China has been estimated at 1.5 cases/million,5 much lower than in other countries. In addition to the English literature, we reviewed 68 papers representing all of the scientific manuscripts on MM in China that were published in the Chinese literature (Supplementary Table 1). These papers reported a total of 1959 MM diagnoses, 293 associated with asbestos exposure (~15%), compared to over 70% in most of the world.1 This low incidence of asbestos-related MM was unexpected because the use of asbestos in China has not been restricted until recently. Amphibole asbestos was banned in 2002; however, the use of chrysotile asbestos continues,3, 6 although it was banned in selected products: as auto brakes (1999), household appliances (2005), ship-building (2007), and mural material (2010). Asbestos mining and asbestos factories are still present in specific provinces and municipalities in China, such as the province of Zhejiang, Eastern China.
We recently discovered that Chinese MMs are surprisingly more common in young women, in the peritoneal localization, and we confirmed that many of these women were not exposed to asbestos.7 These findings are puzzling and their reliability is largely linked to a correct diagnosis. Pathological diagnosis of MM is aid by immunohistochemistry (IHC) and it requires a correct interpretation of the results. We reviewed all the pleural and peritoneal malignancies that were diagnosed as MM during the years 2002–2015 in two medical centers in the Zheijiang province one that sees prevalently non-asbestos exposed patients, and the other that sees prevalently asbestos exposed patients (see below).
MATERIALS AND METHODS
Specimen collection
We collected tissue slides used to diagnose 92 putative MMs in the Zhejiang Cancer Center Hospital (ZJCC), the largest university cancer hospital in the province of Zheijiang located in the city of Hangzhou, and in the Yuyao People’s Hospital (YPH) located 112 km away from Hangzhou, in the town of Yuyao where there is a florid asbestos textile industry. There were a total of 92 diagnoses of MM: 34 in the pleura, 56 in the peritoneum, 2 in the tunica vaginalis. Among confirmed MM 28 were from ZJCC and 24 from Yuyao People Hospital. Demographic and epidemiological information on this set of samples has been presented elsewhere.7 This retrospective study was approved by the Zhejiang Cancer Hospital Ethical Committee and the Zhejiang Academy of Medical Sciences Ethical Committee.
Pathological Review
All initial diagnoses of MM were supported by some, at times limited, immunohistochemistry (IHC). The histopathological review of these MMs –and of the available IHC- was conducted together by pathologists from the ZJCC (Z.G.) and from the University of Hawaii Cancer Center (M.C.) with extensive experience in MM diagnosis. These specimens included tissue biopsies and fine needle biopsies. A set of IHC was ordered based on the histological appearance, sex, and location of the tumor (for example ER and PR stains were ordered only for peritoneal malignancies in women) and final diagnoses were made concurrently by Z.G. and M.C.
IHC
When tissue was available, biopsies were stained for: Calretinin, WT1, D2-40, MOC31, BerEP4, CAM5.2, TTF1, Napsin A, CD15, PAX8 and BAP1, and for ER and PR in peritoneal tumors. These immunostains help differentiate MM from other malignancies.8–11 In addition, those cases in which the differential diagnosis included squamous cell carcinoma (SCC), were stained also for CK5/6, p63 and p40. As control for these IHC, in parallel we stained 10 primary lung adenocarcinomas; 10 primary lung squamous cell carcinomas, and 15 primary carcinosarcomas from: lung (5), esophagus (1) mediastinum (1) breast (1) pancreas (2), cervix (2), liver (1), stomach (1), and duodenum (1). These cancer types were chosen because they often represent a diagnostic challenge in the differential with MM.8–12 IHC was performed using the antibodies listed in Supplementary Table 2.
IHC for BAP1 and INO80
IHC analysis of BAP1 protein expression was performed as previously described using anti-BAP1 antibody (C-4: Santa Cruz Biotechnology, TX).13–15 IHC analysis of INO80 was performed using anti-INO80 antibody (LS-B11393, LSBIo, WA).
Statistical Analyses
Comparisons among proportions were carried using Fisher’s Exact Test or Chi-square Test. Sensitivity, specificity, and predictive values for each immunostains was calculated comparing MM vs. all other non-MM malignancies. A P value < 0.05 was deemed significant. All statistical analyses were carried using GraphPad Prism version 7.00, GraphPad Software, La Jolla California USA.
RESULTS
Diagnostic confirmation of MM related to tumor location
There were a total of 92 MM diagnoses during the years 2002–15 in our two reference centers, ZJCC and YPH. We confirmed the diagnosis of MM in 12/34 (35.3%) of the pleural tumors (10 women and 2 men), in 38/56 (67.9%) of the peritoneal tumors (32 women and 6 men), and in 2/2 (100%) of the tumors of the tunica vaginalis (Table 1). Diagnostic confirmation of MM was statistically more common for peritoneal vs. pleural tumors (Fisher’s Exact Test, P < 0.01). No definitive conclusion could be drawn on tumors of the tunica vaginalis, given their rarity overall and in our sample set. From these results, we can conclude that pleural rather than peritoneal MM is most often misdiagnosed.
Table 1.
Benign and malignant conditions misdiagnosed with MM
| Category | n | Total | |
|---|---|---|---|
| Tumors originally diagnosed as MM | 92 | ||
| MM confirmed diagnoses | 52 | ||
| Pleura | 12/34 | ||
| Peritoneum | 38/56 | ||
| Tunica vaginalis | 2/2 | ||
| Other, non-MM lesions, pleura | 22 | ||
| Metastatic carcinomas of lung | 3 | ||
| Metastatic renal cell carcinoma | 1 | ||
| Poorly differentiated carcinoma, unknown primary | 6 | ||
| Thymic carcinoma | 1 | ||
| Fibrous tumor of pleura | 1 | ||
| Synovial sarcoma | 1 | ||
| Chronic pleuritis | 1 | ||
| Inadequate specimens# | 8 | ||
| Other, non-MM lesions, peritoneum | 18 | ||
| Metastatic carcinomas ovary | 4 | ||
| Solitary fibrous tumor | 2 | ||
| High grade sarcoma peritoneum | 1 | ||
| Carcinosarcoma kidney | 1 | ||
| Inadequate specimens# | 10 | ||
Specimens with extensive necrosis and/or few tumor cells and/or inadequate stains to differentiate between MM, carcinoma, etc.
Sensitivity and Specificity of Immunohistochemistry for MM diagnosis
For 28/52 of the cases diagnosed as MM, and for 19/40 tumors in which the diagnosis of MM was not confirmed, the paraffin blocks used to make the initial diagnosis were available to perform additional IHC studies. As expected,8–11 compared to non-MM tumors, MM cells were characterized by strong membranous staining for cytokeratin (CK) CAM5.2 (Fisher’s Exact Test, P < 0.01), and nuclear staining for calretinin (Fisher’s Exact Test, P < 0.0001) and WT-1 (Fisher’s Exact Test, P < 0.0001) (Table 2). The epithelial markers, CEA, TTF1, MOC31, BerEP4, PAX8, ER and PR were negative in MM (ER/PR stains were used only in peritoneal tumors) (Supplementary Table 3). Full results on sensitivity, specificity, and predictive values of each of the mesothelial immunohistochemical markers of MM are shown in Supplementary Table 4. Based on our results, the chance of finding for example a poorly differentiated carcinosarcoma positive for all three major positive markers of MM (CAM5.2, calretinin, and WT-1) is predicted from our results to be ~1%, whereas ~70% of MMs are expected to have these immunohistochemical characteristics (Fisher’s Exact Test, P < 0.0001, probability of positivity to each marker considered an independent event). From these results, we can conclude that the regular use of all 3 positive MM IHC markers CAM5.2, calretinin, and WT-1 would significantly improve the accuracy of MM diagnosis in China.
Table 2.
Immunostains with mesothelial markers
| Positive Immunostains: | CAM5.2 | % | P value | Calretinin | % | P value | WT-1 | % | P value |
|---|---|---|---|---|---|---|---|---|---|
| MM | 34/36 | 94 | 47/52 | 90 | 40/49 | 82 | |||
| Non-MM | 26/40 | 65 | < 0.01 | 14/40 | 35 | < 0.0001 | 4/40 | 10 | < 0.0001 |
|
| |||||||||
| Carcinosarcoma# | 14/16 | 88 | ns | 5/16 | 31 | < 0.0001 | 1/16 | 6 | < 0.0001 |
| Lung adenocarcinoma | 10/10 | 100 | ns | 0/10 | 0 | < 0.0001 | 0/10 | 0 | < 0.0001 |
| Squamous cell lung cancer | 0/10 | 0 | < 0.0001 | 7/10## | 70 | ns | 0/10 | 0 | < 0.0001 |
| Ovarian adenocarcinoma | 2/4 | 50 | < 0.05 | 2/4## | 50 | ns | 3/4## | 75 | ns |
poorly differentiated biphasic tumors – 3 showed focal calretinin positivity in the epithelial component; 4 showed WT-1 cytoplasmic staining, but no nuclear staining
focal positivity
Identification of MM mimickers
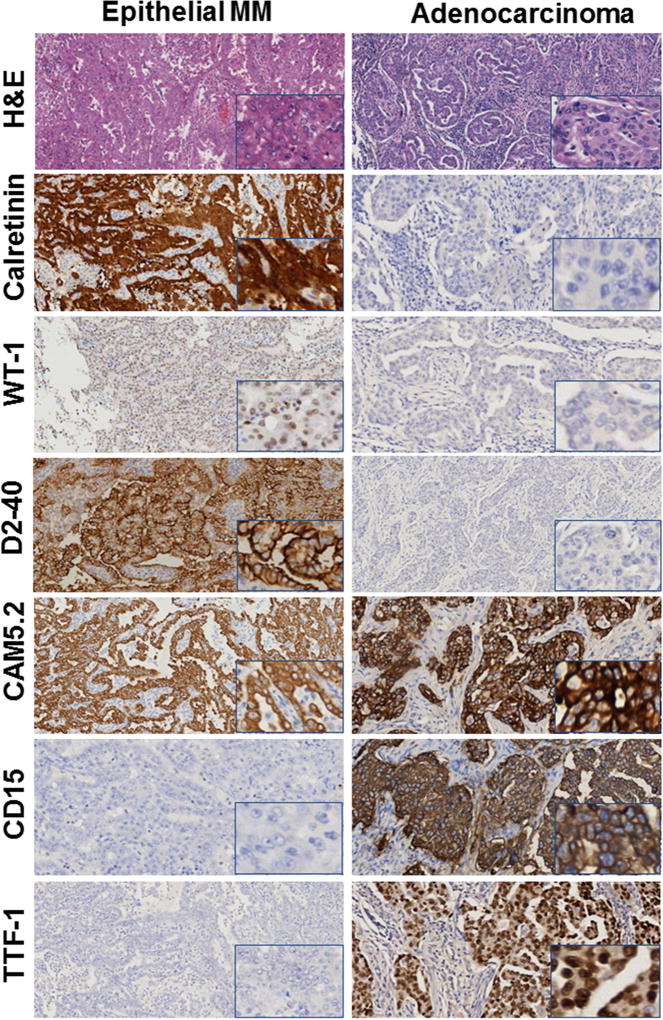
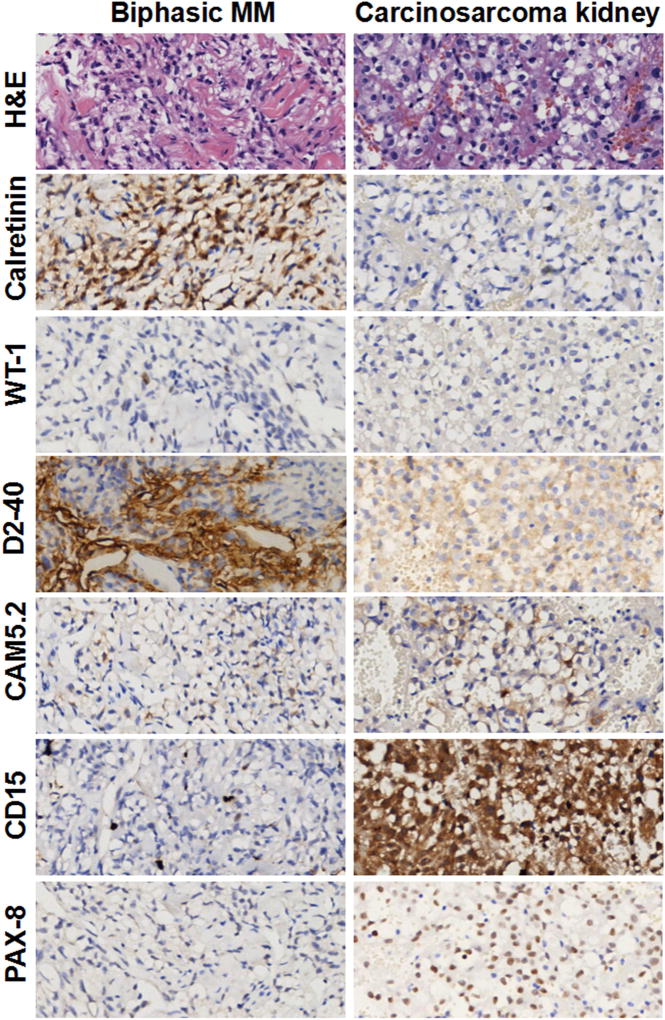
The 40 malignancies (both of the pleura and peritoneum) in which we ruled out the initial diagnosis of MM are shown in Table 1. These included metastatic carcinomas from various organs, including the lung (TTF-1+, Napsin A+, and MOC31+), ovary (BerEP4+, MOC31+, PAX8+, ER+, PR+), and kidney (CD15+, PAX8+); primary fibrous tumors (CD34+); high grade sarcomas (CK−); renal carcinosarcoma (PAX8+); and one case of chronic pleuritis. We could not make a definitive diagnosis in 6 CK+ cases, which we diagnosed as poorly differentiated carcinomas, because of their undifferentiated histological appearance and lack of reactivity for any MM marker. For these 6 cases the paraffin block was not available for additional IHC studies. In 18 additional cases (inadequate specimens, 9 from the pleura and 10 from the peritoneum, Table 1), we could not confirm the MM diagnosis because these were tiny needle biopsies in which invasion by malignant cells could not be identified and/or IHC data were conflicting and no paraffin block was available to perform additional studies. Figures 1, 2 and Supplementary Figures 1 show representative H&E and IHC of these 92 biopsies. Figure 1 shows a representative H&E and immunostains results in a case in which we confirmed the diagnosis of pleural epithelial MM compared to a case we re-diagnosed as lung adenocarcinoma; Figure 2 shows a case we confirmed as biphasic pleural MM compared to a case we re-diagnosed as renal carcinosarcoma metastatic to the pleura; Supplementary Figure 1 shows a case we confirmed as sarcomatoid MM compared to a case we re-diagnosed as primary fibrous tumor of the pleura.
Figure 1. Immunohistochemistry: Epithelial MM and Lung adenocarcinoma.
Left column: Epithelial MM with Calretinin, WT-1, D2-40, CAM5.2 positive,CD15,TTF-1, negative. Right column: Lung Adenocarcinoma, CAM5.2, CD15, TTF-1 positive; Calretinin, WT-1, D2-40 negative. Original magnification 100×. Inserts at right lower corner, original magnification 400×.
Figure 2. Immunohistochemistry: Biphasic MM and Carcinosarcoma of Kidney, both showing a clear cell component.
Left column Biphasic MM, Calretinin and D2-40 positive, WT-1, CAM5.2, CD15,TTF-1 negative. Right column Carcinosarcoma of Kidney, CD15, PAX-8 positive; Calretinin, WT-1, D2-40 negative, CAM5.2 negative/weakly positive. Original magnification, 400×.
BAP1 and INO80 staining
A total of 35 adenocarcinomas, squamous cell carcinomas and primary carcinosarcomas, stained positive for nuclear BAP1 (Supplementary Figure 2, Table 3). Strong nuclear staining was detected in ~100% of the tumor cells in all these tumors except for 2 adenocarcinomas in which some tumor nodules contained cells showing BAP1 nuclear staining and some areas contained tumor nodules that were BAP1 negative, possibly representing tumor sub-clones that had lost BAP1 expression.
Table 3.
BAP1 staining
| BAP1 | |||
|---|---|---|---|
| N | % |
P value vs Total MM |
|
| Total MM | 8/22 | 36 | |
| Epithelial | 3/17 | 18 | |
| Biphasic | 3/2 | 67 | |
| Sarcomatoid | 2/2 | 100 | |
| Total Lung Cancers | 20/20 | 100 | P < 0.0001 |
| Adenocarcinoma | 10/10* | 100 | |
| Squamous cell carcinoma | 10/10 | 100 | |
| Carcinosarcomas | 15/15 | 100 | P < 0.0001 |
| Total non-MM | 35/35 | 100 | P < 0.0001 |
All tumors showed homogeneous BAP1 nuclear staining in 100% of tumor cells, except for 2 lung adenocarcinomas in which positivity was seen in about 70–80% of tumor cells, while some areas contained nodules of tumor cells that entirely lacked BAP1 staining
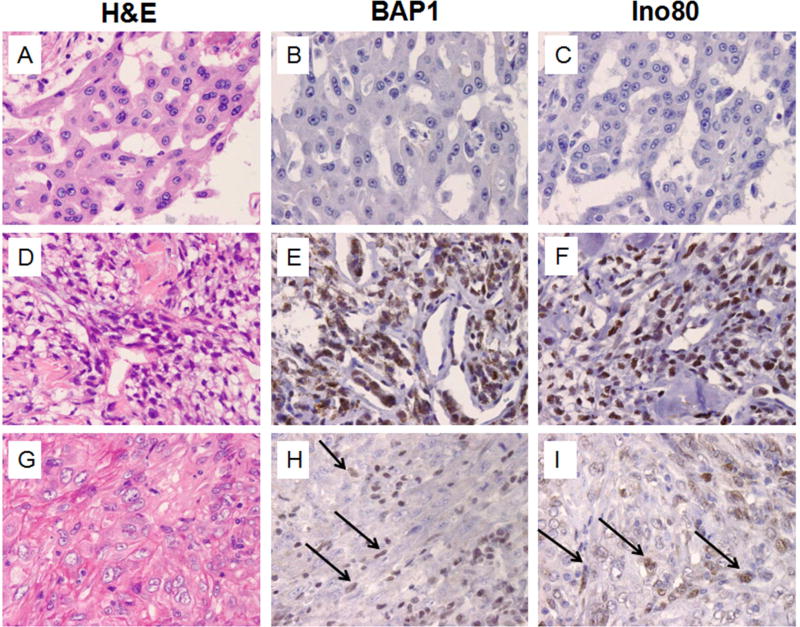
Different results were obtained in parallel immunostains of MM biopsies (Figure 3): among the 17 epithelial type MMs, 2 showed diffuse nuclear BAP1 positivity, 1 was focally positive, 14 were negative. Among the 3 biphasic MM, one was BAP1 positive, one negative and one showed focal positivity in the spindle component only, indicating either polyclonality,16 or that in spite of the morphological appearance the spindle component was benign/reactive and that this was an epithelial-type MM.17, 18 The two sarcomatoid MMs were BAP1 positive. In summary, we found that lack of BAP1 nuclear staining was preferentially associated with MM (Fisher’s Exact Test, P < 0.0001). Consistently, lack of nuclear staining appears to be rare in lung cancer12. In this series 35/35 lung cancers showed positive BAP1 nuclear staining.
Figure 3. Expression of BAP1 and INO80 in MM.
Top row: MM, epithelial type, showing negative nuclear staining for BAP1 and for Ino80. Middle row: Biphasic MM showing homogeneous BAP1 and Ino80 nuclear staining. Bottom row: Sarcomatoid MM showing focal nuclear staining for BAP1 and for Ino80. Original magnification 400×. Arrows point at tumor cells showing nuclear staining.
We stained all these MM biopsies for INO80, a protein involved in DNA replication that requires BAP1 for stabilization and localization on the DNA. It has been suggested that BAP1 mutations result in loss of INO80 nuclear staining.19 The concordance between BAP1 and INO80 staining in our sample set was statistically significant (Figure 3, Chi-square Test, P < 0.0001).
DISCUSSION
A growing number of MMs is expected in rapidly industrializing countries where asbestos use is still allowed, such as China and India1. In these countries, information about the incidence and the prevalence of MM are minimal. We conducted an epidemiological study that revealed unique characteristics of Chinese MMs. In Eastern China, MMs were significantly more common in young individuals, in women, and in the peritoneal localization, compared to US, European and Australian data.7 The mean age of MM diagnosis, in the cases presented here, was 50.6 years old, and the range was 21–71. The mean age of MM in women was 51.2 and the mean age in men was 47.8. The mean age of MM diagnosis among asbestos exposed individuals was 55.2 and among non-asbestos exposed individuals the mean age of diagnosis was 47.3.7 These findings are usually associated with genetic predisposition to MM, such as carriers of germline BAP1 mutations, or are associated with exposure since birth, such as environmental exposure or growing up in families of asbestos workers.20 The latter hypothesis was ruled out.7 Instead, in the Western world, MM occurs prevalently in asbestos exposed individuals at a mean age of 72 years old, as there is a long latency of about 40–50 years from the time of exposure to MM development.21, 22 This log latency, provides an opportunity to identify and develop biomarkers for early detection.23, 24
An additional surprising finding was that among these Chinese MMs, the rate F:M was 4:1, and the peritoneal:pleural ratio was 3:1 which is exactly the opposite as observed in the Western world.7, 21, 22 Except for asbestos exposure, the demographics among the patients from the two Hospitals were very similar: 2/28 (7.1%) MM patients from ZJCC had history of asbestos exposure versus 18/24 (75%) MM from Yuyao People's Hospital.7 Thus, both hospitals MM’s cases comprised mostly young women. Therefore, in Eastern China MMs occur prevalently in a different age group and sex than in the US, and there seems to be no clear association with asbestos exposure: these surprising findings require additional investigations. Ethnicity might be relevant in determining pathogenesis and the molecular characteristics of cancer.25 Here we report that MM in China is often misdiagnosed –especially in its pleural localization where we did not confirm ~2/3 of the diagnoses. In addition to confuse epidemiological and other research studies, cancer misdiagnoses are associated with inappropriate or delayed medical treatment. Moreover, misdiagnoses can lead to unnecessary therapy, e.g. a case described here of chronic pleuritis (a benign condition).
Among the cancer types erroneously identified as pleural MM, the most common were poorly differentiated metastatic carcinomas from the lung, kidney and other organs. Metastatic carcinoma of the ovary was the most common MM mimicker in the peritoneum. This information should help Chinese pathologists to select more appropriate IHC markers to increase the accuracy of the diagnosis.
We confirmed that the most sensitive and specific IHC biomarkers to diagnose Chinese MMs are the same as observed in Western countries, i.e. calretinin and WT-1. We found that D2-40 was not specific as MM marker, as it stained 88% of MMs, but also 80% of carcinosarcomas and lung SCC, and 40% of lung adenocarcinomas. CK5/6 stains epithelial type MMs, but it also stains SCC, which in some of the cases we reviewed caused confusion and mis-diagnoses. CK5/6 does not stain sarcomatoid MM. Thus, we recommend WT-1 and calretinin as MM markers over CK5/6, which remains however a useful additional marker when used in the proper context. Instead, we suggest to include in the diagnostic panel CAM5.2, because it is almost always positive, helps document tumor invasion, and helps eliminate outliers such as melanoma and lymphomas which at times may cause a problem in the differential diagnosis, especially in tumors showing poorly differentiated single small cells among reactive inflammatory cells. Moreover, CAM5.2 is helpful to identify sarcomatoid and biphasic MM, which is almost always CAM5.2 positive –both epithelial and spindle cells, and to rule out various types of sarcomas that can show a morphology identical to MM but that are not stained by CAM5.2. The epithelial markers TTF1, Napsin A, CD15, MOC31, PAX8, P63, P40, ER and PR were consistently (100%) negative in MM; CEA and BerEP4 occasionally stained a small fraction of MM cells. Based on these data, our experience, and statistical considerations, we would recommend that 1) invasion of nearby tissues should be considered a pre-requisite to diagnose MM. This alone would eliminate dubious diagnoses that were made in inadequate specimens (18 cases listed in Table 1); 2) the use of supporting IHC with a minimum panel comprising CAM5.2, calretinin, WT-1 (except in women with peritoneal tumors), and 2 or more epithelial markers which need to be chosen based on location (ER, PR and PAX8 for peritoneal tumors) and histology (TTF1, MOC31, for adenocarcinomas; p63 and p40 when the differential is with squamous cell carcinomas; and PAX8 when the differential includes kidney cancer), would significantly increase the correct identification of MM in China and elsewhere.
Additional recently proposed negative IHC stains for MM diagnoses include BAP1. BAP1 is deubiquitinase and crucial tumor suppressor in MM whose nuclear expression is absent in ~65% of MM.13–15 Loss of the expression of INO80, a protein involved in DNA replication and a BAP1 target, may co-occur with BAP1 loss.19 Our findings, together with recent reports,26 suggests that nuclear BAP1 immunostaining helps differentiate non-small cell lung carcinomas from MM.12
Our study has two main limitations: 1) as our analysis started by reviewing putative MM diagnoses by Chinese pathologists, we do not know the percent of MMs which are misdiagnosed as other cancer types; 2) our analysis was limited to two Hospitals in Eastern China and the results may not be representative of the whole China. ZJCC is the largest Cancer Hospital in the city of Hangzhou –where most of the patients do not have evidence of asbestos exposure – and that YPH is the most important hospital in a nearby area with abundant presence of textile industry that uses chrysotile asbestos7. These two hospitals should be at the forefront of MM diagnosis in China, yet the percent of misdiagnoses was high.
The main reason for inaccurate diagnosis of MM in China appeared to be the use of an incomplete set of immunostains and/or the incorrect interpretation of the stains, as well as an overall tendency to make a definitive diagnosis even when the evidence was inadequate, and a re-biopsy would instead be recommended. Our findings are similar to those of Goldberg et al., 2006 in which a panel of experts reviewed mesotheliomas in France, and confirmed the diagnosis in 67% of the cases (we confirmed the diagnosis in 56.5%, 52/92), ruled it out in 13% (we ruled it out in 17.4%, 16/92) and left it uncertain in 20% of the cases (we left it uncertain in 26%, 24/92)27. Thus, the issue of the accuracy of MM diagnosis is not limited to China. WT1 proved in our study to be the most specific and reliable marker to identify pleural MM. However, since WT1 can stain ovarian tumors, calretinin is much more specific than WT1 to diagnose peritoneal MM.
As the role of genetics in MM has been increasingly being re-evaluated,13, 14, 28 and considering the unique clinico-epidemiological characteristics of Chinese MMs, future studies should address the possible contribution of known and unknown genetic variants to MM in the Chinese population. Genetics studies to investigate whether the same gene alterations found in US/Western world MMs29–34 are found in Chinese MMs would be most informative and might provide clues to the prevalence of peritoneal MM in young Chinese women. Of note, China still uses primary monkey cells to produce polio-vaccines.35 These cells are often heavily contaminated with simian virus 40 (SV40),35 a DNA tumor virus that has been linked to MM.36–38 The process that inactivates polio-vaccine does not inactivate SV40; thus, millions were infected with infectious SV40 until 1963 in the US, the late 70s in the USSR.35 The possible contribution of SV40 to MM in China should be investigated.
In conclusion, the accuracy of MM diagnosis should be improved in China. As the need for adequate MM diagnoses will grow in China in the next decades, it is important to identify and address current pitfalls experienced by local pathologists. IHC represents a powerful tool to help correctly diagnose MM. The suggested set of immunostains proposed, if used routinely, would significantly improve the accuracy of diagnosis.
Supplementary Material
Acknowledgments
Financial Statement. These studies were supported by NCI grants 1R01CA198138-01 and by P30CA071789 to M.C.; by the University of Hawaii Foundation that received generous donations from 1) Honeywell International Inc. to support mesothelioma research by M.C. and 2) from the Riviera United 4-a Cure to support mesothelioma research by H.Y. and M.C.; by NCI-R01 CA160715, DOD CA120355 to H.Y., by donations to support MM research from Belluck and Fox, to H.I.P.; by the Zhejiang Provincial Natural Science Foundation of China (LY16H160036) to Z.G. The Major Science and Technology Project of Medical and Health of Zhejiang Province of China (No. WKJ-ZJ-1403) to D.S. Key Project of Zhejiang Province Science and Technology Plan (2014C03030) to J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, M.C., H.Ya., D.S., W.M.; Methodology, Z.G., M.C.; Validation, M.C., H.I.P., H.Yu., F.W.; Formal analysis, Z.G., M.C., F.W., H.Yu.; Investigation, Z.G., M.C.; Writing, Z.G., M.C., A.N.; Resources, X.Z., D.S., W.S., J.L., Z.G., D.S., J.C., G.Z., J.H., K.C., W.M. Visualization, Z.G.; Supervision, M.C., D.S., H.Ya., H.Y., W.M.; Project administration, M.C., W.M.; Funding acquisition, M.C., W.M.
Competing Interests Statement. MC has a pending patent application on BAP1 and provides consulting expertise on mesothelioma.
References
- 1.Napolitano A, Carbone M. Malignant Mesothelioma: Time to Translate? Trends in Cancer. 2016;2:467–474. doi: 10.1016/j.trecan.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo S, Liu X, Mu S, et al. Asbestos related diseases from environmental exposure to crocidolite in Da-yao, China. I. Review of exposure and epidemiological data. Occup Environ Med. 2003;60:35–41. doi: 10.1136/oem.60.1.35. discussion 41-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai SX, Zhang CH, Zhang X, et al. Epidemiology of occupational asbestos-related diseases in China. Ind Health. 2001;39:75–83. doi: 10.2486/indhealth.39.75. [DOI] [PubMed] [Google Scholar]
- 4.Gao Z, Hiroshima K, Wu X, et al. Asbestos textile production linked to malignant peritoneal and pleural mesothelioma in women: Analysis of 28 cases in Southeast China. Am J Ind Med. 2015;58:1040–1049. doi: 10.1002/ajim.22494. [DOI] [PubMed] [Google Scholar]
- 5.MOH NOfCPaCNCfCRDPaCB. Chinese Cancer Registry Annual Report. Beijing: Military Medical Science Press; 2012. [Google Scholar]
- 6.Courtice MN, Lin S, Wang X. An updated review on asbestos and related diseases in China. Int J Occup Environ Health. 2012;18:247–253. doi: 10.1179/1077352512Z.00000000021. [DOI] [PubMed] [Google Scholar]
- 7.Mao W, Zhang X, Guo Z, et al. Mesothelioma in Eastern China is Mostly Prevalent Among Young Women. JAMA Oncology. 2016 in press. [Google Scholar]
- 8.Ordonez NG. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol. 2006;19:417–428. doi: 10.1038/modpathol.3800544. [DOI] [PubMed] [Google Scholar]
- 9.Addis B, Roche H. Problems in mesothelioma diagnosis. Histopathology. 2009;54:55–68. doi: 10.1111/j.1365-2559.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- 10.Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 11.Comin CE, Novelli L, Cavazza A, et al. Expression of thrombomodulin, calretinin, cytokeratin 5/6, D2-40 and WT-1 in a series of primary carcinomas of the lung: an immunohistochemical study in comparison with epithelioid pleural mesothelioma. Tumori. 2014;100:559–567. doi: 10.1700/1660.18182. [DOI] [PubMed] [Google Scholar]
- 12.Carbone M, Shimizu D, Napolitano A, et al. Positive nuclear BAP1 immunostaining helps differentiate non-small cell lung carcinomas from malignant mesothelioma. Oncotarget. 2016 doi: 10.18632/oncotarget.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10:565–576. doi: 10.1097/JTO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comertpay S, Pastorino S, Tanji M, et al. Evaluation of clonal origin of malignant mesothelioma. J Transl Med. 2014;12:301. doi: 10.1186/s12967-014-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor SM, Dunning R, Hyjek E, et al. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol. 2015;46:1670–1678. doi: 10.1016/j.humpath.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28:1043–1057. doi: 10.1038/modpathol.2015.65. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Lee SA, Hur SK, et al. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat Commun. 2014;5:5128. doi: 10.1038/ncomms6128. [DOI] [PubMed] [Google Scholar]
- 20.Carbone M, Kanodia S, Chao A, et al. Consensus Report of the 2015 Weinman International Conference on Mesothelioma. J Thorac Oncol. 2016;11:1246–1262. doi: 10.1016/j.jtho.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89(716–724):724A–724C. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int J Occup Environ Health. 2013;19:1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostroff RM, Mehan MR, Stewart A, et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS One. 2012;7:e46091. doi: 10.1371/journal.pone.0046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin Cancer Res. 2016;22:3087–3096. doi: 10.1158/1078-0432.CCR-15-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiencke JK. Impact of race/ethnicity on molecular pathways in human cancer. Nat Rev Cancer. 2004;4:79–84. doi: 10.1038/nrc1257. [DOI] [PubMed] [Google Scholar]
- 26.Andrici J, Parkhill TR, Jung J, et al. Loss of expression of BAP1 is very rare in non-small cell lung carcinoma. Pathology. 2016;48:336–340. doi: 10.1016/j.pathol.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg M, Imbernon E, Rolland P, et al. The French National Mesothelioma Surveillance Program. Occup Environ Med. 2006;63:390–395. doi: 10.1136/oem.2005.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone M, Flores EG, Emi M, et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015;11:e1005633. doi: 10.1371/journal.pgen.1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altomare DA, Menges CW, Pei J, et al. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc Natl Acad Sci U S A. 2009;106:3420–3425. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 31.Lo Iacono M, Monica V, Righi L, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol. 2015;10:492–499. doi: 10.1097/JTO.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 32.Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–269. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 33.Ugurluer G, Chang K, Gamez ME, et al. Genome-based Mutational Analysis by Next Generation Sequencing in Patients with Malignant Pleural and Peritoneal Mesothelioma. Anticancer Res. 2016;36:2331–2338. [PubMed] [Google Scholar]
- 34.Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1612074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutrone R, Lednicky J, Dunn G, et al. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;65:10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- 36.Carbone M. Simian virus 40 and human tumors: It is time to study mechanisms. J Cell Biochem. 1999;76:189–193. doi: 10.1002/(sici)1097-4644(20000201)76:2<189::aid-jcb3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Gazdar AF, Carbone M. Molecular pathogenesis of malignant mesothelioma and its relationship to simian virus 40. Clin Lung Cancer. 2003;5:177–181. doi: 10.3816/CLC.2003.n.031. [DOI] [PubMed] [Google Scholar]
- 38.Carbone M, Rizzo P, Pass H. Simian virus 40: the link with human malignant mesothelioma is well established. Anticancer Res. 2000;20:875–877. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.