Abstract
IMPORTANCE
Ophthalmic screening to check for diabetic retinopathy (DR) is important to prevent vision loss in persons with diabetes. The American Academy of Ophthalmology recommends that ophthalmic screening for DR occur beginning at 5 years after initial diabetes diagnosis for youths with type 1 diabetes; the American Diabetes Association recommends screening of youths with type 2 diabetes at the time of initial diagnosis. To our knowledge, it is unknown to what extent youths with diabetes obtain eye examinations in accordance with these guidelines.
OBJECTIVE
To assess the rate of obtaining ophthalmic examinations and factors associated with receipt of eye examinations for youths with diabetes.
DESIGN, SETTING, AND PARTICIPANTS
This retrospective, longitudinal cohort study examined youths 21 years or younger with newly diagnosed diabetes enrolled in a US managed care network from January 1, 2001, through December 31, 2014.
MAIN OUTCOMES AND MEASURES
Kaplan-Meier survival curves estimated the time from initial diabetes diagnosis to first eye examination by an ophthalmologist or optometrist. Multivariable Cox proportional hazards regression models identified factors associated with receiving an ophthalmic examination after initial diabetes diagnosis.
RESULTS
Among 5453 youths with type 1 diabetes (median age at initial diagnosis, 11 years; interquartile range, 8–15 years; 2972 male [54.5%]; 4505 white [82.6%]) and 7233 youths with type 2 diabetes (median age at initial diagnosis, 19 years; interquartile range, 16–22 years; 1196 male [16.5%]; 5052 white [69.9%]), 64.9% of patients with type 1 diabetes and 42.2% of patients with type 2 diabetes had undergone an eye examination by 6 years after initial diabetes diagnosis. Black youths (1367 [10.8%] of the sample) had an 11% and Latino youths (1450 [11.4%] of the sample) had an 18% decreased hazard of undergoing an eye examination by 6 years compared with white youths (black youths: adjusted hazard ratio [HR], 0.89; 95% CI, 0.79–0.99; Latino youths: HR, 0.82; 95% CI, 0.73–0.92). As household net worth increased, youths were increasingly more likely to undergo an eye examination by 6 years after initial diabetes diagnosis (net worth of ≥$500 000 vs <$25 000: HR, 1.50; 95% CI, 1.34–1.68).
CONCLUSIONS AND RELEVANCE
Despite possessing health insurance, many youths with diabetes are not receiving eye examinations by 6 years after initial diagnosis to monitor for DR. These data suggest that adherence to clinical practice guidelines is particularly challenging for racial minorities and youths from less affluent families.
The incidence of diabetes among children and adolescents is increasing worldwide.1–5 Type 2 diabetes, which previously affected few children and adolescents, now constitutes up to 45% of all new diabetes diagnoses among adolescents, concurrent with the increase in childhood obesity.6,7 Projections based on US Census data suggest that the prevalence of type 1 diabetes will triple and type 2 diabetes will quadruple in youth by 2050, with the greatest increases among racial/ethnic minorities.8
Diabetic retinopathy (DR) is a serious complication of diabetes that is often asymptomatic in early and occasionally later stages but may progress to sight-threatening disease.9–12 Ocular complications of diabetes include diabetic macular edema, proliferative DR, tractional retinal detachment, and neovascular glaucoma, all of which can lead to blindness.13 Various clinical practice guidelines for the ophthalmic screening of patients with type 1 diabetes exist, although medical professional societies differ in their recommended timing of monitoring. The American Academy of Ophthalmology (AAO) recommends initial screening 5 years after type 1 diabetes onset.14 The American Diabetes Association (ADA) recommends initial screening 3 to 5 years after type 1 diabetes onset for patients 10 years or older.15 The American Academy of Pediatrics (AAP) recommends the same for patients 9 years or older.16 The AAO and ADA recommend screening of youths with type 2 diabetes at the time of initial diabetes diagnosis, similar to adults with type 2 diabetes.17,18
A prior study19 of youths with diabetes reported that only one-third of those with type 1 diabetes were referred for eye examinations in accordance with the above-mentioned clinical practice guidelines; another study20 found only half of youths with types 2 diabetes obtained eye examinations in accordance with clinical practice guidelines. Factors such as younger age, a diagnosis of type 2 diabetes (compared with type 1 diabetes), and shorter diabetes duration have been associated with poor adherence to guidelines for ophthalmic screening for DR.21 Most previous studies21,22 of factors associated with guideline adherence have been performed in adults, and the few studies19,20 of youths have not investigated sociodemographic factors, which may identify subgroups of children and adolescents particularly at risk for guideline nonadherence. The purpose of this study was to compare the rate of obtaining eye examinations among youths with diabetes in a large, nationwide US managed care network. Specifically, we sought to assess to what extent youths with diabetes obtain eye examinations in accordance with national guidelines and to identify sociodemographic factors associated with receipt of ocular examinations among youths with diabetes.
Methods
Data Source
The Clinformatics DataMart database (OptumInsight) contains detailed records of all enrollees in a nationwide managed care network from January 1, 2001, through December 31, 2014. We accessed data on all beneficiaries 21 years or younger at their initial enrollment date. Medical claims from inpatient and outpatient health care encounters and associated International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes23 for all ocular and nonocular conditions were available, as was information on age, sex, race/ethnicity, household net worth, and urban or rural location of residence. The database also captures pharmacy records of all outpatient medication fills. All persons in the insurance plan were also fully enrolled in the pharmacy plan. This database has been used previously to study patients with ophthalmic diseases.24–26 The University of Michigan Institutional Review Board approved this study involving deidentified data and determined that informed consent was not required.
Inclusion and Exclusion Criteria
We included all youths with continuous enrollment in the medical plan, enrollment for 3 years or more, and 2 or more diabetes diagnoses (ICD-9-CM codes 250.xx or 362.01-362.07) on separate dates. To ensure that all the children had diabetes, those who never filled an insulin or oral hypoglycemic agent prescription were excluded. To help exclude youths with preexisting diabetes (nonincident cases), we required the youths’ first diabetes diagnosis to have occurred 12 months or more after plan enrollment. Children who lacked information on race/ethnicity or household net worth were also excluded.
Classification of Diabetes Type
Youths were classified as having diabetes based on a previously validated algorithm.27 In brief, children younger than 10 years at their first diabetes diagnosis were considered to have type 1 diabetes. Among children 10 years or older, those who were prescribed only insulin in the 730 days after initial diagnosis were also considered to have type 1 diabetes. The remainder were classified as having type 2 diabetes. In this group, patients must have filled an oral hypoglycemic prescription (eg, metformin or sulfonylureas) with or without a concurrent insulin prescription within 730 days of their initial diabetes diagnosis. This algorithm had a sensitivity of 98.6% and a specificity of 78.2% for detecting type 1 diabetes and 83.2% sensitivity and 97.5% specificity for detecting type 2 diabetes in a Canadian study of youths with diabetes.27
Outcome
The primary outcome was receipt of an eye examination by an eye care professional, as identified by Current Procedural Terminology codes 92002, 92004, 92012, 92014, 99201-99205, 99211-99215, 99221-99223, 99231-99233, 99241-99245, 99251-99255, or 99281-99285.
Statistical Analysis
Data analyses were performed using SAS statistical software, version 9.4 (SAS Inc). Kaplan-Meier survival curves were created using GraphPad Prism software, version 6.0 (GraphPad Software). Characteristics of the study population were summarized using medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. Kaplan-Meier survival curves assessed the timing from initial diabetes diagnosis to first eye examination for youth with diabetes; those with type 1 and type 2 diabetes were compared using the log-rank test. Multivariable Cox proportional hazards regression modeling evaluated whether diabetes type and sociodemographic factors affected the hazard for undergoing an eye examination. All enrollees were right-censored 6 years after their initial diabetes diagnosis. Model predictors were diabetes type, age and calendar year at initial diabetes diagnosis, sex, race/ethnicity, household net worth, and residence in an urban or rural community. We performed an additional analysis studying whether an association exists between diabetes type and calendar year to understand whether screening for DR was increasing or decreasing over time for each diabetes type. For all analyses, P < .05 was considered statistically significant.
Results
The study included 5453 youths with type 1 diabetes (median age at initial diagnosis, 11 years; IQR, 8–15 years) and 7233 youths with type 2 diabetes (median age at initial diagnosis, 19 years; IQR, 16–22 years). The median time in the plan after initial diabetes diagnosis for both groups was 2.1 years, with a maximum of 12 years, resulting in a maximum age of 34 years at the end of follow-up. Most youths with type 2 diabetes were female (6037 [83.5%]). Of those with type 1 diabetes, 4505 (82.6%) were white, 445 (8.2%) black, and 392 (7.2%) Latino. Among those with type 2 diabetes, 5052 (69.9%) were white, 922 (12.8%) black, and 1058 (14.6%) Latino (Table 1).
Table 1.
Characteristics of Study Populationa
| Characteristic | Type 1 Diabetes (n = 5453) |
Type 2 Diabetes (n = 7233) |
|---|---|---|
| Age at initial diabetes diagnosis, median (IQR), y | 11 (8–15) | 19 (16–22) |
| Follow-up after diabetes diagnosis, median (IQR), y | 2.1 (0.90–3.93) | 2.1 (1.05–3.64) |
| Sex | ||
| Male | 2972 (54.5) | 1196 (16.5) |
| Female | 2481 (45.5) | 6037 (83.5) |
| Race | ||
| White | 4505 (82.6) | 5052 (69.9) |
| Black | 445 (8.2) | 922 (12.8) |
| Latino | 392 (7.2) | 1058 (14.6) |
| Asian | 111 (2.0) | 201 (2.8) |
| Household net worth, $ | ||
| <25 000 | 787 (14.4) | 1709 (23.6) |
| 25 000–149 999 | 1340 (24.6) | 1880 (26.0) |
| 150 000–249 999 | 873 (16.0) | 976 (13.5) |
| 250 000–499 999 | 1401 (25.7) | 1458 (20.2) |
| ≥500 000 | 1052 (19.3) | 1210 (16.7) |
| Urban or rural residence | ||
| Urban | 4853 (89.0) | 6320 (87.4) |
| Rural | 600 (11.0) | 913 (12.6) |
Abbreviation: IQR, interquartile range.
Data are presented as number (percentage) of patients unless otherwise indicated.
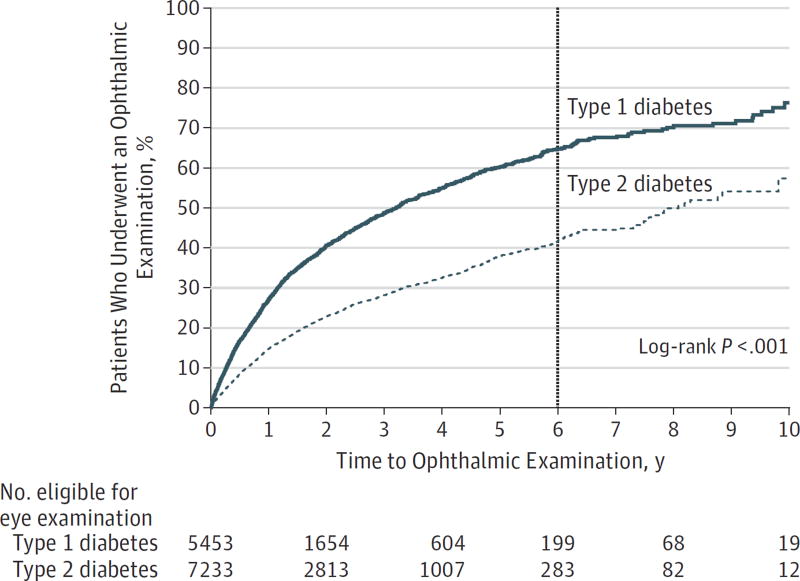
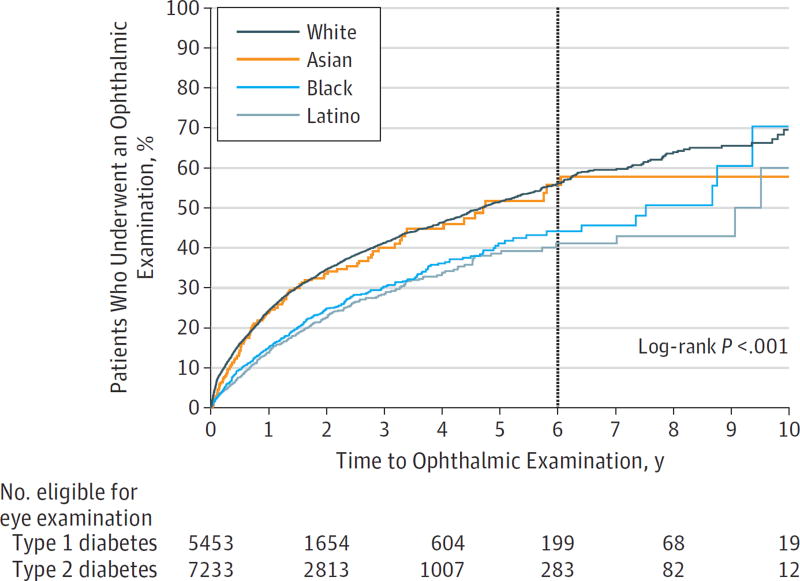
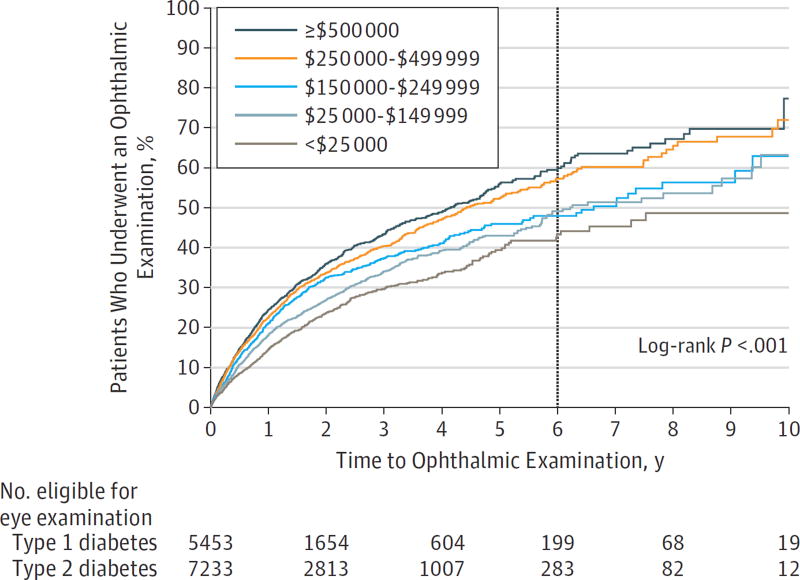
Kaplan-Meier survival analysis evaluated the time to first eye examination after initial diabetes diagnosis for youths with each diabetes type (Figure 1). Youths with type 1 diabetes had a higher rate of obtaining eye examinations compared with those with type 2 diabetes. Survival analysis estimated that by 6 years after initial diabetes diagnosis, 64.9% of youths with type 1 diabetes and 42.2% of youths with type 2 diabetes had undergone an eye examination. White and Asian youths had a higher rate of obtaining eye examinations than did black and Latino youths. By 6 years after initial diabetes diagnosis, 54.7% of white and 57.3% of Asian youths had undergone an eye examination compared with 44.6% of black and 41.6% of Latino youths (Figure 2). Youths from families with higher household net worth were also more likely to obtain an eye examination compared with youths with lower household net worth levels (Figure 3). Overall, 3949 youths with diabetes (31.1%) underwent an eye examination. For 2159 youths (54.7%), the first ocular examination was performed by an ophthalmologist, and for 1790 youths (45.3%), the examination was performed by an optometrist.
Figure 1. Kaplan-Meier Plot of Time to First Eye Examination After Initial Diabetes Diagnosis.
Youths who obtained an eye examination by 6 years after initial diabetes diagnosis are indicated at the vertical line.
Figure 2. Kaplan-Meier Plot of Time to First Eye Examination After Initial Diabetes Diagnosis, Stratified by Race.
Youths who obtained an eye examination by 6 years after initial diabetes diagnosis are indicated at the vertical line.
Figure 3. Kaplan-Meier Plot of Time to First Eye Examination After Initial Diabetes Diagnosis, Stratified by Household Net Worth.
Youths who obtained an eye examination by 6 years after initial diabetes diagnosis are indicated at the vertical line.
In the multivariable regression model, youths with type 1 diabetes were 114% more likely to undergo an eye examination by 6 years after initial DM diagnosis (adjusted hazard ratio [HR], 2.14; 95% CI, 1.97–2.33) compared with those with type 2 diabetes (Table 2). Black youths were 11% less likely (HR, 0.89; 95% CI, 0.79–0.99) and Latino youths were 18% less likely (HR, 0.82; 95% CI, 0.73–0.92) to undergo an eye examination by 6 years compared with white youths. As household net worth increased, youths were increasingly more likely to undergo an eye examination by 6 years (HR, 1.50; 95% CI, 1.34–1.68 for net worth ≥$500 000 vs <$25 000). The likelihood of an ocular examination increased by 2% (HR, 1.02; 95% CI, 1.01–1.02) for every year increase in age at initial diabetes diagnosis. The likelihood of an ocular examination among youths with diabetes decreased by 2% for each calendar year during the study period (HR, 0.98; 95% CI, 0.97–0.99). Separate models stratified by diabetes type revealed that calendar year of initial diagnosis was only a significant predictor for obtaining an ocular examination for youths with type 2 diabetes (HR, 0.97; 95% CI, 0.95–0.98) but not for youths with type 1 diabetes (HR, 1.00; 95% CI, 0.99–1.01). In other words, in later calendar years, the odds of obtaining an eye examination decreased among youths with type 2 diabetes compared with those diagnosed in earlier years.
Table 2.
Predictors for Obtaining Eye Examination Within 6 Years of Initial Diabetes Diagnosis by Cox Proportional Hazards Regression Modeling
| Predictor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Diabetes type | ||
| Type 1 | 2.14 (1.97–2.33) | <.001 |
| Type 2 | 1 [Reference] | NA |
| Age at diabetes diagnosis, per year | 1.02 (1.01–1.02) | <.001 |
| Calendar year of diabetes diagnosis, per year | 0.98 (0.97–0.99) | <.001 |
| Sex | ||
| Male | 0.93 (0.87–1.00) | .05 |
| Female | 1 [Reference] | NA |
| Race | ||
| White | 1 [Reference] | NA |
| Latino | 0.82 (0.73–0.92) | <.001 |
| Black | 0.89 (0.79–0.99) | .04 |
| Asian | 1.09 (0.90–1.32) | .38 |
| Household net worth, $ | ||
| <25 000 | 1 [Reference] | NA |
| 25 000–149 999 | 1.12 (1.01–1.25) | .04 |
| 150 000–249 999 | 1.26 (1.12–1.41) | <.001 |
| 250 000–499 999 | 1.39 (1.25–1.55) | <.001 |
| ≥500 000 | 1.50 (1.34–1.68) | <.001 |
| Urban or rural residence | ||
| Urban | 1 [Reference] | NA |
| Rural | 1.17 (1.06–1.30) | .003 |
Abbreviation: NA, not applicable.
Discussion
In this study of youths with diabetes who were enrolled in a large managed care network, only 64.9% of children and adolescents with type 1 diabetes and 42.2% of those with type 2 diabetes had undergone an eye examination within 6 years after initial diagnosis. This finding indicates that many youths with diabetes were not undergoing screening for DR as frequently as recommended by professional societies, such as the ADA, AAP, and AAO. Factors associated with receipt of a timely ocular examination—by 6 years after initial diabetes diagnosis—included older age at diabetes onset, white race, higher household net worth, and type 1 diabetes (relative to type 2 diabetes).
Previous studies21,22,28,29 estimating adherence to clinical guidelines for eye examinations among youths and adults with diabetes have varied widely, depending on study design and population studied, with adherence rates ranging from 20% to 82%. In a survey21 of 2308 adults with diabetes, 65% self-reported receiving a dilated eye examination in the previous year. In another survey22 of adults with type 1 diabetes, 82% self-reported obtaining an eye examination in the past year. Among 988 adults with diabetes seen in a rural clinic setting, 21% to 30% were reported to have obtained an annual dilated eye examination.28 In the National Long-Term Care Survey, during the 5 years from 1994 to 1998, a mean of 53% of Medicare beneficiaries with diabetes surveyed in each year received annual eye examinations.29
Ocular examination rates among youths with diabetes have also varied widely. For example, a study of 80 youths who had type 1 diabetes for 5 years or more or type 2 diabetes for any duration who were receiving care at a pediatric diabetes clinic at a US academic medical center found that only 38 (35%) were referred for an eye examination.19 An electronic health record review20 of 56 youths with type 2 diabetes from several medical practices in Ohio found documentation that dilated eye examinations within 1 year of initial diagnosis took place for 54% of the patients, although the authors suspected that incomplete record of an eye examination when one actually took place may have led to an underestimation of proportions who had been examined. In a Canadian study30 of 1507 adolescents with diabetes transitioning from pediatric to adult care, 70% to 72% of adolescents had 1 or more eye examination in the 2 years before and after their transition. These prior studies19,20 have typically reported the rate of obtaining an eye examination in a cross-sectional fashion and included patients with diabetes of varying disease duration. These factors can greatly affect whether a patient has undergone an ocular examination. Unlike previous studies,19,20 our study is unique because we captured children and adolescents with newly diagnosed diabetes whom we followed up longitudinally from onset of diabetes to determine whether they received ocular examinations.
Factors previously reported to be associated with obtaining screening ophthalmic examinations in adults with diabetes include possessing health insurance and receiving specialist care, such as from endocrinologists (rather than generalist care, such as from primary care physicians) for diabetes management.22 Younger age, shorter diabetes duration, type 2 diabetes (compared with type 1 diabetes), and having the last eye examination performed by an optometrist or primary care physician (vs an ophthalmologist) have been reported to be associated with increased nonadherence to established ophthalmic screening guidelines.21Our study confirmed some of these previously noted risk factors. In addition, we learned that race and economic status play an important role in adherence to clinical guidelines for obtaining ophthalmic examinations among youths. Despite possessing health insurance, black and Latino youths and youths from lower economic backgrounds were less likely to undergo ophthalmic examinations in accordance with clinical practice guidelines than were white youths and children from more affluent families. This finding is particularly disconcerting because racial minorities are projected to have the greatest increase in diabetes prevalence during the next several decades.8
Barriers to guideline adherence are likely multifactorial, with physician and patient factors playing a role. Patient-related factors that contribute to not obtaining an annual eye examination include limited personal mobility attributable to poor health, forgetfulness, lack of health insurance or financial barriers, long scheduling wait times, and lack of acceptance of the diagnosis of diabetes and its associated indications for regular care.31,32 In one study,31 a small number of patients (5%) reported an aversion to pupil dilation. A survey33 of an Hispanic population identified a lack of knowledge of the potential ophthalmic consequences of diabetes, especially among persons with newly diagnosed diabetes, as a factor contributing to poor guideline adherence. Health care professional factors may also play a role. Although previous studies34,35 of Canadian general practitioners have found that more than 80% to 90% were aware of ophthalmic screening guidelines for adults with type 2 diabetes, it is unclear to what extent primary care physicians are aware that these guidelines also apply to children with type 2 diabetes. One Canadian survey34 reported that only 13% of 645 general practitioners believed that the initial screening for patients newly diagnosed as having type 1 diabetes should be performed 5 years after diagnosis, but 81% believed that the initial screening for patients with newly diagnosed type 1 diabetes was supposed to occur sooner (shortly after initial diagnosis). Although physician knowledge of guidelines seems adequate, there appears to be a discrepancy between knowledge of guidelines and actual referral rate, because one study36 reported that the primary care referral rate for ophthalmologic services for patients with diabetes was 33% or less. Because of our data source, we cannot determine the extent to which failure to obtain an eye examination was attributable to lack of referral for eye care or lack of patient willingness to undergo an eye examination despite referral.
New strategies that use point-of-care ophthalmologic services, especially those that involve nonmydriatic fundus photography, may be a promising option to improve adherence to ophthalmic screening guidelines because they improved screening rates considerably for adults with diabetes.28,37 Pilot studies38,39 of nonmydriatic fundus photography among youths with diabetes have found that gradable or clinically valuable images were obtained in almost all patients screened. More studies are needed to determine whether, how, and in what settings nonmydriatic fundus photography can help remove barriers to adherence to clinical guidelines to improve screening and detection of DR in youths with diabetes, particularly those who are underserved, such as racial minorities, youths from less affluent backgrounds, or those who are uninsured.
Strengths and Limitations
Our study has several strengths. This study included more than 12 000 youths with diabetes throughout the nation, among whom more than 5500 were youths with type 1 diabetes and more than 7000 youths had type 2 diabetes. The diverse population allowed analysis of racial minorities and patients from a range of economic backgrounds. Patients in this study were under the care of numerous practice settings throughout the country and not limited to a single academic center or a few private practices. All participants had health insurance; thus, lack of health insurance could not have been a barrier to adherence to clinical practice guidelines. Determining whether an individual received an eye examination from claims data is also likely more accurate than patient self-report.40
This study also has limitations. Although we relied on a validated algorithm to determine enrollees’ type of diabetes,27 some cases may have been misclassified. Some cases of type 2 diabetes may include patients taking metformin for insulin resistance or prediabetes. Complete data on hemoglobin A1c levels were not available, making it difficult to characterize the severity of diabetes for this cohort. Despite efforts to ensure that the study population included only persons with incident diabetes by requiring 12 months in the plan without a prior diabetes diagnosis, some of these children may have had preexisting diabetes. Thus, we may be underestimating the time from disease onset to first recorded eye examination and overestimating the numbers of youth with diabetes who are adherent to current practice guidelines. Clinical data, such as visual acuity and retinal examination findings, were unavailable in our data set. Although claims data document whether an examination was performed by an eye care professional, we could not discern from claims data alone whether the examination involved pupil dilation to best assess for DR. Thus, we may be overestimating the number of youth with diabetes who are adherent to practice guidelines. Our results may not apply to youth without health insurance who are likely to have even lower ophthalmic screening rates. Although lack of health insurance was not a barrier to adherence in this population, insurance co-payment requirements may have contributed. Finally, our results may not apply to enrollees in other health insurance plans because there may be variation among plans regarding availability and participation in diabetes disease management programs. This particular managed care network has an optional disease management program for diabetes, but we had no information on which youth were in the program.
Conclusions
For youths with type 1 diabetes, for whom there are clear screening guidelines for DR, approximately two-thirds of children and adolescents in our cohort obtained eye examinations to check for DR as recommended by professional societies. Youths with type 2 diabetes were even less likely to undergo screening to check for DR. Groups of youth with diabetes who had a reduced likelihood of undergoing eye examinations included racial minorities and those from less affluent families. Identifying ways to improve adherence to ophthalmic screening guidelines, including for racial minorities and economically disadvantaged youth, can help with timely diagnosis of DR so that sight-threatening consequences of DR can be avoided.
Key Points.
Question
To what extent do youth with diabetes obtain eye examinations to screen for diabetic retinopathy in accordance with established screening guidelines?
Findings
This longitudinal cohort study of 12 686 youth 21 years or younger with newly diagnosed diabetes enrolled in a US managed care network found that only 65% with type 1 diabetes and 42% with type 2 diabetes had undergone an eye examination by 6 years after initial diabetes diagnosis.
Meaning
These data suggest that many youth with diabetes who have health insurance are not receiving timely eye examinations to monitor for retinopathy.
Acknowledgments
Funding/Support: This study was supported by grant R01 EY026641 from the National Eye Institute (Dr Stein), a Research to Prevent Blindness Physician Scientist Award (Dr Stein), the W. K. Kellogg Foundation (Dr Gardner), grants R01EY20582 and DP3DK094292 from the National Eye Institute (Dr Gardner), a grant from the Taubman Institute (Dr Gardner), grant K08DK101755 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr Singer), and the Taubman Emerging Scholars Program (Dr Singer).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Stein had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wang, Andrews, Stein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Wang.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis:Wang, Andrews.
Study supervision: Gardner, Singer, Stein.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Meeting Presentation: Preliminary analyses were presented in poster form at the Association for Research in Vision and Ophthalmology Annual Meeting; May 4, 2016; Seattle, Washington.
Contributor Information
Sophia Y. Wang, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor.
Chris A. Andrews, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Center for Eye Policy and Innovation, University of Michigan, Ann Arbor.
Thomas W. Gardner, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Michigan Diabetes Research Center, University of Michigan, Ann Arbor.
Michael Wood, Division of Endocrinology, Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor.
Kanakadurga Singer, Division of Endocrinology, Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor.
Joshua D. Stein, Department of Ophthalmology and Visual Sciences, University of Michigan Medical School, Ann Arbor; Center for Eye Policy and Innovation, University of Michigan, Ann Arbor; Department of Health Management and Policy, University of Michigan School of Public Health, Ann Arbor.
References
- 1.Green A, Patterson CC EURODIAB TIGER Study Group. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(3 suppl 3):B3–B8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 2.Lin W-H, Wang M-C, Wang W-M, et al. Incidence of and mortality from type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One. 2014;9(1):e86172. doi: 10.1371/journal.pone.0086172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day C. The rising tide of type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1(1):37–43. [Google Scholar]
- 4.Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. 2013;36(6):1597–1603. doi: 10.2337/dc12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 7.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34(suppl 2):S161–S165. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperatore G, Boyle JP, Thompson TJ, et al. SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broe R. Early risk stratification in pediatric type 1 diabetes. Acta Ophthalmol. 2015;93(1):1–19. doi: 10.1111/aos.12702. Thesis 1. [DOI] [PubMed] [Google Scholar]
- 10.Porta M, Allione A. Diabetic retinopathy and its relevance to paediatric age An update. Pediatr Endocrinol Rev. 2004;1(4):404–411. [PubMed] [Google Scholar]
- 11.Thomas RL, Dunstan FD, Luzio SD, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99(1):64–68. doi: 10.1136/bjophthalmol-2013-304017. [DOI] [PubMed] [Google Scholar]
- 12.Lian JX, Gangwani RA, McGhee SM, Chan CK, Lam CL, Wong DS Primary Health Care Group. Systematic screening for diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment among diabetic population. Br J Ophthalmol. 2016;100(2):151–155. doi: 10.1136/bjophthalmol-2015-307382. [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 14.AAO PPP Retina/Vitreous Panel, Hoskins Center for Quality Eye Care. American Academy of Ophthalmology; [Accessed December 12, 2016]. Diabetic Retinopathy PPP - Updated 2016. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2016. Published February 24, 2016. [Google Scholar]
- 15.American Diabetes Association. Diabetic retinopathy. Diabetes Care. 2002;25(suppl 1):s90–s93. [Google Scholar]
- 16.Lueder GT, Silverstein J American Academy of Pediatrics Section on Ophthalmology and Section on Endocrinology. Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics. 2005;116(1):270–273. doi: 10.1542/peds.2005-0875. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Ophthalmology. Diabetic Retinopathy PPP—2014. [Accessed August 22, 2016]; https://www.aao.org/clinical-education.
- 19.Rosenberg JB, Friedman IB, Gurland JE. Compliance with screening guidelines for diabetic retinopathy in a large academic children’s hospital in the Bronx. J Diabetes Complications. 2011;25(4):222–226. doi: 10.1016/j.jdiacomp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Valent D, Pestak K, Otis M, Shubrook J. Type 2 diabetes in the pediatric population: are we meeting ADA clinical guidelines in Ohio? Clin Pediatr (Phila) 2010;49(4):316–322. doi: 10.1177/0009922809344424. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology. 2001;108(3):563–571. doi: 10.1016/s0161-6420(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 22.Dorsey RR, Songer TJ, Zgibor JC, Orchard TJ. Influences on screening for chronic diabetes complications in type 1 diabetes. Dis Manag. 2006;9(2):93–101. doi: 10.1089/dis.2006.9.93. [DOI] [PubMed] [Google Scholar]
- 23.International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [Accessed February 14, 2017]; http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013.
- 24.Schneider EW, Mruthyunjaya P, Talwar N, Harris Nwanyanwu K, Nan B, Stein JD. Reduced fluorescein angiography and fundus photography use in the management of neovascular macular degeneration and macular edema during the past decade. Invest Ophthalmol Vis Sci. 2014;55(1):542–549. doi: 10.1167/iovs.13-13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wubben TJ, Talwar N, Blachley TS, et al. Rates of Vitrectomy among Enrollees in a United States Managed Care Network, 2001–2012. Ophthalmology. 2016;123(3):590–598. doi: 10.1016/j.ophtha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Vanderbeek BL, Zacks DN, Talwar N, Nan B, Musch DC, Stein JD. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011;152(2):273–282.e3. doi: 10.1016/j.ajo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderloo SE, Johnson JA, Reimer K, et al. Validation of classification algorithms for childhood diabetes identified from administrative data. Pediatr Diabetes. 2012;13(3):229–234. doi: 10.1111/j.1399-5448.2011.00795.x. [DOI] [PubMed] [Google Scholar]
- 28.Brown M, Kuhlman D, Larson L, et al. Does availability of expanded point-of-care services improve outcomes for rural diabetic patients? Prim Care Diabetes. 2013;7(2):129–134. doi: 10.1016/j.pcd.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Sloan FA, Bethel MA, Lee PP, Brown DS, Feinglos MN. Adherence to guidelines and its effects on hospitalizations with complications of type 2 diabetes. Rev Diabet Stud. 2004;1(1):29–38. doi: 10.1900/RDS.2004.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakhla M, Daneman D, To T, Paradis G, Guttmann A. Transition to adult care for youths with diabetes mellitus: findings from a Universal Health Care System. Pediatrics. 2009;124(6):e1134–e1141. doi: 10.1542/peds.2009-0041. [DOI] [PubMed] [Google Scholar]
- 31.Puent BD, Nichols KK. Patients’ perspectives on noncompliance with diabetic retinopathy standard of care guidelines. Optometry. 2004;75(11):709–716. doi: 10.1016/s1529-1839(04)70223-7. [DOI] [PubMed] [Google Scholar]
- 32.Hartnett ME, Key IJ, Loyacano NM, Horswell RL, Desalvo KB. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol. 2005;123(3):387–391. doi: 10.1001/archopht.123.3.387. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz B, O’Leary M, Fonseca-Becker F, et al. Knowledge of diabetic eye disease and vision care guidelines among Hispanic individuals in Baltimore with and without diabetes. Arch Ophthalmol. 2008;126(7):968–974. doi: 10.1001/archopht.126.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delorme C, Boisjoly HM, Baillargeon L, Turcotte P, Bernard PM. Screening for diabetic retinopathy: do family physicians know the Canadian guidelines? Can Fam Physician. 1998;44:1473–1479. [PMC free article] [PubMed] [Google Scholar]
- 35.Wiggins MN, Landes RD, Bhaleeya SD, Uwaydat SH. Primary care physicians’ knowledge of the ophthalmic effects of diabetes. Can J Ophthalmol. 2013;48(4):265–268. doi: 10.1016/j.jcjo.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25(11):1946–1951. doi: 10.2337/diacare.25.11.1946. [DOI] [PubMed] [Google Scholar]
- 37.Garg S, Jani PD, Kshirsagar AV, King B, Chaum E. Telemedicine and retinal imaging for improving diabetic retinopathy evaluation. Arch Intern Med. 2012;172(21):1677–1678. doi: 10.1001/archinternmed.2012.4372. [DOI] [PubMed] [Google Scholar]
- 38.Kolomeyer AM, Nayak NV, Simon MA, et al. Feasibility of retinal screening in a pediatric population with type 1 diabetes mellitus. J Pediatr Ophthalmol Strabismus. 2014;51(5):299–306. doi: 10.3928/01913913-20140709-01. [DOI] [PubMed] [Google Scholar]
- 39.Tapley JL, McGwin G, Jr, Ashraf AP, et al. Feasibility and efficacy of diabetic retinopathy screening among youth with diabetes in a pediatric endocrinology clinic: a cross-sectional study. Diabetol Metab Syndr. 2015;7:56. doi: 10.1186/s13098-015-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patty L, Wu C, Torres M, Azen S, Varma R Los Angeles Latino Eye Study Group. Validity of self-reported eye disease and treatment in a population-based study: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(9):1725–1730. doi: 10.1016/j.ophtha.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]