Abstract
Target agents are peculiar oncological drugs which differ from the traditional therapies in their ability of recognizing specific molecules expressed by tumor cells and microenvironment. Thus, their toxicity is generally lower than that associated to chemotherapy, and they represent nowadays a new standard of care in a number of tumors. This paper deals with the relationship between economic costs and toxicity of target agents. At this aim, a cluster analysis-based exploration of the main features of a large collection of them is carried out, with a specific focus on the variables leading to the identification of their toxicity and related costs. The analysis of the toxicity is based on the Severe Adverse Events (SAE) and Discontinuation (D) rates of each target agent considering data published on PubMed from 1965 to 2016 in the phase II and III studies that have led to the approval of these drugs for cancer patients by US Food and Drug Administration. The construction of the dataset represents a key step of the research, and is grounded on the critical analysis of a wide set of clinical studies. In order to capture different evaluation strategies of the toxicity, clustering is performed according to three different criteria (including Voronoi tessellation). Our procedure allows us to identify 5 different groups of target agents pooled by similar SAE and D rates and, at the same time, 3 groups based on target agents’ costs for 1 month and for the median whole duration of therapy. Results highlight several specific regularities for toxicity and costs. This study present several limitations, being realized starting from clinical trials and not from individual patients’ data. However, a macroscopic perspective suggests that costs are rather heterogeneous, and they do not clearly follow the clustering based on SAE and D rates.
Introduction
The present study aims at finding out whether there is a clear connection between the toxicity of novel anticancer drugs and their cost. To this end, we explore the information related to the rate of Severe Adverse Events (SAE) and the discontinuation (D) of a qualified set of oncological drugs. Such rates contribute to the creation of a so-called Toxicity Index (TI). Specifically, we have created a high-quality dataset by investigating the phase III studies in the context of the approval by the US Food and Drug Administration (FDA) of the target agents and of their introduction in the clinical practice.
The motivations for our study are of economic and social nature. In fact, cancer is one of the most costly health conditions to manage worldwide [1]. Anticancer agents have represented the 43% of new drugs approved by the FDA in the last decade [2]. The increase of drug spending in oncology is mainly due to the recent introduction of new targeted and immunotherapy agents [3], which have improved the outcome of cancer patients in terms of Overall Survival (OS) and Progression-Free Survival (PFS) compared to conventional chemotherapy. Although these agents are generally associated with a lower rate of treatment D due to drug toxicity, their impact on patients' Quality of Life (QoL) should not be overlooked. Improving patients’ QoL and their compliance to treatments will represent the challenge for cancer researchers in the future years. Indeed, by a purely economic perspective, reducing the toxic effects of these treatments will allow to decrease the abstention from work days and to increase productivity, hence leading to a wider access to cures due to a better economic status [4–8].
This paper can be properly inserted in the frame of pharmacoeconomics, which is a scientific discipline related to the cost and the value of drugs and provides suggestion for the optimal allocation of the healh care resources. This conceptualization was proposed by Townsend in 1987 [9], who identified the Pharmacoeconomics as “the description and the analysis of costs of therapeutic approch substained by the Health System and Society”. However, the first definition of Pharmacoeconomics dates back to 1977 when Weinstein and Stason [10] published a paper dealing with economic analysis in health field.
On the current scenario of rapidly rising health care costs, pharmaeconomic techniques are becoming increasingly relevant to analyze the cost-effectiveness and economic sustainability of emerging drugs [11]. Among such techniques, cluster analysis plays a relevant role. In fact, cluster analysis is used to identify groups of similar data based on selected variables and is particularly suitable for their comparison. The versatility of such a statistical technique explains also its popularity in many fields of applied science [12–20]. Indeed, cluster analysis seems to be appropiate for performing a global study of the connection between drug effectiveness, toxicity and cost. In this context, it is worth mentioning Perrier et al [14], who explored the transferability of health cost assessment between Italy and France. The authors constructed a hierarchical structure using cluster analysis and identified four different clusters based on diagnosis, surgery, chemotherapy and follow-up. Their findings showed that a high variability was present between this two countries, suggesting a low transferability of cost evaluations across Italy and France. Two years later, Liao et al. [15] performed an observational study on 18,380 patients with end-stage renal disease who initiated hemodialysis. By using K-means and hierarchical cluster analyses with either flexible beta or Ward’s methods, they identified 4 clusters based on sample sizes and change of cost patterns, finding that higher costs were correlated with more increasing comorbidity scores.
In our study we are different from the quoted papers since we first create a dataset containing clinical and economic information about all the oncological target agents approved in clinical practice. In this respect, it is important to recall that a target agent is a drug that is able to recognize one or more specific molecules expressed by tumor cells, immune cells or, more generally, by tumor microenvironment in cancer patients. The identification procedure has been rather complex -it mirrors the complexity of the faced problem- and represents a relevant step of the research.
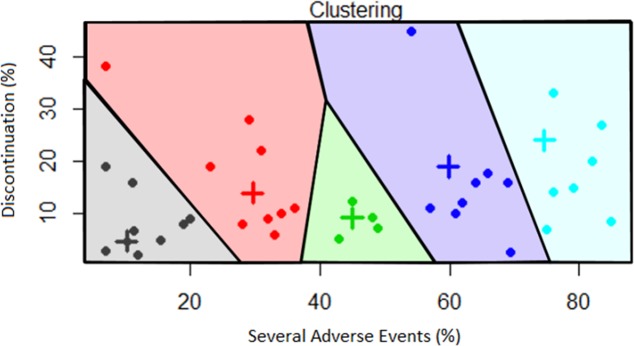
Furthermore, we have employed a method based on Voronoi tessellation [21], which represents a potential visualization of the subgroups identified by the cluster analysis. Voronoi diagram is a kind of decomposition of a given metric space based on the distance (which is Euclidean in the original formulation of Voronoi) to specified sites called centroids [21]. Particularly, each centroid recognizes data that are nearer to it than to the other centroids in accord to the given distance. By applying this technique, we are able to explore the way in which clusters of toxicity and costs overlap, hence giving information on the relationship between drug toxicity and related cost.
As we will see below, to gain more insights we depart from the original formulation of Voronoi and consider also minimum and maximum distances.
Cluster analysis, with a specific Voronoi diagrams approach, has been recently applied in the economic field [22–25]. In 2009, Liu et al. [22] explored the distribution of rural assessment using this technique. They showed that the distance from highways and rivers were the two factors that majorly influenced the distribution of rural settlements. More recently, Vaz et al. [23] reported a significant difference in term of regional innovation patterns as a consequence of istitutional innovation profiles.
As already mentioned above, we here investigate, through a cluster analysis procedure, whether there is a correlation between the cost of molecularly targeted and immnotherapy agents and their toxicity in terms of SAE and D rates.
To the best of our knowledge, this is the first paper dealing with toxicity and cost of target agents in oncology through a cluster analysis. More than this, the construction of the dataset on the basis of an exhaustive literature review is also a novelty in the oncological studies.
The rest of the paper is organized as follows. Section 2 collects the results of the analysis, while Section 3 provides a discussion of them. In Section 4 we present how the used dataset has been constructed and illustrate its main statistical properties. Furthermore, Section 4 contains also the description of the employed methodological tools, with a detailed explanation of the cluster analysis.
Materials and methods
Costruction of the dataset
The construction of the dataset has been implemented through a critical analysis of a wide set of clinical studies.
The selection of the relevant researchs has been carried out according to the instructions contained in the PRISMA [26] (S1 File). The scientific literature of interest has been identified from keywords selections on the PubMed database, in a period ranging from 1965 to 2016. Specifically, the research has been conducted by combining the words "cancer", "neoplasm", "solid tumor" and "clinical trial" with the name of each target agent.
As a second step, we have identified the papers dealing with human studies and randomized trials published in English and meeting the following criteria: 1) phase III studies conducted in patients with cancer; 2) random assignment of participants to treatment with a target therapy or a control (standard of care, placebo or best supportive care). In case of several publications related to the same experiment, only the most recent one or the most complete referring to included trial has been considered. Phase I and phase II trials has been excluded because of their variability and the lack of sufficient controls.
For each of the obtained papers we have reported the scientific study, the name of all authors, the name of the journal, the reference year, the number of the volume and the reference pages.
The resulting list of studies on target agents has been explored to assess the variables of interest related to the specific agent, i.e.: number of patients treated with target agents in the clinical studies, PFS (defined as the time from the start of therapy to disease progression or death), rate of all-grade AE and SAE (which leads to the necessity of medical assistence, hospitalization or drug interruption) and the D rate due to drug toxicity.
For the present research, we consider as variables the rate of SAE and the D rate, leaving the other ones for future studies.
Information on the costs of the target agents has been derived directly from their websites. All costs are expressed in American US Dollars.
Cluster analysis
Cluster analysis and Voronoi tesselation were performed by R software version 3.3.0 for Windows (62 megabytes, 32/64 bit). We have compared the clusters of target agents obtained when taking toxicity and when taking costs.
For what concerns toxicity, we have considered SAE and D rates as relevant variables. They are the parameters concurring in our conceptualization of the Toxicity Index (TI, hereafter).
The procedure of centroids selection has been implemented accordingly to clinical and scientific criteria, in order to represent the most meaningful groups of combinations of the two variables. For this analysis, we have reasonably considered five centroids as follows: ϕ1 = (10,5); ϕ2 = (30,15); ϕ3 = (45,10); ϕ4 = (60,20); ϕ5 = (75,25), where the first component is the SAE value while the second one represents the D rate. In particular, centroid ϕ1 is associated with low rate of SAE and low D rate, which leads to a low TI; ϕ2 has low-medium rate of SAE and medium D rate, which means low-medium TI; ϕ3 has medium rate of SAE and low-medium D rate (medium TI); ϕ4 represents medium-high rate of SAE and medium-high D rate (medium-high TI); ϕ5 identifies a cluster with high rate of SAE and high D rate (high TI). The cluster obtained by centroid ϕh will be denoted by Ch, for each h = 1,2,3,4,5. Moreover, by denoting the observations of SAE and D rates by the variables x and y, respectively, we also denote components of the centroid ϕh = (ϕh,x,ϕh,y), for each h = 1,2,3,4,5.
Clusters are identified by the nearness of the target agent toxicity with the centroids. At this aim, we apply three different concepts of distance: an Euclidean one–in accord to the original model of Voronoi-, the maximum and the minimum. Formally, for any given target agent j = 1,2,…,37 with SAE rate xj and D rate yj, we define
According to the specific metric selected, we derive the clusters of target agents as follows:
For what concerns the costs of the target agents, we have implemented two simple clusterings based on two variables. First, we have grouped the investigated drugs into three groups on the basis of 1-month cost patterns: cost less than 7,000$ (Group A), cost ranging from 7,000 to 11,000$ (Group B) and cost greater than 11,000$ (Group C). In the same way, drugs were grouped according to their costs extimated for the complete treatment for each patient within 3 groups: cost less than 40,000$ (Group D), cost ranging from 40,000$ to 80,000$ (Group E) and cost greater than 80,000$ (Group F).
Results
At the end of text analysis, we have obtained 4,803 studies concerning the use of molecular targeted drugs in cancer patients (the list of drugs is reported in the first column of Table 1).
Table 1. List of target agents employed in oncological patients.
Their characteristics are related to drug efficacy in terms of median Progression-Free Survival (PFS) and drug toxicity in terms of rate of all-grade, severe adverse events and discontinuation rate. BCC = Basal-cell Carcinoma; GIST = Gastrointestinal Stromal Tumor; NSCLC = Non Small Cell Lung Cancer; RCC = Renal Cell Carcinoma.
| Target Agent | First Authors, Year | Reference | Cancer Type | Number of Patients | Median PFS (Months) | All grade Adverse Events (%) | Severe Adverse Events(%) | D Rate (%) |
|---|---|---|---|---|---|---|---|---|
| Abiraterone acetate (first line therapy) | Charles JR, 2013 | 27 | Prostate | 546 | 16.5 | 99 | 48 | 10 |
| Abiraterone acetate (successive line-therapy) | de Bono S, 2011 | 28 | Prostate | 797 | 5.6 | 23 | 7 | 19 |
| Afatinib | Sequist LV, 2013 | 29 | NSCLC | 230 | 11.1 | NA | 49 | 8 |
| Bevacizumab | Friedman HS, 2009 | 30 | Glioblastoma | 82 | 5.6 | 100 | 65.8 | 17.7 |
| Bevacizumab | Escudier B, 2007 | 31 | RCC | 327 | 10.2 | 97 | 29 | 28 |
| Bevacizumab (first line therapy) | Hurwitz H, 2004 | 32 | Colorectal | 411 | 10.6 | NA | 84,9 | 8.4 |
| Bevacizumab (successive line-therapy) | Bennouna J, 2013 | 33 | Colorectal | 409 | 5.7 | 98 | 64 | 16 |
| Cabozantinib | Eisei R, 2013 | 34 | Thyroid | 219 | 11.2 | NA | 69 | 16 |
| Cetuximab | Vermorken JB, 2008 | 35 | Head and Neck | 222 | 5.5 | NA | 82 | 20 |
| Cobimetinib + Vemurafenib | Larkin J, 2014 | 36 | Melanoma | 247 | 9.9 | 95 | 62 | 12 |
| Crizotinib | Shaw AT, 2013 | 37 | NSCLC | 173 | 7.7 | NA | 33 | 6 |
| Enzalutamide (first line therapy) | Beer TM, 2015 | 38 | Prostate | 800 | 8.3 | 34 | 28 | 8 |
| Enzalutamide (successive line-therapy) | Scher HI, 2012 | 39 | Prostate | 872 | 5.7 | 97 | 43 | 6 |
| Erlotinib | Moore MJ, 2007 | 40 | Pancreas | 282 | 3.8 | 100 | 61 | 10 |
| Erlotinib (first line therapy) | Rosell R, 2012 | 41 | NSCLC | 86 | 9.7 | 98 | 45 | 13 |
| Erlotinib (maintainance therapy) | Cappuzzo F, 2010 | 42 | NSCLC | 438 | 2.9 | NA | 11 | 16 |
| Everolimus | Baselga J, 2012 | 43 | Breast | 482 | 7.8 | NA | 23 | 19 |
| Lenvatinib | Schlumberger M, 2015 | 44 | Thyroid | 261 | 14.7 | 97.3 | 75.9 | 14.2 |
| Nivolumab | Brahmer J, 2015 | 45 | Squamous NSCLC | 135 | 3.5 | 58 | 7 | 3 |
| Nivolumab | Borghaei H, 2015 | 46 | Non-Squamous NSCLC | 292 | 2.3 | 69 | 10 | 5 |
| Nivolumab | Robert C, 2015 | 47 | Melanoma | 210 | 5.1 | 74.3 | 11.7 | 2.4 |
| Nivolumab | Motzer RJ, 2015 | 48 | RCC | 410 | 4.6 | 79 | 19 | 8 |
| Palbociclib (+letrozole) | Finn RS, 2015 | 49 | Breast | 84 | 20.2 | 99 | 76 | 33 |
| Palbociclib (+fulvestrant) | Turner NC, 2015 | 50 | Breast | 347 | 9.2 | 97.7 | 69,3 | 2.6 |
| Pembrolizumab | Robert C, 2015 | 51 | Melanoma | 277 | 4.1 | 72.9 | 75 | 6.9 |
| Ramucirumab | Fuchs CS, 2014 | 52 | Gastric | 238 | 2.1 | 94 | 57 | 11 |
| Ramucirumab | Garon EB, 2014 | 53 | NSCLC | 628 | 4.5 | 98 | 79 | 15 |
| Ramucirumab | Tabernero J, 2015 | 54 | Colorectal | 536 | 5.7 | 83 | 36 | 11 |
| Regorafenib | Grothey A, 2013 | 55 | Colorectal | 505 | 1.9 | 93 | 54 | 44.8 |
| Sonidegib | Midgen MR, 2015 | 56 | BCC | 79 | 13.1 | 95 | 31 | 22 |
| Sorafenib | Escudier B, 2007 | 57 | RCC | 451 | 5.5 | NA | 34 | 10 |
| Sunitinib | Motzer RJ, 2009 | 58 | RCC | 375 | 11 | NA | 7 | 38 |
| Sunitinib | Demetri GD, 2006 | 59 | GIST | 207 | 6.4 | 83 | 20 | 9 |
| T-DM1 | Verma S, 2012 | 60 | Breast | 495 | 9.6 | 95.9 | 15,5 | 5 |
| Temsirolimus | Hudes G, 2007 | 61 | RCC | 209 | 3.8 | NA | 11 | 7 |
| Trametinib + Dabrafenib | Long GV, 2014 | 62 | Melanoma | 211 | 9.3 | 95 | 32 | 9 |
| Ziv-Aflibercept | Van Cutsem E, 2012 | 63 | Colorectal | 612 | 6.9 | 99.2 | 83,5 | 26.8 |
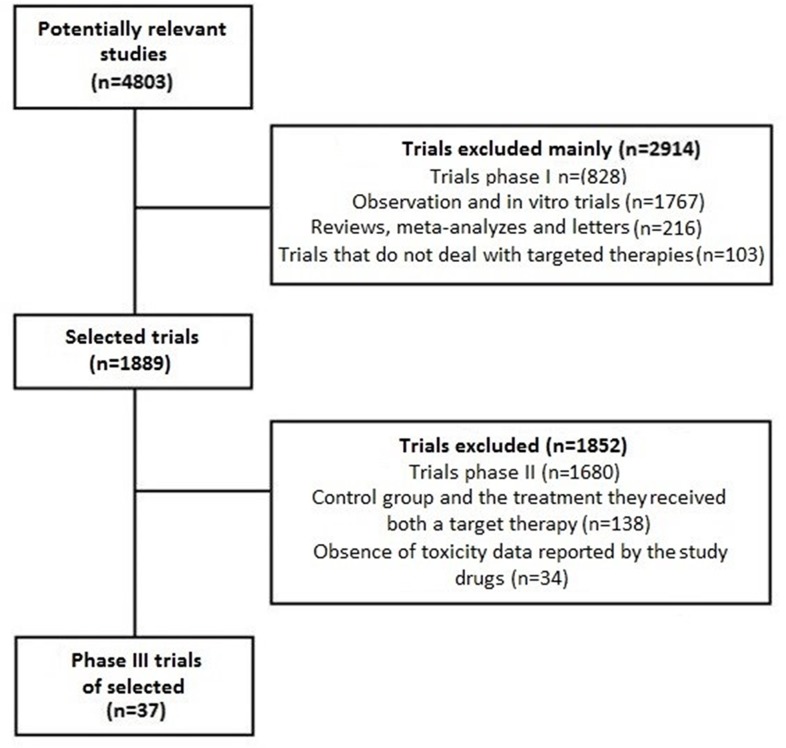
Therefore, 2,914 of the 4,083 original papers have been excluded because of phase I studies, observational, in vitro, reviews or letters about targeted therapies. Of the 1,889 remained studies, 1,852 were excluded because dealing with phase II or because not containing data on the SAE and D rates.
As a result, we have found 23 target agents that are used in 37 different therapeutic settings [27–63] (Table 1).
The identification of the relevant papers is described in Fig 1, where it is presented a block diagram of the PRISMA procedure.
Fig 1. Study selection according to PRISMA statement.
Table 2 contains the main statistical indicators of the dataset. The mean/std. dev. ratio allows additional considerations about the heterogeneity within the clusters, which is low, supporting that each cluster includes similar drugs both in terms of SAE and D rates.
Table 2. Main statistical indicators of the dataset.
| Number of patients | DRUG EFFECTIVENESS | DRUG TOXICITY | |||
|---|---|---|---|---|---|
| Median PFS (months) | All grade adverse events (%) | Severe adverse events (%) | Discontinuation rate (%) | ||
| Mean | 356 | 7.60 | 86 | 44 | 14 |
| Std. Dev. | 205 | 4.14 | 20 | 26 | 10 |
| Mean/Std. Dev. | 1.73 | 1.84 | 4.30 | 1.68 | 1.42 |
| Min | 79 | 1.9 | 23 | 7 | 2.4 |
| Max | 872 | 20.2 | 100 | 84.9 | 45 |
| Median | 292 | 6.4 | 95 | 43 | 11 |
| Skewness | 0.83 | 1.03 | -2.06 | 0.10 | 1.48 |
| Kurtosis | 0.23 | 1.21 | 3.95 | -1.38 | 2.19 |
| Q1 | 211 | 4.6 | 81 | 20 | 8 |
| Q3 | 482 | 9.9 | 98 | 65.8 | 17.7 |
Concerning skewness, it is relevant to note that only the rate of all grade adverse events is negative (-2.06) with a curve of distribution characterized by a longer left tail with a median of patients developing at least an adverse event (95%) that overcross the mean of patients (86%). Further information can be added by observing the leptokurtic distribution of all grade adverse events (curtosis is 3.95), while the distribution of SAE is platykurtic (curtosis is -1.38).
It is also important to observe the response rates reported by target agents, which range from 1% to 80% (Table 2). Such a result underlines the extreme variety of actions of these new generation agents that can improve patient survival without reducing tumour sizes.
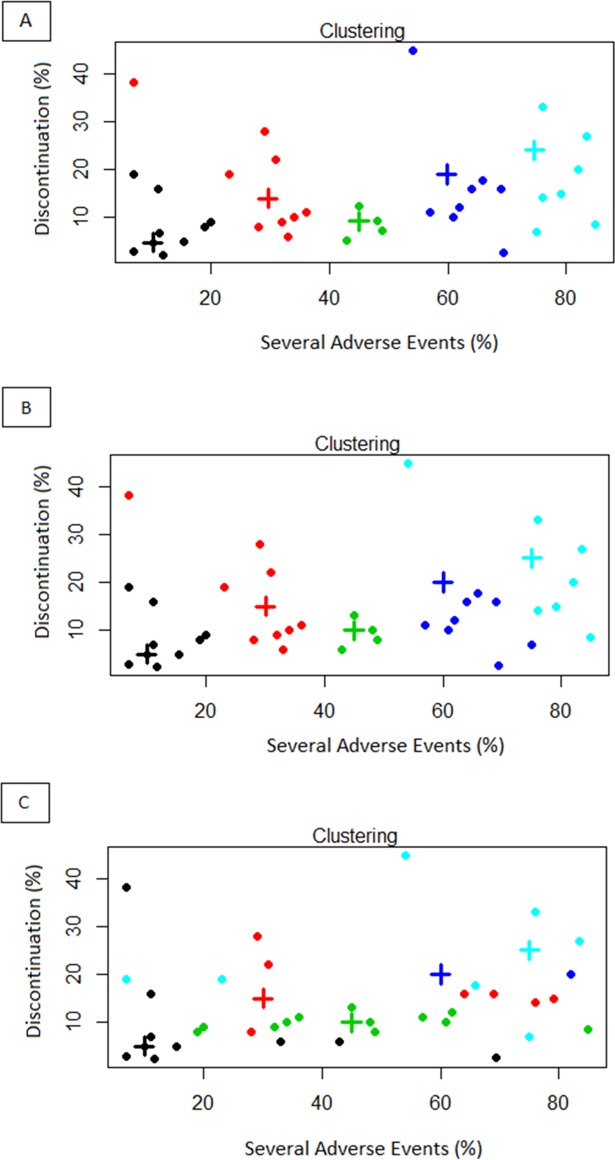
Fig 2A, 2B and 2C show the clusters based on Euclidean distance, maximum distance and minimum distance, respectively. A spatial representation of the dynamic fields related to cluster analysis by Euclidean distance has been obtained by Voronoi diagram as reported in Fig 3.
Fig 2.
Cluster analysis based on Toxicity Index (TI) considering Euclidean distance (A), maximum distance (B) and minimum distance (C). The “+” represent the centroids.
Fig 3. Voronoi tesselation based on Toxicity Index (TI) considering Euclidean distance.
The results of cluster analysis with Euclidean distance show a major similarity with the findings obtained by the maximum distance. In particular, such clustering criteria place in two different clusters only two drugs (Regorafenib, charaterized by SAE and D rates of 54 and 44.8, respectively, and Pembrolizumab, with SAE and D rates of 75 and 6.9, respectively. They belong to cluster 4 based on Euclidean distance and to cluster 5 according to the maximum distance). Differently, the clusters based on minimum distance are markedly different from both the other analyses.
It is interesting to note that the highest cost for a month and per PFS are represented by the combination of Cobimetinib and Vemurafenib and the lowest by Erlotinib (when used for patients with pancreatic cancer). The mean and median montly costs are 9,366 $ and 8,627 $, respectively. On the other hand, the mean and median costs per PFS are 73,154 $ and 49,500 $, respectively (Table 3).
Table 3. List of target agents approved for their use in cancer patients and related costs.
BCC = Basal-cell Carcinoma; GIST = Gastrointestinal Stromal Tumor; NSCLC = Non Small Cell Lung Cancer; RCC = Renal Cell Carcinoma.
| Target Agent | Cancer Type | Monthly cost ($) | Cost per PFS ($) |
|---|---|---|---|
| Abiraterone acetate (first line therapy) | Prostate | 8,627 | 142,346 |
| Abiraterone acetate (successive line-therapy) | Prostate | 8,627 | 48,311 |
| Afatinib | NSCLC | 6,970 | 77,367 |
| Bevacizumab | Glioblastoma | 4,400 | 24,640 |
| Bevacizumab | RCC | 4,400 | 44,880 |
| Bevacizumab (first line therapy) | Colorectal | 2,680 | 28,408 |
| Bevacizumab (successive line-therapy) | Colorectal | 2,680 | 15,276 |
| Cabozantinib | Thyroid | 14,300 | 160,160 |
| Cetuximab | Head and Neck | 7,000 | 38,500 |
| Cobimetinib + Vemurafenib | Melanoma | 26,300 | 260,370 |
| Crizotinib | NSCLC | 11,500 | 88,550 |
| Enzalutamide (first line therapy) | Prostate | 7,450 | 61,835 |
| Enzalutamide (successive line-therapy) | Prostate | 7,450 | 42,465 |
| Erlotinib | Pancreas | 2,450 | 9,310 |
| Erlotinib (first line thrapy) | NSCLC | 3,000 | 29,100 |
| Erlotinib (maintainance therapy) | NSCLC | 3,000 | 8,700 |
| Everolimus | Breast | 7,000 | 54,600 |
| Lenvatinib | Thyroid | 13,945 | 204,992 |
| Nivolumab | Squamous NSCLC | 12,600 | 44,100 |
| Nivolumab | Non-Squamous NSCLC | 12,600 | 28,980 |
| Nivolumab | Melanoma | 12,600 | 64,260 |
| Nivolumab | RCC | 6,984 | 32,126 |
| Palbociclib (+letrozole) | Breast | 9,850 | 198,970 |
| Palbociclib (+fulvestrant) | Breast | 9,850 | 90,620 |
| Pembrolizumab | Melanoma | 23,017 | 94,370 |
| Ramucirumab | Gastric | 13,000 | 27,300 |
| Ramucirumab | NSCLC | 11,000 | 49,500 |
| Ramucirumab | Colorectal | 13,000 | 74,100 |
| Regorafenib | Colorectal | 7,600 | 14,440 |
| Sonidegib | BCC | 12,000 | 157,200 |
| Sorafenib | RCC | 6,600 | 36,300 |
| Sunitinib | RCC | 7,000 | 77,000 |
| Sunitinib | GIST | 7,000 | 44,800 |
| T-DM1 | Breast | 9,800 | 94,080 |
| Temsirolimus | RCC | 2,960 | 11,248 |
| Trametinib + Dabrafenib | Melanoma | 16,300 | 151,590 |
| Ziv-Aflibercept | Colorectal | 11,000 | 75,900 |
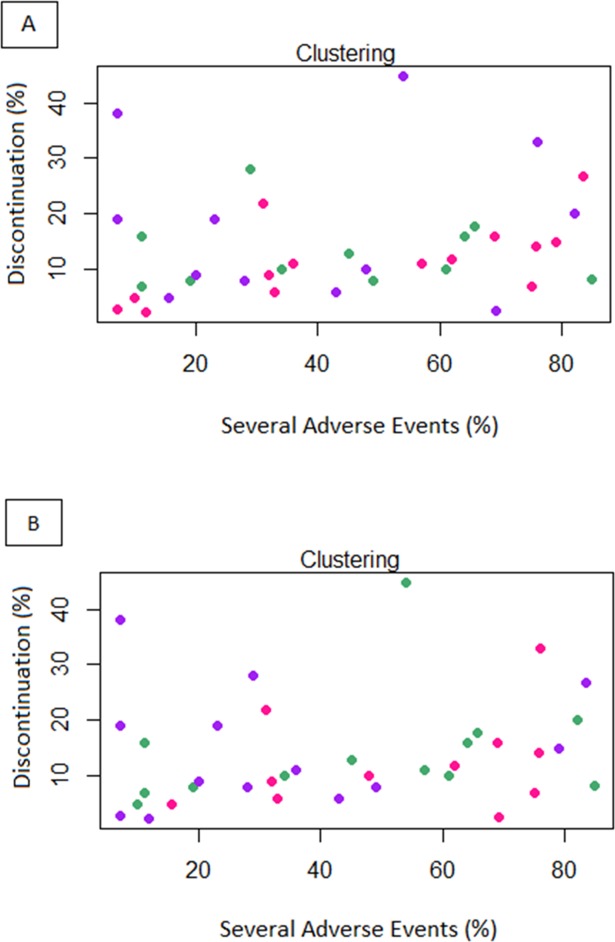
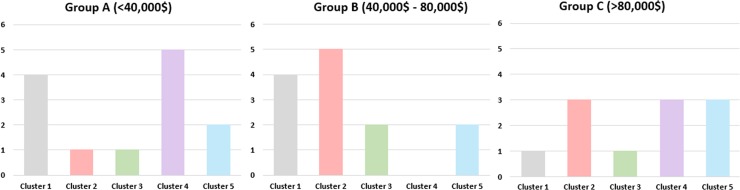
Both Fig 4A and 4B show that heterogeneous cost distribution that doesn’t clearly follow the cluster division based on TI. However, some illustrations of the relationship between toxicity and costs can be carried out at the single clusters level. For instance, as for the 1-month cost, the higher rate of drugs from Group C belongs to Cluster 5, whilst the higher percentage of drugs from Group A are included in Cluster 3. Concerning the total cost estimated for a single patient for the whole treatment, the higher rate of drugs belonging to Group D belongs to Cluster 4, whilst the higher percentage of drugs from Group F are in Cluster 5. The complete distribution of costs within the 5 clusters is reported in Table 4 and Fig 5.
Fig 4.
Cluster analysis based on Euclidean distance considering the drug costs for 1-month (A) or for the median total duration of therapy (B) for a single oncological patient. Green points represent drugs with low cost (Group A), violet points drugs with medium cost (Group B) and pink with high cost for 1-month of treatment (Group C).
Table 4. Distribution of costs within the 5 clusters based on TI.
| 1-month treatment cost | Total cost for a single patient (estimated by PFS) | |||||
|---|---|---|---|---|---|---|
| Group A (%) | Group B (%) | Group C (%) | Group D (%) | Group E (%) | Group F (%) | |
| Cluster 1 | 33 | 33 | 34 | 44 | 44 | 12 |
| Cluster 2 | 22 | 34 | 44 | 11 | 56 | 33 |
| Cluster 3 | 50 | 50 | 0 | 25 | 50 | 25 |
| Cluster 4 | 38 | 24 | 38 | 63 | 0 | 37 |
| Cluster 5 | 14 | 28 | 58 | 29 | 29 | 42 |
Fig 5. The distribution of different clusters into the three cost categories related to the amount for median Progression-Free Survival (PFS).
Discussion
Our paper concerns the study of the relationship between the toxicity and cost of newly approved target agents in the Oncology field. All the drugs approved by FDA have been considered. Variables related to SAE and D rates have been collected from published phase III studies.
Cluster analysis has been employed to explore such a relationship. Specifically, three different clustering criteria based on the Euclidean distance–in accord to the standard Voronoi tessellation definition- and maximum and minimum distances have been considered.
To interpret the outcomes of the analysis, we need to provide an intuitie description of the clustering criteria.
The minimum distance is the one that underestimate the toxicity level, in that it may place a drug in a low-toxicity cluster even if some related parameters are of remarkable high level.
Differently, the maximum distance is more “cautios” and overestimates the level of toxicity, since it may insert an agent into a high-toxicity cluster even when some toxicity parameters exhibit a low value.
The “fair” situation is captured by the Euclidean distance, which is the one used in the original Voronoi model. The comparison among the results of the clustering proedures suggests that taking a definition of toxicity that may imply its overestimation is closer to fairness than dealing with an understimation criterion.
It is important to note that the toxicity associated with oncological drugs implicates a high-cost management. In this regard, previous studies have tried to quantify this amount. For example, Roncato et al. [64] evaluated the economic burden of Irinotecan-related toxicity in patients with metastatic colorectal cancer, revealing that the mean predicted cost per patient was 4,886 €. On the other hand, Arondekar et al. [65] investigated the costs of AEs in 2,621 patients with metastatic melanoma by employing multivariate generalized linear models (GLMs) with a log-link function and gamma distribution. They reported a 30-day incremental cost of over 9,000 $ for metabolic AEs, 8,450 $ for hematologic, 6,476 $ for cardiovascular and 6,338 $ for gastrointestinal AEs [65].
Similarly, Bilir et al. [66] studied the economic burden of toxicities associated with treating metastatic melanoma in the United States. They registered that the highest mean in patient costs for an AE were associated with acute myocardial infarction, sepsis, and coma, ranging from 31,682 $ to 47,069 $. In addition, the mean cost for hospitalization due to other AEs ranged from 19,122 $ to 26,861 $ [66].
The quantification of the economic impact related to the toxicity of target agents will represent a major step forward in the phases of drug approval and cost establishement, representing a fundamental parameter that must be considered during these processes.
In the last years, several techniques of drug reimboursement have been introduced in the pharmacoeconomic scenario and are currently employed in the oncological field. These modalities include: (1) payment by results, which consists in the total refund by the manyfacturer for non-responding patients; (2) risk sharing, which provides for a partial refund for non-responding patients after a clinical/radiological evalutation; (3) cost sharing, which sets an initial discount for all treated patients. These techniques have become even more fundamental after the introduction of immunotherapy in the therapeutic armamentarium of cancer patients. In fact, these agents are characterized by both relevant cost and high efficacy, supporting the research for new tools aimed to optimize the use of economic resources in the health system. In this respect, Russi et al. [67] proposed a new cost-containmet strategy for the use of immunotherapic agent ipilimumab for patients with melanoma in Italy. This model included, by one side, drug-day and centralization of compounding (accounting for a reduction of -11.1% of drug cost) and, by the other side, payback systems designed by AIFA (resulting in additional -6.2%) [67].
Our study present several limitations. First of all it is a systematic review realized starting from clinical trials and not from individual patients’ data. Thus, data on drug toxicity might be influenced by confounding factors such as the presence of different tumors, patients’ comorbidities or simultaneous treatments. Furthermore, patients eligible for clinical trials mostly show fair organ functions, leading to a potential underestimation of drug toxicity compared to clinical practice. Finally, we are awared that the various toxicities considered in our analysis may have a different impact on patient QoL and a wide range of clinical consequences, although we considered only SAEs that lead to patient hospitalization and/or medical interventions and the D rate.
In face of these limitations, at a macroscopic level, our analysis highlights that there is a not straightforward relationship between the toxicity of target agents and their relative costs for 1-month or the whole treatment duration. However, we can notice that the number of target agents with high costs results more relevant in the clusters associated with the worst drug-tolerability (high SAE and D rates), although they belong also to the cluster characterized by better safety (low SAE and D rates).
Interestingly, data on kurtosis and skewness underline that a high percentage of cancer patients treated with molecularly target agents do experience at least one all grade adverse event. The toxicity of these drugs, altough lower than that associated with chemotherapy, suggest that the costs of management of adverse events must be considered during the phases of approval and price negotiation.
To sum up, the relationship between the cost and the efficacy and toxicity of new generation drugs does not follows a regular path. However, the constructed database and the findings here obtained can be efficiently used for the development of a unified theory on the cost management of treating cancer patients and on the study of the impact of these agents on their QoL.
Supporting information
List of the indications provided by the “PRISMA statment” for the realization of meta-analyses and systematic review.
(DOC)
Acknowledgments
We thank Dr. Matteo Santoni for his support in the revision of the oncological data employed in this analysis.
Data Availability
Data are available directly on the paper, and have been collected by a critical reading of the scientific literature of interest. The source of the publications is the PubMed database.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Soni A. Trends in the Five Most Costly Conditions among the U.S. Civilian Institutionalized Population, 2002 and 2012 Statistical Brief 470. Rockville, Md: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 2.http://www.fda.gov; accessed on December 10th, 2016
- 3.Bradley CJ, Yabroff KR, Warren JL, Zeruto C, Chawla N, Lamont EB. Trends in the Treatment of Metastatic Colon and Rectal Cancer in Elderly Patients. Med Care. 2016;54: 490–497. doi: 10.1097/MLR.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 4.Shih YC, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J Clin Oncol. 2015;33: 2190–2196. doi: 10.1200/JCO.2014.58.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekwueme DU, Yabroff KR, Guy GP Jr, Banegas MP, de Moor JS, Li C, et al. Medical costs and productivity losses of cancer survivors United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2014;63: 505–510. [PMC free article] [PubMed] [Google Scholar]
- 6.Guy GP Jr, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriguez JL, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31: 3749–3757. doi: 10.1200/JCO.2013.49.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy GP Jr, Yabroff KR, Ekwueme DU, Smith AW, Dowling EC, Rechis R, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff (Millwood). 2014;33: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy GP Jr, Yabroff KR, Ekwueme DU, Virgo KS, Han X, Banegas MP, et al. Healthcare Expenditure Burden Among Non-elderly Cancer Survivors, 2008–2012. Am J Prev Med. 2015;49: S489–497. doi: 10.1016/j.amepre.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend RJ. Post-marketing drug research and development. Drug Intell Clin Pharm. 1987;21: 134–136. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein MC, Stason WB. Foundations of costo-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296: 716–721. doi: 10.1056/NEJM197703312961304 [DOI] [PubMed] [Google Scholar]
- 11.Zanfina A, Hansoo K, Ella Z, Christopher MR, Bruce H, Danny L. Overview of pharmacoeconomic modelling methods. Br J Clin Pharmacol. 2013;75: 944–950. doi: 10.1111/j.1365-2125.2012.04421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Řezanková Hana. Cluster Analysis of Economic Data. STATISTIKA 94(1); 2014. [Google Scholar]
- 13.Eisen MB, Spellman PT, Brown PO, Botstein D. Genetics Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. pp.14863–14868; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lionel P, Alessandra B, Giuseppe M, Patrick SB, Françoise D, Petrus JP, et al. Transferability of health cost evaluation across locations in oncology: cluster and principal component analysis as an explorative tool. BMC Health Serv Res. 2014;14: 537 doi: 10.1186/s12913-014-0537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao M, Li Y, Kianifard F, Obi E, Arcona S. Cluster analysis and its application to healthcare claims data: a study of end-stage renal disease patients who initiated hemodialysis. BMC Nephrol. 2016;17: 25 doi: 10.1186/s12882-016-0238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Requia WJ, Koutrakis P, Roig HL, Adams MD, Santos CM. Association between vehicular emissions and cardiorespiratory disease risk in Brazil and its variation by spatial clustering of socio-economic factors. Environ Res. 2016;150: 452–460. doi: 10.1016/j.envres.2016.06.027 [DOI] [PubMed] [Google Scholar]
- 17.Blanco MR, Martin JS, Kahlscheuer ML, Krishnan R, Abelson J, Laederach A, et al. Single Molecule Cluster Analysis dissects splicing pathway conformational dynamics. Nat Methods. 2015;12: 1077–1084. doi: 10.1038/nmeth.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber S, Horenko I. Improving clustering by imposing network information. Sci Adv. 2015;1: e1500163 doi: 10.1126/sciadv.1500163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailly S, Destors M, Grillet Y, Richard P, Stach B, Vivodtzev I, et al. scientific council and investigators of the French national sleep apnea registry (OSFP). Obstructive Sleep Apnea: A Cluster Analysis at Time of Diagnosis. PLoS One. 2016;11: e0157318 doi: 10.1371/journal.pone.0157318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong NS, Huang S, Chen L, Zhao P, Tucker JD, Yang LG, et al. Spatiotemporal clusters of primary and secondary syphilis cases in south China: an observational study. Lancet. 2016;388 Suppl 1: S90. [Google Scholar]
- 21.Voronoi GF. Nouvelles applications des paramtres continus la thorie de formes quadratiques, Journal fÄur die reine und angewandte Mathematik. 1908;134: 198–287. [Google Scholar]
- 22.Liu XT, Zheng XQ, Li DB. Voronoi Diagram-Based Research on Spatial Distribution Characteristics of Rural Settlements and Its A®ecting FactorsA Case Study of Changping District, Beijing. Journal of Ecology and Rural Environment 2,007; 2009. [Google Scholar]
- 23.Vaz E, de Noronha Vaz T, Galindo PV, Nijkamp P. Modelling innovation support systems for regional developmentanalysis of cluster structures in innovation in Portugal. Entrepreneurship and Regional Development. 2014;26: 23–46. [Google Scholar]
- 24.https://howmuch.net/articles/us-economy-summarized-in-one-diagram; accessed on December 10th, 2016
- 25.http://www.relativelyinteresting.com/world-economy-visualized-one-brilliant-diagram; accessed on December 10th, 2016
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: 25–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368: 138–48. doi: 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364: 1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung denocarcinoma with EGFR mutations. J Clin Oncol. 2013;31: 3327–3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 30.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in current glioblastoma. J Clin Oncol. 2009;27: 473–4740. [DOI] [PubMed] [Google Scholar]
- 31.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007; 370: 2103–2111. doi: 10.1016/S0140-6736(07)61904-7 [DOI] [PubMed] [Google Scholar]
- 32.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350: 2335–2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 33.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14: 29–37. doi: 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 34.Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013. (29):3639–3646. doi: 10.1200/JCO.2012.48.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359: 1116–1127. doi: 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371: 1867–1876. doi: 10.1056/NEJMoa1408868 [DOI] [PubMed] [Google Scholar]
- 37.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368: 2385–2394. doi: 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 38.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371: 424–33. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367: 1187–1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 40.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25: 1960–1966. doi: 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 41.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13: 239–246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 42.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11: 521–529. doi: 10.1016/S1470-2045(10)70112-1 [DOI] [PubMed] [Google Scholar]
- 43.Baselga J1, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366: 520–529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372: 621–630. doi: 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 45.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373: 123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373: 1627–1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372: 320–330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 48.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373: 1803–1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16: 25–35. doi: 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 50.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373: 209–219. doi: 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 51.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372: 2521–2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 52.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383: 31–39. doi: 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 53.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384: 665–673. doi: 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 54.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16: 499–508. doi: 10.1016/S1470-2045(15)70127-0 [DOI] [PubMed] [Google Scholar]
- 55.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381: 303–312. doi: 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 56.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16: 716–728. doi: 10.1016/S1470-2045(15)70100-2 [DOI] [PubMed] [Google Scholar]
- 57.Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, et al. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13: 1801–1809. doi: 10.1158/1078-0432.CCR-06-1432 [DOI] [PubMed] [Google Scholar]
- 58.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27: 3584–3590. doi: 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368: 1329–1338. doi: 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 60.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367: 1783–1791. doi: 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356: 2271–2281. doi: 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 62.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371: 1877–1888. doi: 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 63.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;(28): 3499–3506. doi: 10.1200/JCO.2012.42.8201 [DOI] [PubMed] [Google Scholar]
- 64.Roncato R, Cecchin E, Montico M, De Mattia E, Giodini L, Buonadonna A, et al. Cost Evaluation of Related Toxicities Associated With the UGT1A1*28 Patient Genotype. Clin Pharmacol Ther. 2017; doi: 10.1002/cpt.615 [DOI] [PubMed] [Google Scholar]
- 65.Arondekar B, Curkendall S, Monberg M, Mirakhur B, Oglesby AK, Lenhart GM, et al. Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm. 2015;(2): 158–164. doi: 10.18553/jmcp.2015.21.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilir SP, Ma Q, Zhao Z, Wehler E, Munakata J, Barber B. Economic Burden of Toxicities Associated with Treating Metastatic Melanoma in the United States. Am Health Drug Benefits. 2016;(4): 203–213. [PMC free article] [PubMed] [Google Scholar]
- 67.Russi A, Chiarion-Sileni V, Damuzzo V, Di Sarra F, Pigozzo J, Palozzo AC. Case study on an Ipilimumab cost-containment strategy in an Italian hospital. Int J Technol Assess Health Care. 2017;13: 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the indications provided by the “PRISMA statment” for the realization of meta-analyses and systematic review.
(DOC)
Data Availability Statement
Data are available directly on the paper, and have been collected by a critical reading of the scientific literature of interest. The source of the publications is the PubMed database.