Abstract
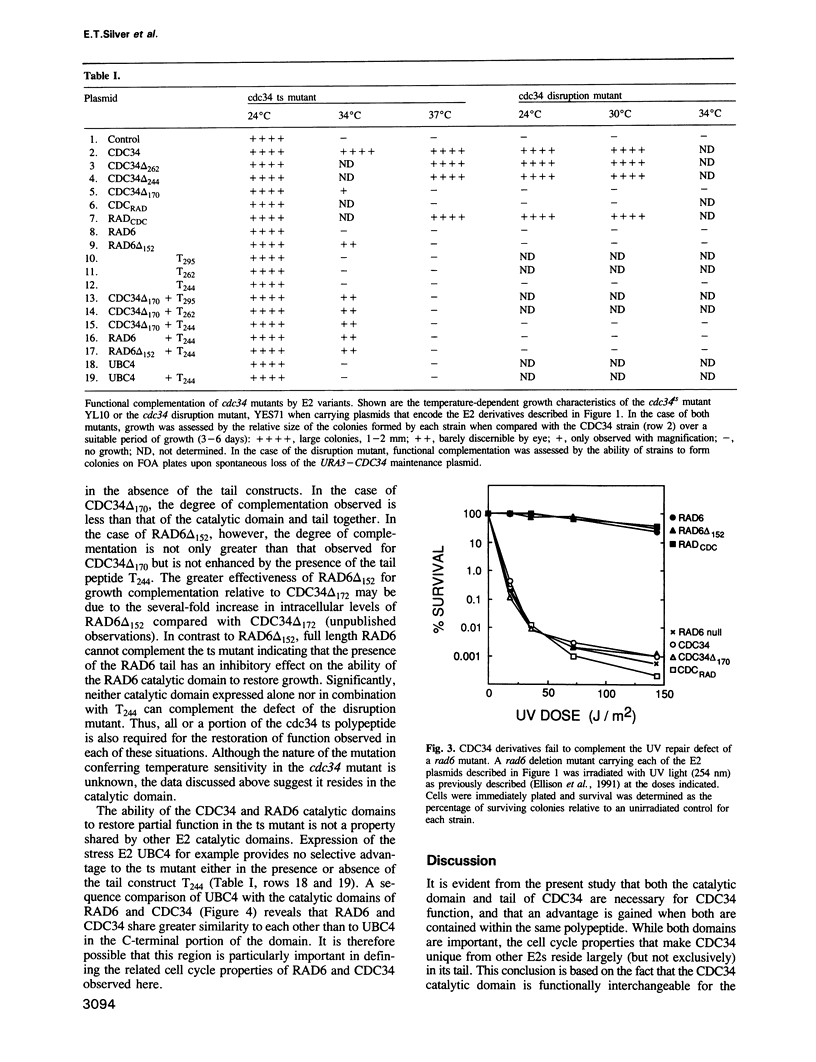
The CDC34 (UBC3) protein from Saccharomyces cerevisiae has a 125 residue tail that contains a polyacidic region flanked on either side by sequences of mixed composition. We show that although a catalytic domain is essential for CDC34 activity, a major cell cycle determinant of this enzyme is found within a 74 residue segment of the tail that does not include the polyacidic stretch or downstream sequences. Transposition of the CDC34 tail onto the catalytic domain of a functionally unrelated E2 such as RAD6 (UBC2) results in a chimeric E2 that combines RAD6 and CDC34 activities within the same polypeptide. In addition to the tail, the cell cycle function exhibited by the chimera and CDC34 is probably dependent on a conserved region of the catalytic domain that is shared by both RAD6 and CDC34. Despite this similarity, the CDC34 catalytic domain cannot substitute for the DNA repair and growth functions of the RAD6 catalytic domain, indicating that although these domains are structurally related, sufficient differences exist to maintain their functional individuality. Expression of the CDC34 catalytic domain and tail as separate polypeptides are capable of only partial function; thus, while the tail displays autonomous structural characteristics, there is considerable advantage gained when both domains coexist within the same polypeptide. The ability of these and other derivatives to restore partial function to a cdc34 temperature-sensitive mutant but not to a disruption mutant suggests that interaction between two CDC34 polypeptides is a requirement of CDC34 activity. Based on this idea we propose a model that accounts for the initiating steps leading to multi-ubiquitin chain synthesis.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmair A., Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989 Mar 24;56(6):1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989 Mar 24;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Chen Z., Pickart C. M. A 25-kilodalton ubiquitin carrier protein (E2) catalyzes multi-ubiquitin chain synthesis via lysine 48 of ubiquitin. J Biol Chem. 1990 Dec 15;265(35):21835–21842. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen R. J., Madura K., Bartel B., Varshavsky A. The N-end rule is mediated by the UBC2(RAD6) ubiquitin-conjugating enzyme. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7351–7355. doi: 10.1073/pnas.88.16.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Khan M. I., Marsh J., Butt T. R., Crooke S. T. Chemical synthesis and expression of a cassette adapted ubiquitin gene. J Biol Chem. 1987 Mar 15;262(8):3524–3527. [PubMed] [Google Scholar]
- Ellison K. S., Gwozd T., Prendergast J. A., Paterson M. C., Ellison M. J. A site-directed approach for constructing temperature-sensitive ubiquitin-conjugating enzymes reveals a cell cycle function and growth function for RAD6. J Biol Chem. 1991 Dec 15;266(35):24116–24120. [PubMed] [Google Scholar]
- Ellison M. J., Hochstrasser M. Epitope-tagged ubiquitin. A new probe for analyzing ubiquitin function. J Biol Chem. 1991 Nov 5;266(31):21150–21157. [PubMed] [Google Scholar]
- Goebl M. G., Yochem J., Jentsch S., McGrath J. P., Varshavsky A., Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988 Sep 9;241(4871):1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- Gregori L., Poosch M. S., Cousins G., Chau V. A uniform isopeptide-linked multiubiquitin chain is sufficient to target substrate for degradation in ubiquitin-mediated proteolysis. J Biol Chem. 1990 May 25;265(15):8354–8357. [PubMed] [Google Scholar]
- Haas A. L., Reback P. B., Chau V. Ubiquitin conjugation by the yeast RAD6 and CDC34 gene products. Comparison to their putative rabbit homologs, E2(20K) AND E2(32K). J Biol Chem. 1991 Mar 15;266(8):5104–5112. [PubMed] [Google Scholar]
- Hershko A., Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985 May 16;128(3):1079–1086. doi: 10.1016/0006-291x(85)91050-2. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Jentsch S., McGrath J. P., Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987 Sep 10;329(6135):131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- Koken M. H., Reynolds P., Jaspers-Dekker I., Prakash L., Prakash S., Bootsma D., Hoeijmakers J. H. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M., Reynolds P., Bootsma D., Hoeijmakers J., Prakash S., Prakash L. Dhr6, a Drosophila homolog of the yeast DNA-repair gene RAD6. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3832–3836. doi: 10.1073/pnas.88.9.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman C. J., Toth J., Gonda D. K. Identification of a portable determinant of cell cycle function within the carboxyl-terminal domain of the yeast CDC34 (UBC3) ubiquitin conjugating (E2) enzyme. EMBO J. 1992 Aug;11(8):3081–3090. doi: 10.1002/j.1460-2075.1992.tb05380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Kent J. C., Hartwell L. H. Genetic analysis of the mitotic transmission of minichromosomes. Cell. 1985 Feb;40(2):393–403. doi: 10.1016/0092-8674(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Morrison A., Miller E. J., Prakash L. Domain structure and functional analysis of the carboxyl-terminal polyacidic sequence of the RAD6 protein of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Mar;8(3):1179–1185. doi: 10.1128/mcb.8.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Nakajima B., Nomura M., Arfin S. M. Cloning and characterization of a Saccharomyces cerevisiae gene encoding a new member of the ubiquitin-conjugating protein family. J Biol Chem. 1991 Aug 15;266(23):15549–15554. [PubMed] [Google Scholar]
- Reynolds P., Koken M. H., Hoeijmakers J. H., Prakash S., Prakash L. The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae. EMBO J. 1990 May;9(5):1423–1430. doi: 10.1002/j.1460-2075.1990.tb08258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Weber S., Prakash L. RAD6 gene of Saccharomyces cerevisiae encodes a protein containing a tract of 13 consecutive aspartates. Proc Natl Acad Sci U S A. 1985 Jan;82(1):168–172. doi: 10.1073/pnas.82.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Eckerskorn C., Lottspeich F., Schweiger M. The human ubiquitin carrier protein E2(Mr = 17,000) is homologous to the yeast DNA repair gene RAD6. EMBO J. 1990 May;9(5):1431–1435. doi: 10.1002/j.1460-2075.1990.tb08259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990 Feb;9(2):543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. L., Vierstra R. D. A ubiquitin carrier protein from wheat germ is structurally and functionally similar to the yeast DNA repair enzyme encoded by RAD6. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9861–9865. doi: 10.1073/pnas.86.24.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Berleth E., Pickart C., Prakash S., Prakash L. Yeast RAD6 encoded ubiquitin conjugating enzyme mediates protein degradation dependent on the N-end-recognizing E3 enzyme. EMBO J. 1991 Aug;10(8):2187–2193. doi: 10.1002/j.1460-2075.1991.tb07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Prakash S., Prakash L. The RAD6 protein of Saccharomyces cerevisiae polyubiquitinates histones, and its acidic domain mediates this activity. Genes Dev. 1988 Nov;2(11):1476–1485. doi: 10.1101/gad.2.11.1476. [DOI] [PubMed] [Google Scholar]