Abstract
Direct and quantitative detection of recombinant RNA molecules by polymerase chain reaction (PCR) provides a novel method for studying recombination in RNA viruses without selection for viable progeny. The parental poliovirus strains used in this study contained polymorphic marker loci approximately 600 bases apart; both exhibited wild-type growth characteristics. We established conditions under which the amount of PCR product was linearly proportional to the amount of input template, and the reproducibility was high. Recombinant progeny were predominantly homologous and arose at frequencies up to 2 x 10(-3). Recombination events increased in frequency throughout replication, indicating that there is no viral RNA sequestration or inhibition of recombination late in infection as proposed in earlier genetic studies. Previous studies have demonstrated that poliovirus recombination occurs by a copy-choice mechanism in which the viral polymerase switches templates during negative-strand synthesis. Varying the relative amount of input parental virus markedly altered reciprocal recombination frequencies. This, in conjunction with the kinetics data, indicated that acceptor template concentration is a determinant of template switching frequency. Since positive strands greatly outnumber negative strands throughout poliovirus infection, this would explain the bias toward recombination during negative-strand synthesis.
Full text
PDF

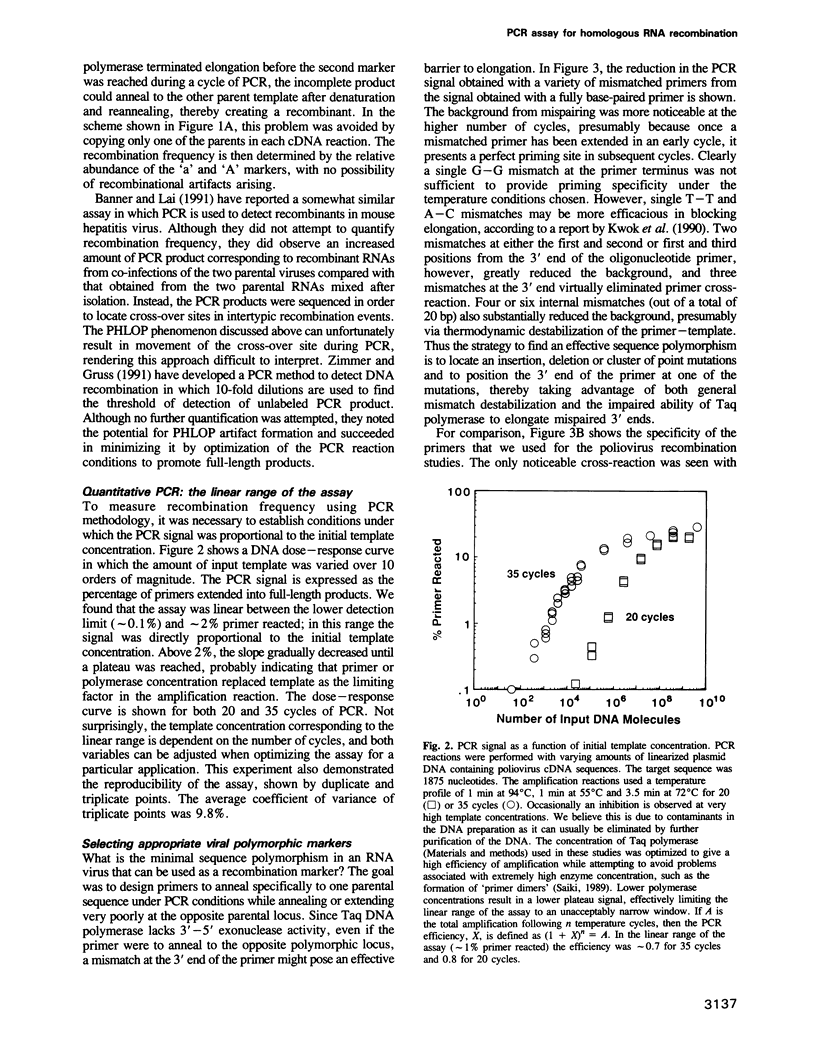


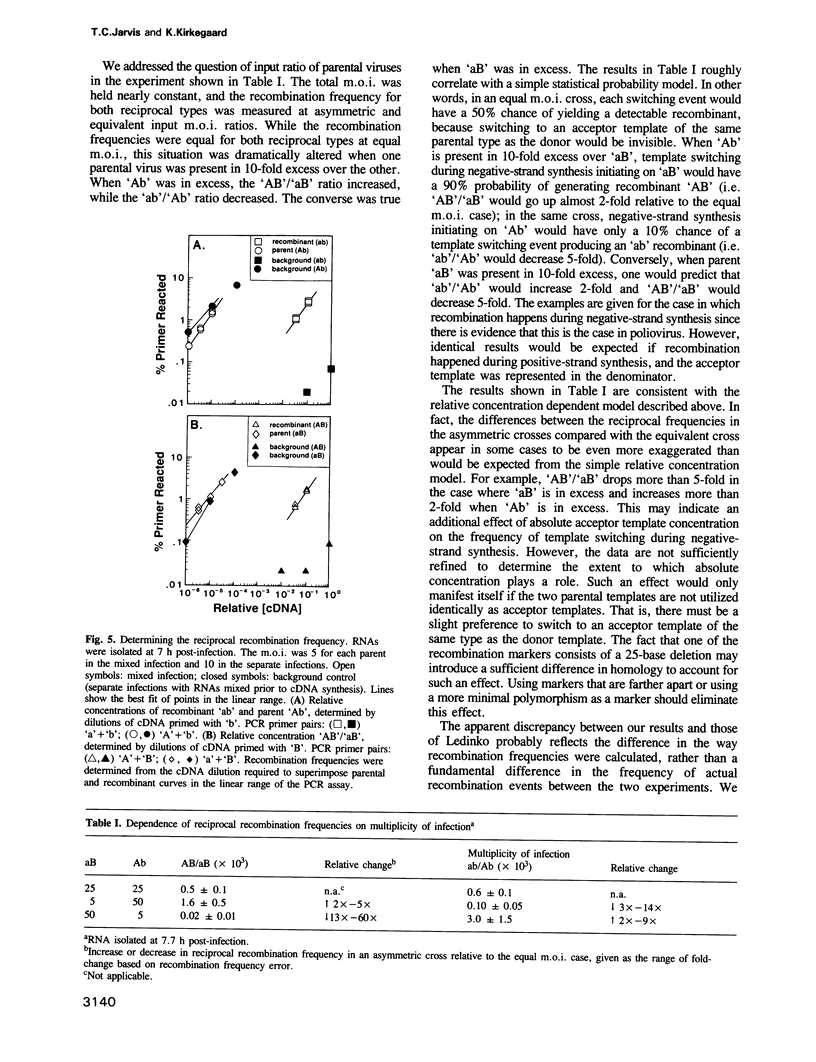





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Bujarski J. J. Molecular studies of brome mosaic virus using infectious transcripts from cloned cDNA. Adv Virus Res. 1987;32:215–242. doi: 10.1016/s0065-3527(08)60478-9. [DOI] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Banner L. R., Lai M. M. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology. 1991 Nov;185(1):441–445. doi: 10.1016/0042-6822(91)90795-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987 Sep;160(1):220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology. 1980 Jan 30;100(2):390–399. doi: 10.1016/0042-6822(80)90530-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G., Evans B., Merriman M. Poliovirus replicative intermediate: structural basis of infectivity. J Mol Biol. 1969 Dec 14;46(2):235–249. doi: 10.1016/0022-2836(69)90419-7. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone P. J., Carpenter C. D., Li X. H., Simon A. E. Recombination between satellite RNAs of turnip crinkle virus. EMBO J. 1990 Jun;9(6):1709–1715. doi: 10.1002/j.1460-2075.1990.tb08294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton S. R., Nelsen B., Kirkegaard K. Temperature-sensitive poliovirus mutant fails to cleave VP0 and accumulates provirions. J Virol. 1990 Sep;64(9):4067–4075. doi: 10.1128/jvi.64.9.4067-4075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D. A genetic map of poliovirus temperature-sensitive mutants. Virology. 1968 Aug;35(4):584–596. doi: 10.1016/0042-6822(68)90287-0. [DOI] [PubMed] [Google Scholar]
- Gauss P., Gayle M., Winter R. B., Gold L. The bacteriophage T4 dexA gene: sequence and analysis of a gene conditionally required for DNA replication. Mol Gen Genet. 1987 Jan;206(1):24–34. doi: 10.1007/BF00326532. [DOI] [PubMed] [Google Scholar]
- Guinea R., Carrasco L. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 1990 Jun;9(6):2011–2016. doi: 10.1002/j.1460-2075.1990.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Lustig S., Strauss E. G., Strauss J. H. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Jarvis T. C., Kirkegaard K. The polymerase in its labyrinth: mechanisms and implications of RNA recombination. Trends Genet. 1991 Jun;7(6):186–191. doi: 10.1016/0168-9525(91)90434-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M. Preferred sites of recombination in poliovirus RNA: an analysis of 40 intertypic cross-over sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11705–11723. doi: 10.1093/nar/16.24.11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDINKO N. Genetic recombination with poliovirus type 1. Studies of crosses between a normal horse serum-resistant mutant and several guanidine-resistant mutants of the same strain. Virology. 1963 May;20:107–119. doi: 10.1016/0042-6822(63)90145-4. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Baric R. S., Makino S., Keck J. G., Egbert J., Leibowitz J. L., Stohlman S. A. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985 Nov;56(2):449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M. RNA recombination in animal and plant viruses. Microbiol Rev. 1992 Mar;56(1):61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Taylor J. Template switching by reverse transcriptase during DNA synthesis. J Virol. 1990 Sep;64(9):4321–4328. doi: 10.1128/jvi.64.9.4321-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology. 1988 Oct;166(2):415–422. doi: 10.1016/0042-6822(88)90512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahon D., Slade W. R. A sensitive method for the detection and isolation of recombinants of foot-and-mouth disease virus. J Gen Virol. 1981 Apr;53(Pt 2):333–342. doi: 10.1099/0022-1317-53-2-333. [DOI] [PubMed] [Google Scholar]
- Mindich L., Qiao X., Onodera S., Gottlieb P., Strassman J. Heterologous recombination in the double-stranded RNA bacteriophage phi 6. J Virol. 1992 May;66(5):2605–2610. doi: 10.1128/jvi.66.5.2605-2610.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J. E., Kirkegaard K. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J Virol. 1991 Jun;65(6):3384–3387. doi: 10.1128/jvi.65.6.3384-3387.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palasingam K., Shaklee P. N. Reversion of Q beta RNA phage mutants by homologous RNA recombination. J Virol. 1992 Apr;66(4):2435–2442. doi: 10.1128/jvi.66.4.2435-2442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Richards O. C., Martin S. C., Jense H. G., Ehrenfeld E. Structure of poliovirus replicative intermediate RNA. Electron microscope analysis of RNA cross-linked in vivo with psoralen derivative. J Mol Biol. 1984 Mar 5;173(3):325–340. doi: 10.1016/0022-2836(84)90124-4. [DOI] [PubMed] [Google Scholar]
- Rott M. E., Tremaine J. H., Rochon D. M. Comparison of the 5' and 3' termini of tomato ringspot virus RNA1 and RNA2: evidence for RNA recombination. Virology. 1991 Nov;185(1):468–472. doi: 10.1016/0042-6822(91)90801-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarnow P. Role of 3'-end sequences in infectivity of poliovirus transcripts made in vitro. J Virol. 1989 Jan;63(1):467–470. doi: 10.1128/jvi.63.1.467-470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolskaya E. A., Romanova L. I., Blinov V. M., Viktorova E. G., Sinyakov A. N., Kolesnikova M. S., Agol V. I. Studies on the recombination between RNA genomes of poliovirus: the primary structure and nonrandom distribution of crossover regions in the genomes of intertypic poliovirus recombinants. Virology. 1987 Nov;161(1):54–61. doi: 10.1016/0042-6822(87)90170-x. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Cascone P. J., Simon A. E. Recombination between satellite and genomic RNAs of turnip crinkle virus. Virology. 1991 Oct;184(2):791–794. doi: 10.1016/0042-6822(91)90454-j. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]