Abstract
The regulation of β-catenin turnover is the central mechanism governing activation of the Wnt signaling pathway. All components of the pathway are present in the early embryo of Xenopus laevis, and Xenopus egg extracts have been used to recapitulate complex biological reactions such as microtubule dynamics, DNA replication, chromatin assembly, and phases of the cell cycle. Herein, we describe a biochemical method for analyzing β-catenin degradation using radiolabeled and luciferase-fusion proteins in Xenopus egg extracts. We show that in such a biochemical system, cytoplasmic β-catenin degradation is regulated by soluble components of the Wnt pathway as well as small molecules.
Keywords: Xenopus laevis, Ubiquitin, Proteasome, Egg extract, Axin, β-Catenin destruction complex
1 Introduction
1.1 The Wnt Signaling Pathway
Wnt signaling is a highly conserved pathway critical for metazoan development and tissue homeostasis in adult organisms. Dysregulation of Wnt signaling has been associated with several types of cancers in humans, including colorectal cancer [1]. In the absence of a Wnt ligand, β-catenin is maintained at low levels via the assembly of a destruction complex comprised of the scaffold protein, Axin, the tumor suppressor adenomatous polyposis coli (APC), and the kinases glycogen synthase kinase-3 (GSK3) and casein kinase 1α (CK1α) [2]. This complex acts to phosphorylate β-catenin at specific sites and target it for polyubiquitination by the SCFβ-TRCP complex and subsequent degradation by the protea-some. Wnt signaling is activated when Wnt ligands bind to the receptors Frizzled (Fz) and low-density lipoprotein-related receptor 5/6 (LRP 5/6), thereby triggering a cascade that leads to inhibition of β-catenin degradation. Stabilized β-catenin accumulates in the cytoplasm and subsequently translocates into the nucleus to initiate a Wnt-specific transcriptional program. Thus, the regulation of β-catenin turnover is critical for the downstream activation of Wnt target genes.
The importance of β-catenin degradation is highlighted by the fact that mutations that inhibit β-catenin degradation by affecting the stability of this complex (e.g., APC and β-catenin) are found in ~90 % of nonhereditary cases of colorectal cancer [3]. Several decades of research have highlighted the critical role of Wnt signaling in the earliest events of embryonic development of the African clawed frog, Xenopus laevis. Not surprisingly, Xenopus egg extract has been shown to contain all the essential components of the β-catenin destruction complex, and it offers a robust in vitro system for studying the biochemistry of cytoplasmic Wnt regulation [4–12].
1.2 Use of Xenopus Egg Extracts to Study the Wnt Signaling Pathway
Extracts are a cell-free, biologically active cytoplasm that mimic the cellular environment. Xenopus eggs are collected, and through a series of centrifugation steps, the cytoplasmic fraction is isolated in its undiluted form. Xenopus egg extract contains all of the cytoplasmic proteins, organelles, and other components at or near physiological levels, such that complex cellular pathways often remain intact.
An advantage of Xenopus egg extract is that β-catenin levels can be readily measured without interference from changes in its steady-state levels because these extracts lack the capacity to support transcription or translation in the absence of supplementation. Conversely, components of the pathway (e.g., Axin) can be added as recombinant proteins into the system to influence the kinetics of β-catenin degradation [4, 7]. In addition, components can be immunodepleted using antibodies to conduct loss-of- function studies. Accordingly, Xenopus egg extracts have provided important insight into mechanistic details of Wnt signaling.
1.3 Xenopus Egg Extract as a Tool for Drug Discovery
Studies have successfully used Xenopus egg extracts as a tool to identify small-molecule effectors [8, 13–16]. This system is ideal for small molecule discovery as it contains native forms of all of the relevant protein components of the Wnt pathway. Extract-based assays targeting the Wnt pathway have identified molecules such as pyrvinium, a potent Wnt inhibitor that promotes β-catenin degradation via enhancement of CK1α activity [13]. Thus, Xenopus egg extracts provide a novel and useful tool for the discovery of modulators of the Wnt pathway.
2 Materials
Pregnant mare serum gonadotropin (PMSG): 250 U/ml stock.
20× Marc’s Modified Ringer solution (MMR): 2 M Sodium chloride, 40 mM potassium chloride, 40 mM calcium chloride, 20 mM magnesium chloride, and 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4.
Human chorionic gonadotropin (HCG): 1000 U/ml.
2%l-Cysteine, pH 8.1.
Leupeptin, pepstatin, aprotinin mixture (LPA).
Cytochalasin D.
Anti-Axin antibody.
Protein A beads.
[35S] Methionine.
In vitro translation kit.
20× Energy regeneration mix: 150 mM Creatine phosphate, 20 mM ATP, 600 μg/ml creatine phosphokinase, 20 mM MgCl2.
3 Methods
3.1 Harvesting Xenopus Eggs
3.1.1 Inject Female Xenopus laevis
Hold the female Xenopus frog firmly with one hand, with its head facing towards your wrist and one leg between your thumb and index finger, and the other leg between your index and middle fingers.
Locate the site of injection, the dorsal lymph sac, which is adjacent to the lateral line and around 1 cm from the midline.
Using a hypodermic syringe with a 25 G needle, inject 100 U of freshly made PMSG subcutaneously into the frog. Be sure to insert the needle with the bevel side up, from the rear, with the tip of the needle pointing towards the head of the frog. Once the puncture is made with the needle, insert it about 1 cm forward from the puncture site between the skin and the muscle. Gently expel the PMSG solution. Once injection is complete, slowly pull out the needle. Inject 10–20 female frogs.
After injection, return the primed frogs to 16 °C water for 5–10 days. In the meantime, make fresh 0.5× MMR solution from a 20× stock. Prepare 4 L tanks filled with 16 °C 0.5× MMR. At the end of priming with PMSG, place one frog into each of the 4 L tanks. Do not place more than one frog per tank to eliminate the time-consuming task of removing poor-quality eggs in case one of the frogs should lay a bad batch.
After 5–10 days, inject 1000 U of HCG in the same manner as done for the PMSG. Place the frogs in a 16 °C incubator and wait for 16 h before the females start laying eggs directly into the tank water. In order to obtain large amounts of eggs, gently massage the abdomen and sides of the frogs to expel more eggs.
3.1.2 Harvest Xenopus Eggs
After the frogs have laid the eggs, remove the eggs and most of the MMR from the tank, leaving approximately 1–200 ml of MMR plus the eggs in the tank (see Note 1).
Evaluate the quality of the eggs and remove any poor-quality ones with a transfer pipette. In general, discard the entire batch if poor-quality eggs exceed 10 % of the total. After discarding bad eggs from each of the 4 L tanks, collect all remaining good eggs and combine them in a 500 ml beaker. Remove as much MMR as possible while at the same time keeping the eggs fully submerged. To wash the eggs, add two egg volumes of 0.5× MMR and swirl the beaker, removing any debris and remaining poor-quality eggs. Repeat this washing step at least once.
To de-jelly the eggs, pour out as much MMR as possible and add 100 ml of 2 % cysteine, pH 8.1 solution to the beaker, mix by swirling, and allow the eggs to settle for 1–2 min. The detached jelly will float to the top of the eggs and make the eggs more compact. Pour off the cysteine solution with the detached jelly. Repeat the process of adding the cysteine solution, allowing the eggs to de-jelly, and pouring off the solution until the eggs have become tightly packed. This usually happens at the end of the third cysteine treatment.
Wash the eggs with 100 ml 1× MMR a total of ten times. The solution should become increasingly clear, typically at the end of the second wash. Finally, wash with 30 ml of 0.1× MMR and pour off the MMR solution. Maintain the eggs in 0.1× MMR.
3.2 Preparation of Xenopus Egg Extract
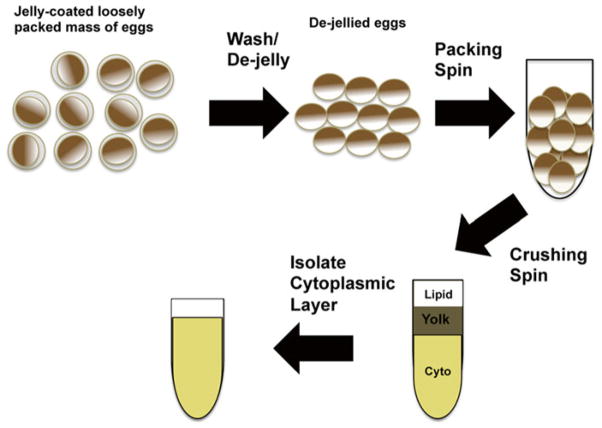
A summary of Xenopus egg extract preparation is shown in Fig. 1.
Fig. 1.
Preparation of Xenopus egg extract. Flow chart indicating individual steps to prepare Xenopus egg extract. Frog eggs are covered with a sticky jelly coat that must be removed prior to extract preparation. Harvested eggs are washed and de-jellied with cysteine before performing a packing spin to remove residual buffer. The crushing spin separates the egg extract into a cytoplasmic, yolk, and lipid layers. The cytoplasmic layer is isolated and used to perform β-catenin degradation assays
3.2.1 Centrifugation of Xenopus Eggs
Add the LPA protease inhibitor at 10 μg/ml and Cytochalasin D at 20 μg/ml into 20 ml of 0.1× MMR. Add this 0.1× MMR solution directly to the eggs, swirl, and incubate at 16 °C for 5 min.
Use a transfer pipette to transfer the eggs to pre-chilled 50 ml centrifuge tubes at 4 °C. Wait for the eggs to settle and then remove any extra buffer from the top. Repeat the process by adding additional eggs to the centrifuge tube until it is filled.
For the packing spin, spin the centrifuge tubes at 400 × g for 30 s at 4 °C. After spinning, remove any extra buffer from the top of the centrifuge tubes.
For the crushing spin, spin the centrifuge tubes at 15,000 × g for 5 min at 4 °C.
3.2.2 Collect and Prepare the Cytoplasmic Layer of Extract
To collect the cytoplasmic layer of the extract, use a P1000 pipette tip to puncture through the lipid layer, which is the topmost layer in the centrifuge tube. Then, using a new P1000 pipette tip, proceed through the punctured lipid layer to reach the cytoplasmic layer and begin withdrawing.
Collect the cytoplasmic layer in a pre-chilled centrifuge tube at 4 °C.
Spin the tubes containing the cytoplasmic layer at 15,000 × g for 10 min at 4 °C.
Using the same method, collect the cytoplasmic layer again and repeat the spinning step to further isolate the layer. At the end of this step, the cytoplasmic extract should be straw colored and uniform (see Note 2).
Add LPA and Cytochalasin D to the cytoplasmic extract with a final concentration of 10 μg/ml for each.
Snap freeze the tubes in liquid nitrogen and transfer to a −80 °C freezer for long-term storage.
3.3 Depletion of Extracts
In order to experimentally control perturbations of the Wnt signaling pathway, Xenopus egg extracts can be depleted of individual protein components. This depletion allows the investigator to add back known amounts of the component in order to determine its kinetic effect on β-catenin degradation.
3.3.1 Deplete Xenopus Egg Extracts of Endogenous Axin
Conjugate anti-Axin (rabbit IgG) to protein A beads as per the manufacturer’s protocol.
Wash conjugated beads 3× with compatible wash buffer (e.g., 50 mM Tris pH 7.4, 150 mM NaCl, 0.1 % Triton X-100).
Thaw previously prepared Xenopus egg extracts on ice.
Discard wash buffer after the last spin. Add extract directly to washed beads in a 1:10 ratio.
Incubate the extract with the beads on a rotating platform for 1 h at 4 °C.
After incubation, spin the extract-bead mixture at 12,000 × g for at least 1 min at 4 °C.
The supernatant contains extract depleted of endogenous Axin protein. Transfer this depleted extract to a new microcentrifuge tube on ice and confirm depletion of Axin by immunoblotting using an appropriate anti-Axin antibody. Depleted extract may be aliquoted and snap frozen prior to storage at −80 °C.
3.4 Degradation Assay
The use of extracts to determine the kinetics of β-catenin degradation has played an important role in our understanding of Wnt signaling under normal and pathological conditions. Further, depletion of extracts to determine the effects of individual components of the pathway on β-catenin degradation has provided many mechanistic insights into the pathway.
3.4.1 Prepare Radiolabeled β-catenin
Prepare [35S] β-catenin protein using a commercially available in vitro translation kit as per the manufacturer’s protocol.
Confirm successful translation of radiolabeled protein by performing autoradiography. Subject the in vitro-translated (IVT) protein (0.5–1 μl) to SDS-PAGE gel. Fix the gel in 50 % meth-anol/20 % acetic acid, and dry the gel on Whatman paper. Perform autoradiography to assess successful incorporation of [35S] into the IVT protein.
The IVT-protein may be stored at −80 °C for up to 2 months without significant loss of radioactivity.
3.4.2 Prepare Egg Xenopus Extract for Degradation Assay
Thaw Xenopus egg extracts quickly in hands, and then place immediately on ice. Thaw 20× energy regeneration mix and store on ice.
Add 10 μl of 20× ER per 200 μl Xenopus egg extract. Mix thoroughly. Spin down the extract quickly and place on ice until ready for use in the degradation assay. Aliquots can also be stored at −80 °C and thawed immediately before use.
3.4.3 Perform Degradation Assay
Add 1–5 μl of [35S] radiolabeled β-catenin, depending on radiolabeling efficiency, to 20 μl of Xenopus egg extract (containing energy regeneration mix). If small molecules or proteins are being tested, add appropriate volumes such that the volume of such components is less than 10 % of the total reaction volume.
Mix the reaction mixture by vortexing in short bursts (2–3×) and pulse spinning. Place mixture on ice.
Begin degradation assay by incubating tubes at room temperature.
Perform time points by removing 1–5 μl of the mixture and stopping the reaction with SDS sample buffer. Vortex the tubes vigorously and boil the samples at 95 °C.
Run degradation assay samples on SDS-PAGE gels and perform autoradiography with suitable film (Fig. 2). Results may be quantified using imaging software such as ImageJ.
Fig. 2.
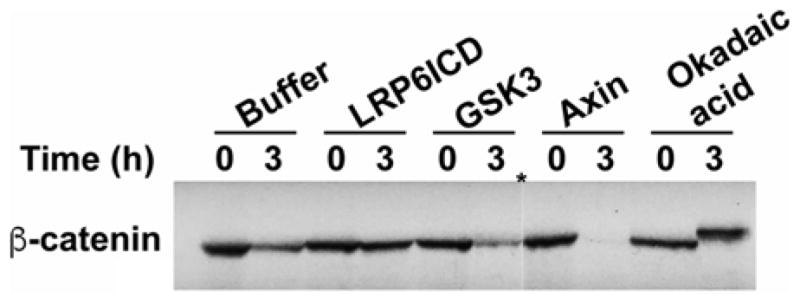
Reconstitution of radiolabeled β-catenin degradation in Xenopus egg extracts. LRP6ICD inhibits, whereas GSK3 and Axin stimulate, the turnover of radio-labeled β-catenin in Xenopus egg extracts. In vitro-translated [35S] β-catenin was added to Xenopus egg extracts and incubated with buffer, LRP6ICD (1.5 μM), GSK3 (1 μM), Axin (50 nM), and okadaic acid (1 μM). At the indicated times, aliquots were removed and subjected to SDS-PAGE/autoradiography. LRP6ICD is the intracellular domain of the Wnt co-receptor, LRP6. *, Indicate intervening lanes removed
3.4.4 Perform β-Catenin Luciferase Degradation Assay
Make IVT luciferase-tagged β-catenin as per the manufacturer’s protocol.
-
Confirm successful translation of non-radiolabeled, luciferase-tagged β-catenin by performing a luciferase assay to measure activity. As controls, perform the luciferase assay using translated luciferase protein or untranslated IVT reaction mixture.
Commercial kits are available for measuring luciferase activity.
Add luciferase-tagged β-catenin to 20 μl of Xenopus egg extract (containing energy regeneration mix) such that the luciferase activity is ~50,000 relative luciferase units (RLU)/μl of extract as measured by the luciferase assay in Subheading 3.4.4, step 2. Mix well mixture by vortexing in short bursts (2–3×) and pulse spinning.
Perform the degradation assay at room temperature and remove aliquots of the reaction at desired time points. The volume of aliquots removed is typically calculated as the volume that is sufficient to yield at least 5000 RLU at the start of the reaction. Snap freeze these aliquots in liquid nitrogen for storage at −80 °C.
To analyze samples, thaw the samples on ice, and immediately measure luciferase activity as described in Subheading 3.4.4, step 2 (Fig. 3).
Fig. 3.
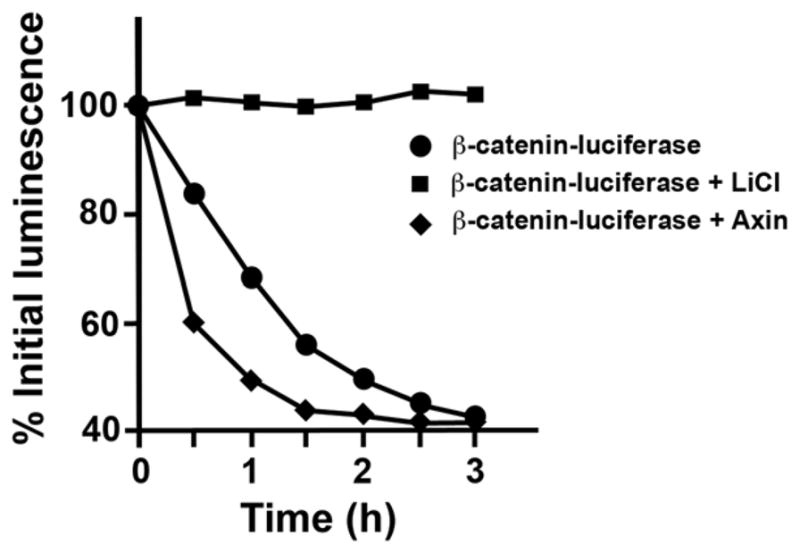
Reconstitution of β-catenin-luciferase degradation in Xenopus egg extracts. In vitro-translated β-catenin-luciferase fusion was incubated in Xenopus egg extracts in the presence of buffer, Axin (50 nM), or lithium chloride (20 mM; inhibitor of GSK3). At the indicated times, an aliquot was removed and luciferase activity measured. Background signal observed in the β-catenin-luciferase degradation assay is due to free luciferase protein, which degrades slowly
Acknowledgments
We thank Laurie Lee for critical reading of the manuscript. ASH is supported by a fellowship from the NIH Molecular Endocrinology Training Program. EL is supported by the NIH grants R01GM081635 and R01GM103926.
Footnotes
High-quality eggs should have a clear separation between the darkly pigmented animal hemisphere and the lightly pigmented vegetal hemisphere. Poor-quality eggs are blotchy, and puffy, and/or are part of a large, stringy mass of eggs.
If contamination from the lightly colored lipid layer or the darkly colored pigmented layer should occur repeat the spinning and extraction protocol.
References
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E. The way Wnt works: components and mechanism. Growth Factors. 2013;31:1–31. doi: 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 4.Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 5.Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phos-phorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci U S A. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hang BI, Thorne CA, Robbins DJ, Huppert SS, Lee LA, Lee E. Screening for small molecule inhibitors of embryonic pathways: sometimes you gotta crack a few eggs. Bioorg Med Chem. 2012;20:1869–1877. doi: 10.1016/j.bmc.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 11.Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proc Natl Acad Sci U S A. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jernigan KK, Cselenyi CS, Thorne CA, Hanson AJ, Tahinci E, Hajicek N, Oldham WM, Lee LA, Hamm HE, Hepler JR, Kozasa T, Linder ME, Lee E. Gbetagamma activates GSK3 to promote LRP6-mediated beta-catenin transcriptional activity. Sci Signal. 2010;3:ra37. doi: 10.1126/scisignal.2000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorne CA, Lafleur B, Lewis M, Hanson AJ, Jernigan KK, Weaver DC, Huppert KA, Chen TW, Wichaidit C, Cselenyi CS, Tahinci E, Meyers KC, Waskow E, Orton D, Salic A, Lee LA, Robbins DJ, Huppert SS, Lee E. A biochemical screen for identification of small-molecule regulators of the Wnt pathway using Xenopus egg extracts. J Biomol Screen. 2011;16:995–1006. doi: 10.1177/1087057111416657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salic A, King RW. Identifying small molecule inhibitors of the ubiquitin-proteasome pathway in Xenopus egg extracts. Methods Enzymol. 2005;399:567–585. doi: 10.1016/S0076-6879(05)99038-1. [DOI] [PubMed] [Google Scholar]
- 15.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM, III, Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, Kopelovich L, Jasin M, Baer R, Paull TT, Gautier J. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2008;4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]