Abstract
Background
Preoperative anemia is a well-established risk factor for short-term mortality in patients undergoing non-cardiac surgery, but appropriate thresholds for transfusion remain uncertain. The objective of this study was to determine long-term outcomes associated with anemia, hemorrhage and red blood cell transfusion in patients undergoing non-cardiac surgery.
Methods
We performed a long-term follow-up study of consecutive subjects undergoing hip, knee, and spine surgery between November 1, 2008 and December 31, 2009. Clinical data were obtained from administrative and laboratory databases, and retrospective record review. Pre-operative anemia was defined as baseline hemoglobin <13 g/dL for men and <12 g/dL for women. Hemorrhage was defined by ICD-9 coding. Data on long-term survival were queried from the Social Security Death Index (SSDI) database. Logistic regression models were used to identify factors associated with long-term mortality.
Results
3,050 subjects underwent orthopedic surgery. Pre-operative anemia was present in 17.6% (537) of subjects, hemorrhage occurred in 33 (1%), and 766 (25%) received 1 red blood cell transfusion. Over 9,015 patient-years of follow up, 111 deaths occurred. Anemia (HR 3.91, CI 2.49 –f 6.15) and hemorrhage (HR 5.28, CI 2.20 – 12.67) were independently associated with long-term mortality after multivariable adjustment. Red blood cell transfusion during the surgical hospitalization was associated with long-term mortality (HR 3.96, CI 2.47 – 6.34), which was attenuated by severity of anemia (no anemia [HR 4.39], mild anemia [HR 2.27], and moderate/severe anemia [HR 0.81], P for trend 0.0015).
Conclusions
Preoperative anemia, perioperative bleeding and red blood cell transfusion are associated with increased mortality at long-term follow up after non-cardiac surgery. Strategies to minimize anemia and bleeding should be considered for all patients and restrictive transfusion strategies may be advisable. Further investigation into mechanisms of these adverse events is warranted.
Keywords: anemia, hemorrhage, red blood cells, transfusion, surgery, mortality
Introduction
Orthopedic surgery accounts for nearly one quarter of all operating room procedures in the United States, with more than 1.5 million hip, knee, and spine surgeries performed each year.1–3 Up to a third of patients undergoing orthopedic surgery have pre-operative anemia,4, 5 a well-established risk factor for short-term mortality and cardiovascular complications in patients undergoing non-cardiac surgery.4, 6–11 Red blood cell transfusion remains the cornerstone of treatment for anemia and approximately one-third of orthopedic patients receive an allogeneic blood transfusion in the perioperative period.5, 12, 13 Despite the theoretical advantages of perioperative red blood cell transfusion, the benefits of this practice remain unproven for patients without life-threatening hemorrhage or symptomatic anemia. In a randomized trial of older patients with hip fractures and anemia, a liberal transfusion strategy using a hemoglobin threshold of <10 g/dL did not reduce the frequency of in-hospital acute coronary syndrome or death compared with a restrictive strategy using a threshold of <8 g/dL.14 Retrospective studies of red blood cell transfusion in orthopedic surgery have reported conflicting short-term findings.15–17 Appropriate thresholds for perioperative transfusion in orthopedic surgery remain uncertain. Moreover, long-term outcomes associated with anemia and red blood cell transfusion following orthopedic surgery remain poorly characterized. We investigated the association between anemia, bleeding and transfusion on long-term mortality in a large, retrospective study of patients undergoing major orthopedic surgery.
Methods
Study Design
We performed a retrospective cohort study of consecutive adults undergoing knee, hip, or spine surgery between November 1, 2008 and December 31, 2009 at 2 hospitals within a large tertiary care academic medical center. The full methods have been described previously.18 Clinical data were obtained from administrative, blood bank and laboratory databases, and retrospective record review. The study was approved by the Institutional Review Board with an informed consent waiver.
Patients & Outcomes
Patient demographics, clinical history, and baseline comorbidities were evaluated using a hospital administrative dataset. International Classification of Disease (ICD)-9 codes were used to identify spinal fusion (81.0×), refusion of spine (81.3×), joint replacement of lower extremity (81.5×), and other procedures on spine (81.6×). Comorbidities present on admission were determined using ICD-9 diagnosis codes, including diabetes mellitus (250.×), stroke (V12.54, 438.0–438.9), heart failure (402.01, 402.11, 402.91, 428, 428.1, 428.2, 428.22, 428.23, 428.3, 428.32, 428.33, 428.4, 428.42, 428.43, 428.9), or coronary artery disease (412, 414.×).
Complete blood counts obtained prior to surgery were reviewed. Pre-operative anemia was defined as baseline hemoglobin <13 g/dL for men and <12 g/dL for women, according to the World Health Organization definition.19 Anemia was sub-classified as mild (Hgb ≥ 11 g/dL), moderate (9 – 11 g/dL), and severe (<9 g/dL). Red blood cell transfusion during the surgical admission was abstracted from a hospital blood bank dataset. Leukocyte-reduced units of packed red blood cells comprised approximately 50% of all transfusions during the study period, but patient-level data on leukocyte reduction were not available for analysis. Postoperative hemorrhage was identified by discharge diagnosis code (998.11). Long-term mortality was determined from the Social Security Death Index (SSDI) database, with a query performed on July 13, 2012. Long-term survival was not available for 32 (1.0%) subjects; 7 subjects had birth dates incongruent with data from the SSDI and 25 lacked social security numbers. These patients were excluded from the present analysis.
Statistical Analysis
Normally distributed continuous variables were displayed as means ± standard deviation (SD) and were compared using the Student’s t test. Categorical variables were displayed as frequencies and percentages and were compared by Chi-square and Fisher exact tests. Baseline characteristics associated with outcomes were estimated with univariate logistic regression models and reported as hazard ratios (HR) with 95% confidence intervals (CI). Time-to-event Cox proportional hazards analyses were used to examine the association between anemia, hemorrhage, transfusion, and mortality, while controlling for potential demographic, clinical, and procedural confounders. Associations between red blood cell transfusion and long-term mortality were performed with anemia as a dichotomous variable and stratified by severity. A sensitivity analysis was performed after excluding patients with hemorrhage. The likelihood ratio test was used to compare hazard ratios for equality. Kaplan-Meier plots were generated for long-term mortality stratified by the presence of pre-operative anemia and perioperative red blood cell transfusion.
In order to control for treatment-selection bias for red blood cell transfusion, we assembled two equal-sized cohorts of patients with similar baseline characteristics, one with and one without perioperative red blood cell transfusion, using propensity-score matching. The propensity score is the conditional probability of a particular exposure given a set of covariates measured at baseline. Propensity scores were determined for patients who had complete data, including baseline hemoglobin values, using a logistic regression model with demographic, clinical, and procedural covariates. Matching was performed using a 1:1 protocol without replacement with a caliper width equal to 0.2 of the standard deviation of the log odds of the estimated propensity score. Two-tailed p-values < 0.05 were considered to be statistically significant for all tests. Statistics were calculated using SPSS 20 (IBM SPSS Statistics, Armonk, NY) and R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
3,050 subjects underwent orthopedic surgery of the spine, hip, or knee during the study period and were included in the analysis. Mean age was 60.8 years, 59% of subjects were female. Coronary artery disease was present in 10.7% of patients. Full demographic, clinical, and procedural characteristics are displayed in Table 1. Mean pre-operative hemoglobin (Hgb) concentration was 13.4±1.6 g/dL. Hemorrhage was coded in 33 patients (1.07%). Perioperative red blood cell transfusion was administered in 766 (25.1%) subjects. A complete blood count was obtained in 2,722 subjects (89.2%) prior to surgery. Patients with and without pre-operative hemoglobin measurements had similar post-operative bleeding, transfusion, and mortality (Supplemental Table 1).
Table 1.
Baseline characteristics
| Overall Population (n=3050) | |
|---|---|
| Age (yr), mean | 60.84 ± 13.25 |
| Female | 1806 (59.2) |
| Race | |
| White | 1986 (65.1) |
| Black | 433 (14.2) |
| Hispanic | 414 (13.6) |
| Other | 217 (7.1) |
| Admission Type | |
| Elective | 2876 (94.3) |
| Emergency or Urgent Surgery | 174 (5.7) |
| Procedure | |
| Spine | 1144 (37.5) |
| Knee | 992 (32.5) |
| Hip | 914 (30.0) |
| RCRI Score (Lee et. al, 1999) | |
| Coronary artery disease | 326 (10.7) |
| Heart Failure | 86 (2.8) |
| Stroke/Transient ischemic attack | 18 (0.6) |
| Creatinine > 2 mg/dL | 15 (0.6) |
| Diabetes Mellitus | 445 (14.6) |
| Hemoglobin, mean (SD) | 13.4 ± 1.6 |
| Anemia | 537 (17.6) |
BMI: body mass index; RCRI: Revised Cardiac Risk Index,
Mean follow-up was 3.0 ± 0.5 years following surgery. During 9,015 patient-years of follow up, 111 (3.6%) deaths occurred, with 53 (48%) deaths within 12 months of surgery and 37 (33%) deaths between 12–24 months. Subjects who died during follow up were older, more likely to be male, had lower BMI, greater burden of cardiovascular risk factors, and were more likely to have undergone urgent or emergent surgery. Univariate associations with mortality are displayed in Table 2.
Table 2.
Univariate analysis of baseline characteristics, procedural risk factors, and long-term mortality
| Death (n=111) | No Death (n=2939) | Univariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | |||
| Age (yr), mean | 69.71 ± 15.4 | 60.51 ± 13.0 | 1.06 (1.04,1.08) | <0.001 |
| Female | 54 (48.6) | 1752 (59.6) | 0.64 (0.44,0.94) | 0.014 |
| Race | ||||
| White | 82 (73.9) | 1904 (64.8) | 1 | |
| Black | 8 (7.2) | 425 (14.5) | 0.44 (0.21,0.91) | 0.03 |
| Hispanic | 12 (10.8) | 402 (13.7) | 0.69 (0.38,1.28) | 0.39 |
| Other | 9 (8.1) | 208 (7.1) | 1.01 (0.50, 2.03) | 0.68 |
| Admission Type | ||||
| Elective | 65 (58.6) | 2811 (95.6) | 1 | |
| Emergency or Urgent Surgery | 46 (41.4) | 128 (4.4) | 15.54 (10.24, 23.59) | <0.001 |
| Procedure | ||||
| Spine | 50 (45.0) | 1094 (37.2) | 1 | |
| Knee | 19 (17.1) | 973 (33.1) | 0.43 (0.25, 0.73) | 0.002 |
| Hip | 42 (37.8) | 872 (29.7) | 1.05 (0.69, 1.60) | 0.81 |
| RCRI Score | ||||
| Coronary artery disease | 27 (24.3) | 299 (10.2) | 2.84 (1.81,4.45) | <0.001 |
| Heart Failure | 18 (16.2) | 68 (2.3) | 8.17 (4.67,14.29) | <0.001 |
| Stroke/Transient ischemic attack | 2 (1.8) | 16 (0.5) | 3.35 (0.76,14.7) | 0.14 |
| Creatinine > 2 mg/dL | 3 (2.7) | 12 (0.4) | 6.95 (1.93,25.08) | 0.015 |
| Diabetes Mellitus | 26 (23.4) | 419 (14.3) | 1.84 (1.17,2.90) | 0.01 |
| Hemoglobin (g/dL) | 11.9 ± 1.9 | 13.5 ± 1.5 | 0.53 (0.47 – 0.60) | <0.001 |
| Pre-Operative Anemia | ||||
| None | 38 (40.0%) | 2147 (81.7%) | 1 | |
| Mild Anemia | 27 (28.4%) | 373 (14.2%) | 4.09 (2.47–6.78) | <0.001 |
| Moderate Anemia | 25 (26.3%) | 96 (3.7%) | 14.71 (8.54–25.36) | <0.001 |
| Severe Anemia | 5 (5.3%) | 11 (0.4%) | 25.68 (8.51–77.52) | <0.001 |
| Baseline Hgb Not Available | 16 (14.4%) | 312 (10.6%) | ||
| Blood Transfusion (any) | 71 (64.0) | 695 (23.6) | 5.57 (3.78 – 8.20) | <0.001 |
| Blood Transfusion (within 48 hours of surgery) | ||||
| None | 51 (45.9) | 2414 (81.4) | 1 | |
| 1U Packed Red Blood Cells | 21 (18.9) | 198 (6.7) | 4.87 (2.93 – 8.09) | <0.001 |
| 2U Packed Red Blood Cells | 15 (13.5) | 173 (5.9) | 4.00 (2.25 – 7.11) | <0.001 |
| 3+U Packed Red Blood Cells | 24 (21.6) | 179 (6.0) | 6.10 (3.75 – 9.90) | <0.001 |
| Hemorrhage (ICD9 Coded) | 7 (6.3) | 26 (0.9) | 7.27 (3.38 – 15.64) | <0.001 |
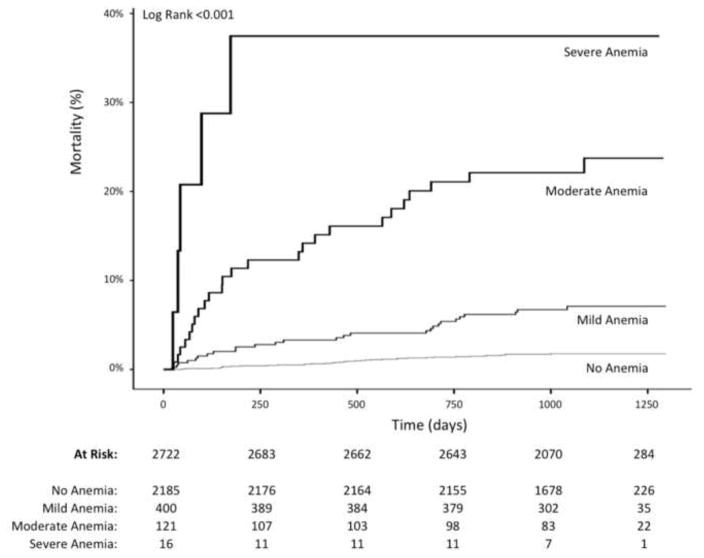
Pre-operative anemia was present in 17.6% (537) of subjects overall (19.7% of subjects with pre-operative hemoglobin measurements recorded). Age, sex, and multiple cardiovascular risk factors were significantly associated with baseline anemia in multivariable analysis (Table 3). Among patients with anemia at baseline, long-term mortality was higher than those without pre-operative anemia (10.6% vs. 1.7%, p<0.001). After adjusting for demographic and clinical variables in multivariable models, anemia at baseline remained independently associated with long-term mortality (HR 3.91, CI 2.49 – 6.15). The severity of pre-operative anemia was also associated with long-term mortality, with mild (6.8%, adjusted HR 3.30, CI 1.97–5.53), moderate (20.7%, adjusted HR 4.63, CI 2.52 – 8.49), and severe (31.3%, adjusted HR 9.15, CI 3.37 – 25.82) anemia demonstrating an incremental hazard for mortality after multivariable adjustment (p for trend = <0.001), (Table 4). Kaplan Meier curves of long-term survival by degree of pre-operative anemia are shown in Figure 1.
Table 3.
Multivariate odds of anemia, hemorrhage, and transfusion adjusted for baseline cardiovascular risk factors
| Odds Ratio (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Anemia | p-value | Major Bleeding | p-value | Transfusion | p-value | |
| Age (yr), mean | 1.02 (1.01 – 1.03) | <0.001 | 1.01 (0.98–1.04) | 0.53 | 1.02 (1.01 – 1.03) | <0.001 |
| Female | 1.39 (1.12–1.73) | 0.003 | 1.10 (0.51 – 2.37) | 0.79 | 1.84 (1.50 – 2.25) | <0.001 |
| Admission Type | ||||||
| Urgent or Emergent | 3.07 (2.14–4.40) | <0.001 | 1.49 (0.50 – 4.41) | 0.47 | 3.06 (2.13 – 4.40) | <0.001 |
| Procedure | ||||||
| Spine | 1 | 1 | 1 | |||
| Knee | 0.92 (0.72–1.18) | 0.30 | 0.07 (0.01–0.55) | 0.01 | 1.00 (0.80 – 1.28) | 0.99 |
| Hip | 0.89 (0.69 – 1.15) | 0.42 | 0.81 (0.37 – 1.78) | 0.59 | 1.56 (1.24 – 1.96) | <0.001 |
| RCRI | ||||||
| Coronary artery disease | 1.97 (1.47 – 2.66) | <0.001 | 5.70 (2.40 – 13.60) | <0.001 | 2.33 (1.76 – 3.10) | <0.001 |
| Heart Failure | 1.70 (0.99–2.93) | 0.06 | 4.05 (1.22 – 13.39) | 0.01 | 2.04 (1.18 – 3.52) | 0.01 |
| Stroke/Transient ischemic attack | 1.81 (0.58 – 5.65) | 0.31 | - | 1.00 | 4.64 (1.35 – 15.97) | 0.02 |
| CKD (Cr > 2 mg/dL) | 9.09 (2.96–27.90) | <0.001 | - | 1.00 | 8.60 (2.61 – 28.28) | <0.001 |
| Diabetes Mellitus | 1.77 (1.36–2.29) | <0.001 | 0.38 (0.11 – 1.36) | 0.14 | 1.13 (0.88 – 1.46) | 0.34 |
Table 4.
Multivariate Cox Proportional Hazards regression analysis of anemia and long-term mortality
| Mild Anemia | Moderate Anemia | Severe Anemia | Any Anemia | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Unadjusted | 3.97 (2.43–6.51) | <0.001 | 13.39 (8.08 – 22.18) | <0.001 | 24.64 (9.70 – 62.63) | <0.001 | 6.43 (4.26–9.69) | <0.001 |
| Adjusted for age, sex, race | 3.33 (2.02 – 5.48) | <0.001 | 9.96 (5.86–16.91) | <0.001 | 21.53 (8.38–55.27) | <0.001 | 5.13 (3.37–7.82) | <0.001 |
| Adjusted for age, sex, race, admission type, procedure | 3.13 (1.90–5.16) | <0.001 | 4.95 (2.78–8.84) | <0.001 | 9.10 (3.39–24.40) | <0.001 | 3.82 (2.48–5.89) | <0.001 |
| Multivariable adjusted** | 3.30 (1.97–5.53) | 0.001 | 4.63 (2.52 – 8.49) | <0.001 | 9.15 (3.37–24.81) | <0.001 | 3.91 (2.49–6.15) | <0.001 |
Adjusted for age, sex, race, admission type (elective vs. emergency/urgent surgery), procedure, coronary artery disease, heart failure, stroke/transient ischemic attack, creatinine > 2 mg/dL, diabetes mellitus
Figure 1.
Preoperative anemia and long-term mortality following surgery.
Perioperative hemorrhage occurred in 33 subjects (1.1%), and was more common among patients with pre-operative anemia (2.8% vs. 0.6%, p<0.001; multivariable adjusted HR 3.29, CI 1.45 – 7.46). Perioperative hemorrhage was associated with substantially higher long-term mortality than subjects without hemorrhage (21.2% vs. 3.4%, p<0.001), and a significant hazard for mortality after multivariable adjustment (HR 5.28, CI 2.20 – 12.67).
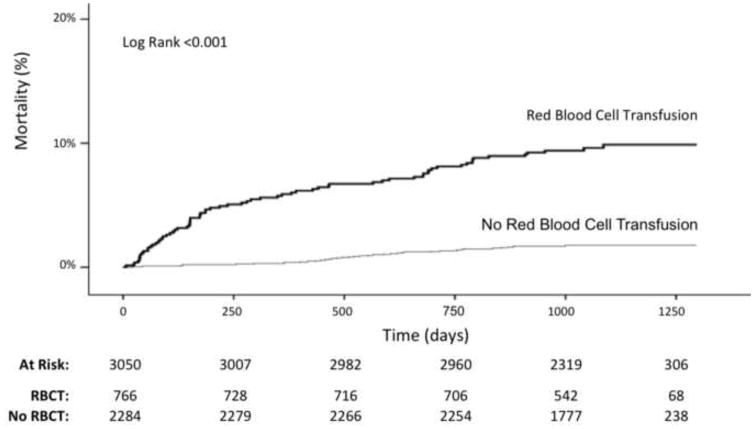
During the surgical hospitalization, 766 (25.1%) subjects received ≥1 red blood cell transfusion, with 606 subjects transfused within the first 48 hours of surgery. Patients who required red blood cell transfusion during their hospitalization had an increased long-term mortality (9.3% vs. 1.8%, p<0.001 for transfusion overall), and red blood cell transfusion was more common in those who died during follow up (64.0% vs. 23.7%, p<0.001 for transfusion overall). Findings were similar for patients who received red blood cell transfusion within 48 hours of surgery. Kaplan Meier curves of long-term survival by red blood cell transfusion are shown in Figures 2. Red blood cell transfusion was associated with a significant hazard for mortality after multivariable adjustment (HR 3.96, CI 2.47 – 6.34). When stratified by severity of anemia at baseline, red blood cell transfusion in patients with the highest pre-operative hemoglobin was associated with the greatest degree of hazard for mortality after multivariable adjustment. The association between red blood cell transfusion and long-term mortality was attenuated by the severity of anemia (Table 6). Similar findings were observed for red blood cell transfusion administered within 48 hours of surgery (data not shown) and after the exclusion of patients with hemorrhage (Supplemental Table 2). In the propensity-matched cohort of 1,108 patients, red blood cell transfusion was also associated with significantly higher 1-year (3.2% vs. 0.4%, p=0.0007) and long-term mortality (6.3% vs. 2.5%, p=0.002), (Supplemental Table 3, Figure 1).
Figure 2.
Red blood cell transfusion and long-term mortality following surgery.
Table 6.
Association between red blood cell transfusion and long term mortality stratified by severity of baseline anemia.
| Long Term Mortality, n (%) | Multivariate Analysis** | ||
|---|---|---|---|
| Hazard Ratio (95% CI) ^ | P value | ||
| Moderate/Severe Anemia | |||
| Red Blood Cell Transfusion | 26/105 (24.8%) | 0.81 (0.24–2.73) | 0.73 |
| No Transfusion | 4/32 (12.5%) | ||
| Mild Anemia | |||
| Red Blood Cell Transfusion | 20/185 (10.8%) | 2.27 (0.86–6.04) | 0.10 |
| No Transfusion | 7/215 (3.3%) | ||
| No Anemia | |||
| Red Blood Cell Transfusion | 18/400 (4.5%) | 4.39 (2.14 – 8.98) | <0.001 |
| No Transfusion | 20/1785 (1.1%) | ||
P for trend = 0.0015
Adjusted for age, sex, race, admission type (elective vs. emergency/urgent surgery), procedure, coronary artery disease, heart failure, stroke/transient ischemic attack, creatinine > 2 mg/dL, diabetes mellitus
Discussion
In this large, retrospective study of patients undergoing orthopedic surgery, we demonstrate a strong association between pre-operative hemoglobin, anemia, and long-term mortality that persisted after adjustment for demographic and clinical variables. Even mild anemia was associated with a three-fold increased hazard for long-term mortality. In multivariable adjusted models, red blood cell transfusion was associated with long-term mortality, with the greatest hazard among patients with higher pre-operative hemoglobin values. In contrast, red blood cell transfusion was not associated with mortality in patients with the lowest pre-operative hemoglobin levels.
The findings of the present study are consistent with data on the risks of pre-operative anemia and in-hospital and 30-day outcomes. In a retrospective study of 1,958 patients undergoing non-cardiac surgery who refused transfusion, there was a strong association between preoperative hemoglobin and 30-day mortality, with substantially greater risks in patients with comorbid cardiovascular disease.6 Subsequent large retrospective studies of patients undergoing major non-cardiac surgery confirmed these findings.4, 7–11 In an analysis of non-cardiac surgery from the Department of Veterans Affairs National Surgical Quality Improvement Program database, a 1.6% increase in 30-day mortality was associated with each percentage point deviation of hematocrit below the normal range.8 In the subgroup of patients who underwent orthopedic surgery, anemia was associated with an even greater short-term mortality. Thus, even mild preoperative anemia is implicated in adverse outcomes after surgery. In the present study, we demonstrate durable associations between pre-operative anemia and mortality at 3 years of follow-up. The mechanism of this association remains uncertain, although it may be related to underlying systemic disease, inflammation, inadequate oxygen delivery leading to organ dysfunction during periods of stress, or a combination of factors.
The effect of perioperative transfusion and outcomes has been subject to much controversy. Since the first successful human blood transfusion in 1818, red blood cell transfusions have become the standard of care in the management of anemia in patients undergoing surgery.20 In fact, 40–70% of all red cell units are transfused in the perioperative setting given the prevalence of anemia and the risks of bleeding after surgery.21, 22 Still, transfusion targets and the effects of allogeneic red blood cells in the perioperative period remain uncertain. To date, only one large randomized trial of patients undergoing non-cardiac surgery has been powered to evaluate the effect of optimal transfusion targets on mortality. The Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial randomized 2,016 older patients (mean age 81.6 years) undergoing hip fracture repair with anemia (Hgb <10 g/dL) and cardiovascular disease or risk factors to a liberal (Hgb <10 g/dL) or restrictive (Hgb < 8 g/dL) transfusion strategy.14 Although patients in the liberal transfusion arm were more likely to receive red blood cell transfusion (96.7% vs. 41.0%), there were no differences in the occurrence of acute coronary syndrome or short-term mortality between the two groups and there was no compelling evidence to support higher transfusion targets.14 These findings are consistent with large randomized trials of transfusion targets in critically ill, non-surgical populations.23, 24 Observational studies of the effects of transfusion in patients undergoing non-cardiac surgery have reported conflicting findings. Multiple orthopedic surgery studies report that red blood cell transfusions are associated with approximately two-fold higher short-term mortality compared to non-transfused patients.16, 17, 25 In a propensity-matched analysis of a large claims database of patients undergoing non-cardiac surgery, red blood cell transfusion was also associated with a greater than two-fold increase in 30-day perioperative stroke and myocardial infarction.26 A large, retrospective multicenter study of 162,190 patients undergoing orthopedic procedures demonstrated patients who received perioperative transfusion had higher 30-day (HR 2.32, CI 1.91–2.83) and 1-year (HR 1.75, CI 1.60 – 1.91) mortality after multivariable adjustment.25 However, when participating hospitals were categorized by hospital-specific rates of perioperative transfusion (ranging from 10.3% to 57.9%), surgeries performed at centers with the highest transfusion rates were not associated with an increased mortality after multivariable adjustment, raising questions whether anemia requiring transfusion is simply a proxy for the burden of unmeasured confounders that may underlie observed associations between transfusion and mortality. Furthermore, a separate retrospective cohort study of 8,787 older adults undergoing orthopedic surgery for hip fracture found no association between perioperative transfusion in patients with hemoglobin levels ≥8 g/dL and 30 or 90-day mortality after adjusting for nadir hemoglobin and other risk factors.15 Based on the totality of data from studies of perioperative and critically ill populations, current guidelines recommend lower hemoglobin values (6–8 g/dL) as thresholds for red blood cell transfusion.27, 28
The findings of the present study are consistent with previous reports that red blood cell transfusion has a narrow therapeutic window and may be independently associated with an increased hazard for perioperative stroke, MI, and mortality. We identified the greatest hazard for mortality associated with transfusions in patients who had the highest pre-operative hemoglobin levels. red blood cell transfusion had no significant effect on mortality among patients with baseline anemia. Thus, restrictive transfusion strategies may be most appropriate for patients without pre-operative anemia who undergo orthopedic surgery.
Plausible mechanisms exist to explain the observed hazard associated with transfusion. Red blood cell transfusion can result in transfusion-induced immunomodulation related to the infusion of cytokines, allogeneic leukocytes and other bioactive compounds. Immunomodulation has been postulated to cause aberrant immune activity that may be detrimental.29 Transfusion has been associated with an increased risk of serious infection in multiple clinical settings. In a systematic review of randomized trials of patients undergoing orthopedic surgery, restrictive red blood cell transfusion strategies were associated with a reduced hazard for acute health care associated infections (HR 0.70, CI 0.54 – 0.91).30 Platelet aggregation may be another unintended consequence of transfusion.31 Administration of packed red blood cells is associated with increased platelet reactivity in healthy volunteers, although increased incidence of thrombotic events has not been observed in randomized trials of transfusion targets.31 Finally, risks of transfusion may not be uniform among all units of blood. Stored red cells undergo structural and functional changes over time, with nitric oxide depletion and increased adhesion to the vascular endothelium potentially impairing microvascular function.32, 33 Further studies are necessary to fully understand the mechanisms of adverse outcomes associated with red blood cell transfusions.
There are a number of limitations to this study. First, this is a retrospective observational study of consecutive orthopedic surgeries. All surgeries were conducted over a short 1-year time period, minimizing the effect of changes to routine health care practices. Medical comorbidities and clinical variables were identified through hospital databases and unmeasured confounders and misclassification bias cannot be excluded. Second, preoperative hemoglobin measurements were performed at the discretion of providers and data was available for 89.3% of patients. Nonetheless, outcomes of hemorrhage, transfusion, and mortality were similar between the groups. Nadir hemoglobin levels and estimates of intra-operative blood losses were not evaluated. Prior studies suggest that the benefits of perioperative transfusion may be limited to patients with >500 mL of operative blood loss.34 Third, the administration of red blood cell transfusion was determined by the treating physicians. Standardized transfusion thresholds were not enforced. Distinctions between allogeneic and autologous red blood cells were not available, nor was the duration of blood cell storage prior to transfusion. Fourth, medical management therapy was not recorded. Therefore, the long-term impact of bleeding and transfusion on anti-platelet or anticoagulant therapy prescribing could not be assessed. Fifth, hemoglobin values were not available during follow up, and the association between long-term hemoglobin trends and mortality could not be assessed. Consequently, we were unable to identify the precise mechanisms through which pre-operative anemia was associated with long-term adverse outcomes. However, worsening anemia during long-term follow-up may have impaired the function of other organ systems and/or adversely impacted the delivery of life-saving medical and surgical interventions during follow up. Finally, causes of death were not available for analysis and adjudication of outcomes was not performed.
In conclusion, among patients undergoing major non-cardiac surgery, pre-operative anemia, bleeding, and red blood cell transfusion are associated with increased mortality at long-term follow up. Strategies to minimize pre-operative anemia and surgical bleeding should be considered for all patients. Restrictive transfusion strategies may be advisable in the absence of a compelling clinical indication for transfusion. Further investigation into mechanisms of adverse events associated with erythrocyte transfusion and trials to improve perioperative outcomes are needed.
Supplementary Material
Table 5.
Association between anemia, bleeding, transfusion and long-term mortality
| n (%) | Long Term Mortality | Multivariate Analysis** | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | |||
| Anemia | 537 (19.7%^/17.6%) | 57 (10.6%) | 3.91 (2.49–6.15) | <0.001 |
| No Anemia | 2185 (80.3%^/71.6%) | 38 (1.7%) | ||
| Hemorrhage | 33 (1.1%) | 7 (21.2%) | 5.28 (2.20 – 12.66) | <0.001 |
| No Hemorrhage | 3017 (98.9%) | 104 (3.4%) | ||
| Transfusion | 766 (25.1%) | 71 (9.3%) | 3.96 (2.47 – 6.34) | <0.001 |
| No Transfusion | 2284 (74.9%) | 40 (1.8%) | ||
Multivariate Cox Proportional Hazards regression analysis adjusted for age, sex, race, admission type (elective vs. emergency/urgent surgery), procedure, coronary artery disease, heart failure, stroke/transient ischemic attack, creatinine > 2 mg/dL, diabetes mellitus
Excluding patients without pre-operative hemoglobin values.
Acknowledgments
Funding Source: Investigator-initiated.
Dr. Berger was partially funded by Grant RO1HL114978 from the National Heart and Lung Blood Institute of the National Institutes of Health and Grant 13CRP14410042 from the American Heart Association Clinical Research Program. The authors report no relevant disclosures or conflicts of interest.
Abbreviations List
- BMI
body mass index
- Hgb
Hemoglobin
- ICD
International Classification of Disease
- RCRI
Revised Cardiac Risk Index
- SSDI
Social Security Death Index
Footnotes
Disclosures: None
Conflicts of interest: None
All authors had access to the data and a role in drafting of the final manuscript.
References
- 1.Steiner C, Andrews R, Barrett M, Weiss A. Report # 2012-03. U.S. Agency for Healthcare Research and Quality; [Accessed November 1, 2014]. HCUP Projections: Mobility/Orthopedic Procedures 2003 to 2012. http://www.hcup-us.ahrq.gov/reports/projections/2012-03.pdf. Published September 20, 2012. [Google Scholar]
- 2.Haralson RH, 3rd, Zuckerman JD. Prevalence, health care expenditures, and orthopedic surgery workforce for musculoskeletal conditions. JAMA. 2009;302:1586–1587. doi: 10.1001/jama.2009.1489. [DOI] [PubMed] [Google Scholar]
- 3.Weiss AJ, Elixhauseer A. HCUP Statistical brief #171. Agency for Healthcare Research and Quality; Rockville, MD: Mar, 2014. Trends in operating room procedures in US Hospitals, 2001–2011. [Google Scholar]
- 4.Seicean A, Seicean S, Alan N, Schiltz NK, Rosenbaum BP, Jones PK, Kattan MW, Neuhauser D, Weil RJ. Preoperative anemia and perioperative outcomes in patients who undergo elective spine surgery. Spine. 2013;38:1331–1341. doi: 10.1097/BRS.0b013e3182912c6b. [DOI] [PubMed] [Google Scholar]
- 5.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, Noveck H, Strom BL. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 9.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: A single-center cohort study. Anesthesiology. 2009;110:574–581. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 10.Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, Soweid A, Hoballah JJ, Taher AT, Jamali FR. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 11.Saager L, Turan A, Reynolds LF, Dalton JE, Mascha EJ, Kurz A. The association between preoperative anemia and 30-day mortality and morbidity in noncardiac surgical patients. Anesth Analg. 2013;117:909–915. doi: 10.1213/ANE.0b013e31828b347d. [DOI] [PubMed] [Google Scholar]
- 12.Vuille-Lessard E, Boudreault D, Girard F, Ruel M, Chagnon M, Hardy JF. Red blood cell transfusion practice in elective orthopedic surgery: A multicenter cohort study. Transfusion. 2010;50:2117–2124. doi: 10.1111/j.1537-2995.2010.02697.x. [DOI] [PubMed] [Google Scholar]
- 13.Audet AM, Andrzejewski C, Popovsky MA. Red blood cell transfusion practices in patients undergoing orthopedic surgery: A multi-institutional analysis. Orthopedics. 1998;21:851–858. doi: 10.3928/0147-7447-19980801-08. [DOI] [PubMed] [Google Scholar]
- 14.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J Investigators F. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson JL, Duff A, Berlin JA, Lawrence VA, Poses RM, Huber EC, O’Hara DA, Noveck H, Strom BL. Perioperative blood transfusion and postoperative mortality. JAMA. 1998;279:199–205. doi: 10.1001/jama.279.3.199. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen AB, Mehnert F, Overgaard S, Johnsen SP. Allogeneic blood transfusion and prognosis following total hip replacement: A population-based follow up study. BMC Musculoskelet Disord. 2009;10:167. doi: 10.1186/1471-2474-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–292. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 18.Oberweis BS, Nukala S, Rosenberg A, Guo Y, Stuchin S, Radford MJ, Berger JS. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J. 2013;165:427–433. e421. doi: 10.1016/j.ahj.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Report of a who scientific group. 1968. Nutritional anaemias. [PubMed] [Google Scholar]
- 20.Blundell J. Experiments on the transfusion of blood by the syringe. Med Chir Trans. 1818;9:56–92. doi: 10.1177/09595287180090p107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells AW, Mounter PJ, Chapman CE, Stainsby D, Wallis JP. Where does blood go? Prospective observational study of red cell transfusion in north england. BMJ. 2002;325:803. doi: 10.1136/bmj.325.7368.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker B, Schlumpf K, Schulman J, Green J. Report of the us department of health and human services. The 2009 national blood collection and utilization survey report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. [Google Scholar]
- 23.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, canadian critical care trials group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 24.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettila V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Muller RG, Moller MH, Steensen M, Tjader I, Kilsand K, Odeberg-Wernerman S, Sjobo B, Bundgaard H, Thyo MA, Lodahl D, Maerkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A the TTG, the Scandinavian Critical Care Trials G. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 25.Karkouti K, Stukel TA, Beattie WS, Elsaadany S, Li P, Berger R, Wijeysundera DN. Relationship of erythrocyte transfusion with short- and long-term mortality in a population-based surgical cohort. Anesthesiology. 2012;117:1175–1183. doi: 10.1097/ALN.0b013e318271604e. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock EL, Kim H, Auerbach AD. Harms associated with single unit perioperative transfusion: Retrospective population based analysis. BMJ. 2015;350:h3037. doi: 10.1136/bmj.h3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Society of Anesthesiologists Task Force on Perioperative Blood T, Adjuvant T. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the american society of anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B Clinical Transfusion Medicine Committee of the A. Red blood cell transfusion: A clinical practice guideline from the aabb*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 29.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: Fact or fiction? Blood. 2001;97:1180–1195. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 30.Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, Hickner A, Rogers MA. Health care-associated infection after red blood cell transfusion: A systematic review and meta-analysis. JAMA. 2014;311:1317–1326. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvain J, Abtan J, Kerneis M, Martin R, Finzi J, Vignalou JB, Barthelemy O, O’Connor SA, Luyt CE, Brechot N, Mercadier A, Brugier D, Galier S, Collet JP, Chastre J, Montalescot G. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: The transfusion-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry) J Am Coll Cardiol. 2014;63:1289–1296. doi: 10.1016/j.jacc.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Rao SV, Califf RM. Is old blood bad blood? Am Heart J. 2010;159:710–712. doi: 10.1016/j.ahj.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 34.Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, Mor V, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252:11–17. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.