Abstract
Skeletal muscle precursor cells (MPCs) are considered a key candidate for cell therapy in the treatment of skeletal muscle dysfunction due to injury, disease, or age. However, expansion of a sufficient number of functional skeletal muscle cells in vitro from a small tissue biopsy has been challenging due to changes in phenotypic expression of these cells under traditional culture conditions. Thus, the aim of the study was to develop a better culture system for the expansion and myo-differentiation of MPCs that could further be used for therapy. For this purpose, we developed an ideal method of tissue decellularization and compared the ability of different matrices to support MPC growth and differentiation. Porcine-derived skeletal muscle and liver and kidney extracellular matrix (ECM) were generated by decellularization methods consisting of distilled water, 0.2 mg/mL DNase, or 5% fetal bovine serum. Acellular matrices were further homogenized, dissolved, and combined with a hyaluronic acid-based hydrogel decorated with heparin (ECM-HA-HP). The cell proliferation and myogenic differentiation capacity of human MPCs were assessed when grown on gel alone, ECM, or each ECM-HA-HP substrate. Human MPC proliferation was significantly enhanced when cultured on the ECM-HA-HP substrates compared to the other substrates tested, with the greatest proliferation on the muscle ECM-HA-HP (mECM-HA-HP) substrate. The number of differentiated myotubes was significantly increased on the mECM-HA-HP substrate compared to the other gel-ECM substrates, as well as the numbers of MPCs expressing specific myogenic cell markers (i.e., myosin, desmin, myoD, and myf5). In conclusion, skeletal mECM-HA-HP as a culture substrate provided an optimal culture microenvironment potentially due to its similarity to the in vivo environment. These data suggest a potential use of skeletal muscle-derived ECM gel for the expansion and differentiation of human MPCs for cell-based therapy for skeletal muscle dysfunction.
Keywords: : skeletal muscle precursor cells, differentiation, extracellular matrix, urinary incontinence
Introduction
Skeletal muscle dysfunction, whether due to injury, disease, or aging, is a major cause of disability. Stress urinary incontinence (SUI) primarily effects perimenopausal and postmenopausal women, and is a good example of muscle dysfunction, which benefits from cell therapy.1 The urethral musculature along with the surrounding tissues and pelvic floor musculature are substantial contributors to urinary continence in women and injury to these muscles is a primary cause of SUI.2 The external urethral sphincter (EUS) is significantly weakened in women and animal models of SUI2 and is primarily caused by vaginal delivery, which results in injuries to the muscles of the pelvic floor and disruption of innervation of the EUS.3 Therefore, the restoration of functional skeletal muscle within the EUS is key to ameliorating SUI. Cell-based therapy is a promising alternative method to restore urethral sphincter function for the treatment of SUI.
Skeletal muscle precursor cells (MPCs) are a population of adult stem cells that promote muscle repair in response to injury, aging, or disease.4 Currently, MPCs are a commonly used cell source for the treatment of SUI; however, urinary incontinence appears to persist or even recur after MPC implantation.5 One reason for this lack of success is mal-differentiation of MPCs into a fibroblast-like phenotype following cell expansion using current culture conditions. To generate a large amount of MPCs in vitro, cells must be cultured for several passages. However, like other functional cells (i.e., hepatocytes or kidney cells), MPCs lose their capacity for myogenic differentiation rapidly and obtain a fibroblast-like phenotype after leaving the original host niche.6–9 Such non- or low-functioning MPCs reduce the therapeutic benefits of cell therapy. Therefore, an approach is needed that retains the myogenic capacity of MPCs during cell culture, expansion in vitro and administration in vivo, to improve the efficiency of cell therapy.
Each tissue consists of unique cell types and a specific extracellular matrix (ECM). To retain cellular function in vitro, cells should be grown in the same or a similar condition as their in vivo environment. Tissue-specific ECM provides the niche (microenvironment) from which the cells are derived and supports cell growth and differentiation in vitro.10 Binding ECM proteins with a biodegradable hydrogel can further promote cell growth and myogenesis. Although many hydrogels are available for these purposes, hyaluronic acid (HA) gel has been demonstrated to better improve grafted cell survival and proliferation as it has a unique ability to hold in moisture (1,000 mL of water per gram of HA) in soft tissue. In addition, this hydrogel, decorated with heparin (HA-HP), more efficiently promotes cell differentiation due to the presence of growth factors retained within the ECM that can be slowly and sustainably released.10
Our previous data showed that liver tissue-specific ECM, compared to muscle or skin ECMs, significantly promoted hepatocyte growth and, even more importantly, supported the retention of the hepatocyte phenotype in vitro by growth factors and cytokines within the ECM.10 The liver ECM combined with HA-HP gel achieved better outcomes in the production of albumin with differentiated hepatocytes than HA gel or liver ECM alone.11 In other studies, we demonstrated that implanted human urine-derived stem cells within HA-HP, in combination with extra growth factors, had better survival, distribution, and differentiation of cells within the tissue and improved engraftment in an athymic mouse model.12 However, the effect of skeletal muscle ECM (mECM), combined with an HA-based hydrogel decorated with heparin (mECM-HA-HP), on human MPC proliferation and myogenic differentiation in vitro is unknown.
Based on these data, we hypothesized that mECM-HA-HP can provide a more favorable niche for MPC growth and myogenesis compared to ECMs from other tissues or gel alone. The goal of this study was to determine whether mECM-HA-HP can enhance cell expansion with the retention of the myogenic phenotype and induce functional myogenic differentiation. To address this, we optimized fabrication methods of muscle tissue-specific ECM by lyophilization, homogenization, decellularization, purification and gel processes. We also tested different ECM proteins on the proliferation and differentiation of MPCs in vitro. This is an initial study for the in vitro expansion and myogenic differentiation of human MPCs, which could be further used as cell therapy in in vivo experimental models of skeletal muscle injuries such as urethral sphincter muscle dysfunction.
Materials and Methods
Decellularization of ECM
Decellularized ECM was isolated from fresh human skeletal muscle from the biceps femoris of the legs or kidney and liver tissues of adult pigs. Porcine tissues were obtained from a local slaughterhouse and human skeletal muscle tissue was obtained from surgical waste materials in our institute. Each tissue specimen was cut into small cubes (<1 cm3) after the attached fat and connective tissues were removed. These tissue fragments were frozen at −80°C for 24 h, lyophilized for 48 h, and homogenized into tissue particles (<1 mm3) with a gentleMACS™ Dissociator (Miltenyi Biotec, San Diego, CA) for different time periods (3, 10, 20, and 30 min).
To optimize the decellularization protocol, three methods were used after homogenization: (1) deionized water, (2) 0.2 mg/mL DNase, or (3) 5% fetal bovine serum (FBS, v/v) (GE Healthcare, Logan, UT) (Fig. 1). To prevent bacterial contamination, each tissue-specific suspension was washed in a solution containing 1% penicillin and streptomycin for 3 days, followed by a deionized water changed every 3–6 h, and final centrifugation at 3,000 rpm × 5 min. To evaluate residual DNA concentrations within the decellularized tissue, samples were smeared on glass slides for histologic assessment by hematoxylin and eosin and 4,6-Diamidino-2-phenylindole (DAPI) staining. The final tissue samples were frozen at −80°C, lyophilized, and stored at −80°C for further use.
FIG. 1.
Gross view of milky ECM suspension, milk-like ECM particle solution after being homogenized for 20 min. (A) After homogenization. (B) After decellularization. ECM, extracellular matrix. Color images available online at www.liebertpub.com/tea
Fabrication of tissue-specific ECM
The lyophilized tissue samples were ground into fragments with a freezer mill (SPEX™ SamplePrep 6870 Freezer/Mill™, Metuchen, NJ). One gram of ECM was mixed with 100 mg pepsin from porcine gastric mucosa containing 3,400 units of protein (Fisher Scientific, Fair Lawn, NJ) and sterilized by gamma irradiation (1 Mrad). Hydrochloric acid (0.1 N, 45 mL) was added to the sterilized biomaterials and then incubated at 37°C on a shaking bed for 48 h. The resulting mixture was transferred to conical tubes and centrifuged at 3,000 rpm for 15 min. The supernatant was transferred to a new tube and the pellet discarded. The supernatant solution was neutralized with 0.1 N NaOH and stored at −80°C until further use. To detect DNA concentrations, 10 μL of neutralized solution of each tissue or ECM was measured using a NanoDrop™ spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA).
Optimization of mECM solution
The HA-HP gel (EsiBio, Alameda, CA) was prepared as follows: Heprasil (thiolated and heparinized HA-based hydrogel derivative) and Gelin-S (thiolated gelatin) were dissolved in sterile water to make 2% w/v solutions. Extralink, a polyethylene (glycol) diacrylate (PEGDA) crosslinker, was dissolved in water to make a 4% w/v solution. Heprasil, Gelin-S, and Extralink solutions were then mixed in a 2:2:1 ratio by volume. To optimize the concentration of ECM solution for cell culture, 0%, 2.5%, 5%, 10%, 25%, 50%, 75%, and 100% mECM solutions (v/v) in HA-HP hydrogel were tested. To establish a base for cell cultures, thicker HA-HP gel (0.5 mL/3.8 cm2) with muscle, kidney, and liver ECM proteins was coated in the 24-well culture plates or chambers, and incubated at 37°C for 30 min for congealing before seeding cells.
Culture of human MPCs
The protocol for obtaining human skeletal muscle samples was approved by the Wake Forest University Institutional Review Board.4 Surgical waste material was obtained during plastic surgery on the gracilis muscle from the normal inner thigh tissue from the donor. Immediately following surgical removal, muscle tissue specimens were placed in a sterile bottle containing 10–20 mL transport solution, that is, phosphate-buffered saline (PBS) with penicillin/streptomycin, at 4°C. A 2 × 2 cm2 sample was dissected from the specimen, weighed, and rinsed in three changes of wash solution (PBS with 10 μg/mL gentamicin).
The muscle tissue was further minced into ∼1 × 1 mm pieces and incubated in 10 mL collagenase solution/gram of tissue (1.2 × 105 Collagen Digestion Activity Units/mL Collagenase HA; Vitacyte, Indianapolis, IN) and 3.3 × 104 Neutral Protease Units/mL BP Protease (Vitacyte) in Dulbecco's modified Eagle's medium (DMEM)/F12 (PeproTech, Burlington, NC) for 45 min at 37°C. The enzymatic reaction was terminated by rinsing in PBS and centrifugation. Large tissue fragments were removed by passing through a SteriFlip filter (100 μM pore size). The resulting cell pellets from centrifugation (300 × g, 5 min) were resuspended in 15 mL myogenic growth medium (DMEM/F12, 15% FBS, 10 ng/mL recombinant human epidermal growth factor, 1 ng/mL recombinant human basic fibroblast growth factor, 10 μg/mL recombinant human insulin, 0.4 μg/mL dexamethasone, and 10 μg/mL gentamicin),4 plated in 10 mm collagen type I-coated 100-mm culture dishes, and then incubated for 24 h at 37°C in 5% carbon dioxide (CO2). This population of cells was considered passage 0 (p0).
To reduce fibroblast contamination in culture, the supernatant of the cell suspension solution containing nonadherent MPCs after initial culture (p0) was transferred to a collagen type I-coated 100-mm culture dish and expanded. This MPC population was considered p1. Cultured cells were detached by trypsin at ∼70% confluency. MPCs were resuspended in the growth medium and plated in tissue culture dishes at a seeding density of 3–4 × 103 viable cells/cm2 for the following passages. Cells at p3 were frozen in CryoServ (Belleville, ON) at 1.5 × 107 cells/vial for further experiments.
Assessment of cell proliferation
To determine the effect of different tissue ECMs on the proliferation of MPCs, cells were seeded on HA-HP hydrogels alone or containing 10% muscle, liver, or kidney ECM solutions and then incubated at 37°C in 5% CO2 in growth media. Matrigel® (BD Biosciences, San Jose, CA) served as a positive control, and cells grown in without any coating (blank) were negative controls. MTS cell proliferation assays were performed to analyze the effects of ECM on MPC growth. Before seeding cells, HA-HP-ECMs (100 μL/well) were precoated in 96-well plates.
MPCs at p3 were seeded at 1 × 104 cells/well and incubated at 37°C in 5% CO2 in the MPC growth medium, with the medium changed every 3 days. The MTS reagent, CellTiter 96RAQueous One Solution Cell Proliferation Assay (Promega, Madison, WI), was added directly into the cell culture media (20 μL/well) for 2 h and detected by measuring the absorbance at 490 nm on days 0, 1, 3, 5, 7, and 9 at the same time of the day. To set up a standard curve, 0, 1 × 104, 2 × 104, 4 × 104, 8 × 104, 1.6 × 105, 3.2 × 105, 6.4 × 105, 1.3 × 106, and 2.6 × 106 cells/well in a 96-well plate were seeded and absorbance detected on day 0. Each concentration and time point was performed in triplicate, and mean values were evaluated. Cell growth curves were drawn according to the absorbance and standard curves.
Myogenic differentiation and myotube formation
To induce myogenic differentiation of MPCs in vitro, a thick layer of mECM-HA-HP, skeletal mECM solution, 0.1% gelatin, or Matrigel was loaded into 24-well plates (0.5 mL gel/well), incubated at 37°C for at least 2 h, and rinsed with PBS thrice. These gels firmly attached to the culture wells. Human MPCs were seeded on precoated dishes at a density of 105 cells in a myogenic differentiation medium (DMEM; Thermo Fisher Scientific) containing 5% FBS, 2% horse serum (GE Healthcare), 1% antibiotic antimycotic solution (AA; Hyclone, Logan, UT), 250 nM dexamethasone (Lonza, Allendale, NJ), and 1% insulin-transferrin-selenium solution (Lonza). The medium was refreshed every 2 days and after 2 weeks of induction, cells were collected for further analysis.
Immunofluorescence staining
The morphologic characteristics of MPCs cultured on HA-HP-ECM hydrogels were observed using a phase contrast microscope (Zeiss) and quantitated with Image J software. To determine the ratio or percentage of expression of myogenic proteins, MPCs on each ECM-HA-HP gel cultured in a chamber for 5 days were fixed with 4% paraformaldehyde for 15 min at room temperature, and blocked with serum-free block solution (Dako, Denmark) for 15 min. Fixed cells were incubated with primary antibodies to desmin (Dako, 1:1,000), myosin (Abcam, 1:1,000), myf5 (Abcam, 1:1,000), and myoD (BD Biosciences, 1:1,000) overnight at 4°C. Next, cells were incubated with secondary antibodies (Vector Laboratories, Burlingame, CA, 1:200) for 1 h and phalloidin (Abcam, 1:40) for 20 min, and counterstained with the antifade mounting medium (Vector Laboratories) containing DAPI. Images were obtained using a fluorescence microscope (Leica DM 4000B, Germany) or a confocal microscope (Olympus FluoView Fv10i, Olympus Life Science, Shinjuku, Tokyo, Japan). Investigators who conducted the immunofluorescent analyses of myogenic proteins were blinded to experimental groups. The percentage of positive cells was scored as follows: 0 = no staining; 1 + = 0–25%; 2 + = 26–50%; 3 + = 51–75%; and 4 + = 75% above. Samples were assessed in triplicate. Both stained cells per section and the percentage of the areas stained compared to the total area were calculated by computerized image analysis using Image-Pro Plus software.
Western blot analyses
Western blot analyses were performed to quantitate the amount of myogenic proteins from MPCs grown on mECM-HA-HP gels compared to MPCs grown on other coating gels (matrix gel, gelatin gel, mECM coating, and blank). MPCs were collected 2 weeks after induction of differentiation, and total protein was extracted using radio-immunoprecipitation assay reagent (Pierce, Rockford, IL) containing a 1% protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). Protein samples (10 μg total protein) in different coating conditions were separated on 10% precast gels (Bio-Rad Laboratories, Hercules, CA). A 100 V column ran for 60 min and then a 12 V charge was used to transfer the proteins onto a nitrocellulose membrane.
Protein samples were blocked with 5% nonfat milk at room temperature for 40 min, and then the following primary antibodies were added: myosin (Abcam, 1:1,000), desmin (Abcam, 1:1,000), Myf5 (Santa Cruz, 1:100), myogenin (Abcam, 1:1,000), and MyoD (Santa Cruz, 1:100), and incubated overnight at 4°C. Blots were rinsed thrice with PBS solution containing 0.1% Tween 20 (PBST) and then incubated with secondary antibodies (anti-mouse/rabbit, Abcam, 1:5,000) at room temperature for 60 min. Finally, samples were rinsed with PBST solution thrice and developed with Supersignal® West Femto Maximum Sensitive Substrate (Thermo) at room temperature for 1 min.
Images were analyzed with Fujifilm LAS-3000 Luminescent Image Analyzer system. Data were calculated and analyzed as gray values using ImageJ software. All experiments were repeated thrice.
Statistical analyses
Statistical analyses were performed with Student's t-test (p-values) for comparisons of cell proliferation and myogenesis among the six types of different tested culture systems, with significance being p < 0.05. Analyses of variance were used to assess differences in myogenic differentiation.
Results
Analysis of decellularized ECM
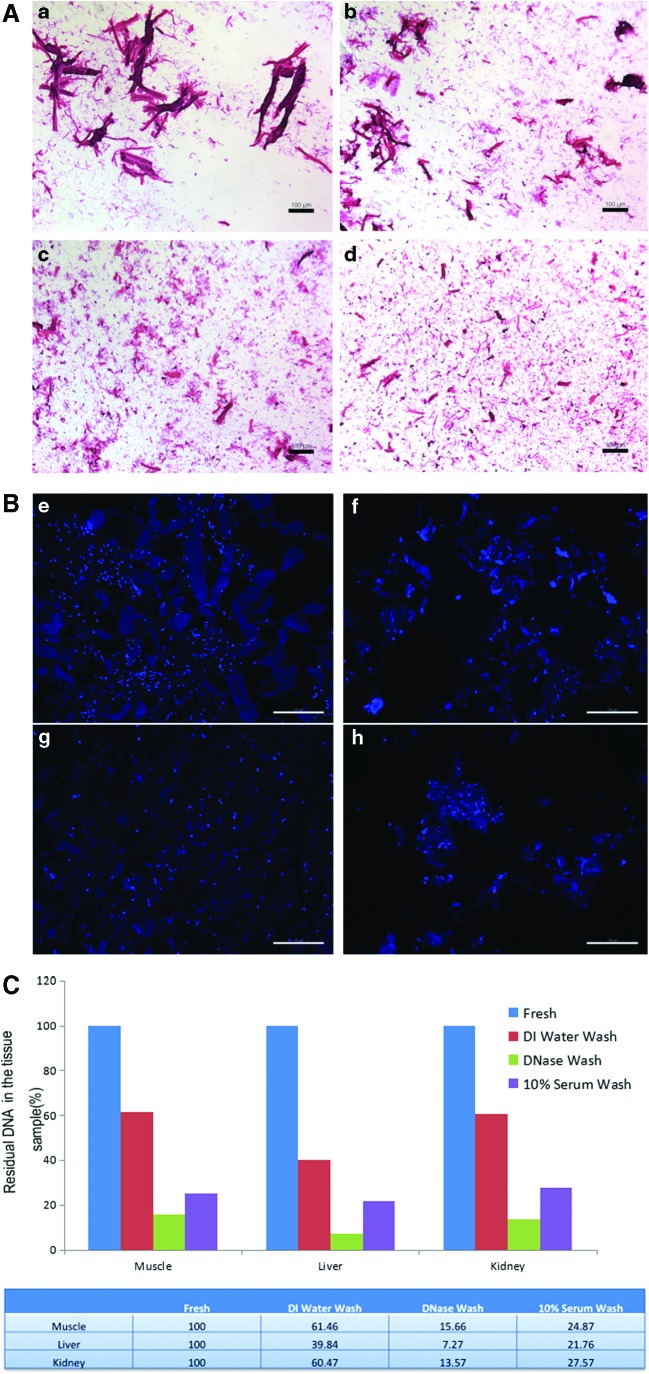
To increase the decellularization efficiency, each tissue ECM was minced into small fragments. Increased homogenization time resulted in smaller ECM particles, with a higher percentage of the ECM fragments at <100 μm diameter after 20 and 30 min (Fig. 2A; Table 1). Therefore, we used ECM that had been homogenized for 20 min for the described experiments.
FIG. 2.
The size of ECM particulars after homogenization and DNA concentration after decellularization. (A) Hematoxylin and eosin staining showed ECM particles in decellularized, homogenized ECM sample suspension. (a) After 3-min homogenization. (b–d) After 10, 20, and 30 min of homogenization. Scale bar = 100 μm. (B) DAPI fluorescent staining showed residual nuclei/DNA components in decellularized, homogenized ECM sample suspension. (e)After 3-min homogenization. (f–h) After 10, 20, and 30 min of homogenization. Scale bar = 100 μm. (C) The changes in DNA concentration (%) with different stages of treatment with each fresh tissue starting at 100%. Quantification of DNA remaining in decellularized, homogenized ECM samples after different methods of decellularization. DAPI, 4,6-Diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
Table 1.
Size of Pig Muscle Particle with Homogenize Mince
| Time for homogenization | Size of particulars | Percentage of finer particulars (5–20 μm) | Degree of uniform |
|---|---|---|---|
| 3 min | 10–500 μm | 50% | ++ |
| 10 min | 10–150 μm | 70% | +++ |
| 20 min | 5–100 μm | 80% | ++++ |
| 30 min | 5–80 μm | 85% | ++++ |
DNase removed >80% of cell components from the homogenized tissue, with remaining DNA (ng/mL) of 15.7% in mECM, 7.3% in the liver ECM, and 13.6% in the kidney ECM compared to nonhomogenized fresh tissue. Washes with deionized water and 5% FBS removed >70% of cell components, with residual DNA of 24.9% in mECM, 21.8% in the liver ECM, and 27.6% in the kidney ECM, compared to fresh tissue. In contrast, after washing with only deionized water, 61.46% of DNA was retained in mECM, 39.84% in the liver ECM, and 60.47% in the kidney ECM (Fig. 2B, C). No significant difference was found between washing for 2 and 4 days based on residual DNA levels.
Optimal concentration of mECM-HA-HP
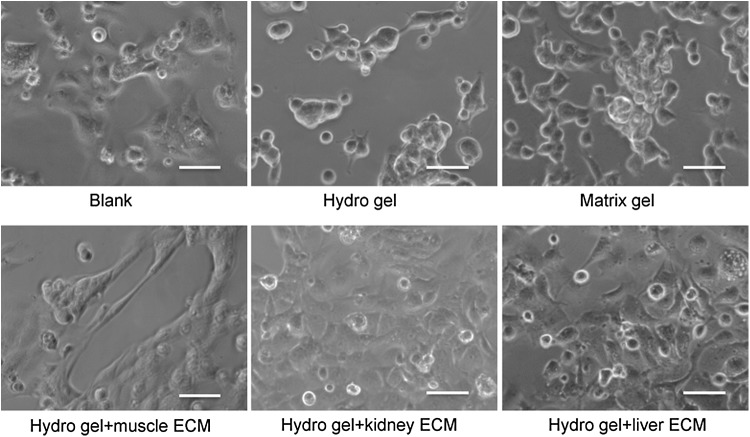
The viability of MPCs was similar when cells were cultured on different concentrations of mECM-HA-HP gels (0%, 2.5%, 5%, 10%, and 25% mECM), with no dead cells observed 3 days after seeding (data not shown). On an HA hydrogel, with or without 2.5% mECM, MPCs were distributed evenly, but no myotube formation was seen (data not shown). The numbers of myotubes significantly increased on the gels with 5% and 10% mECM (p < 0.05), with the highest concentration found on the gels with 10% mECM. Grossly, MPCs seeded in the gel formed cell spheroids on 25% mECM-HA-HP, without myotube formation. When 50% and higher mECM were mixed with HA-HP, the gels did not set, and no myotubes appeared (data not shown). Importantly, MPCs started to expand and form spindle shapes at 3 h postseeding on thicker gels with 10% mECM-HA-HP (Fig. 3). Therefore, 10% mECM-HA-HP gel was considered the most suitable substrate concentration for MPC cultures in further experiments.
FIG. 3.
hMPCs cultured on different ECM gels in myogenic differentiation medium 3 h after seeding (initial concentration: 100,000/cm2 in 8-well chambers. Phase contrast, scale bar = 50 μm). hMPC, human muscle progenitor cell.
Characterization of MPCs
Phenotypes of MPCs at passage 2 (p2) and p5 showed the following: MyoD decreased from 64.7 ± 11.1% to 41.3 ± 6.9%, desmin decreased from 77.1 ± 6.3% to 59.0 ± 8.9%, and myosin heavy chain increased from 5.9 ± 3.8% to 7.8%, assessed by Attune flow cytometer. Growth kinetics: study showed an average doubling time of 29 h over four passages in 16 days.
MPCs cultured on different substrates
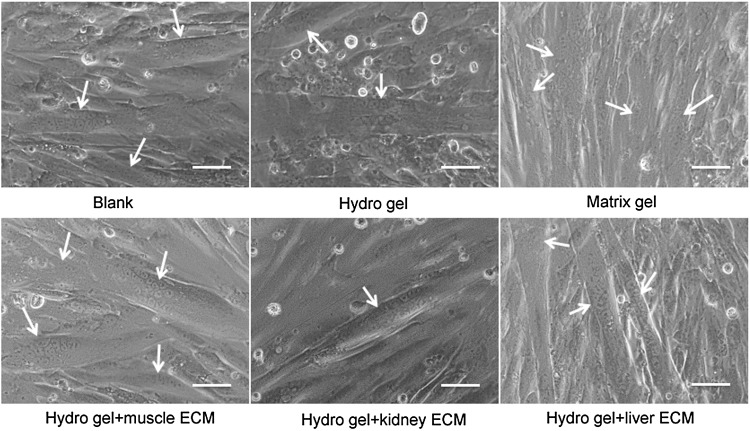
To identify the capacity and specificity of mECM to support expansion and the myogenic differentiation of MPCs, cells were cultured on mECM-HA-HP and compared to those cultured on no gel (blank), kidney ECM-HA-HP, liver ECM-HA-HP, Matrigel, and HA-HP gel alone as control. The mECM-HA-HP gel substrate significantly enhanced MPC proliferation on day 6 in culture, compared to liver ECM-HA-HP, kidney ECM-HA-HP, or non-ECM gel substrates (Fig. 4) (p < 0.05). The numbers and size of multinucleated myotubes are considered key identifiers of myogenesis in vitro. Myoblasts were detected fused into myotube structures with multiple centrally located nuclei under all conditions (Fig. 5).
FIG. 4.
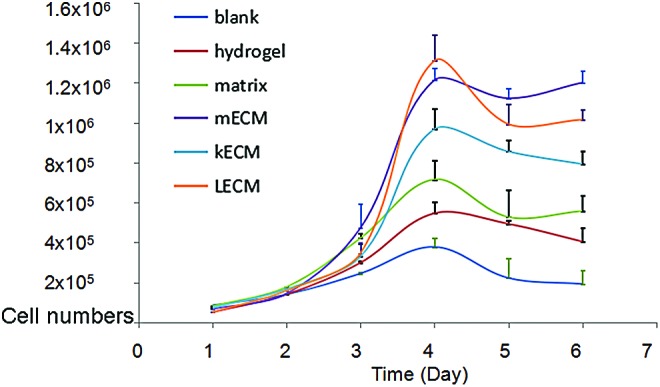
hMPC growth in different tissue-derived, decellularized, and homogenized ECM. kECM, kidney ECM; LECM, liver ECM; mECM, muscle ECM. Color images available online at www.liebertpub.com/tea
FIG. 5.
Myotube formation of hMPCs. Phase micrographs representing the morphology of human multinuclear myoytube formation when PMCs were cultured on skeletal muscle-, kidney-, liver-ECM-HA-HP gel, matrix, and noncoated dishes on Day 3 (Scale bar = 50 μm). White arrows indicate myotubes with multinuclei.
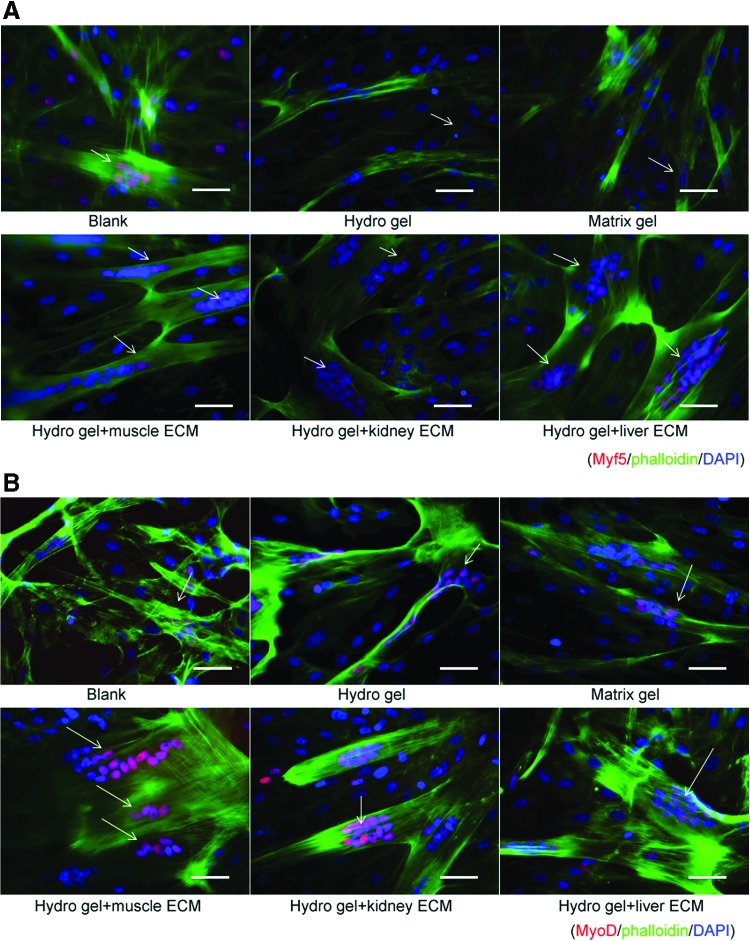
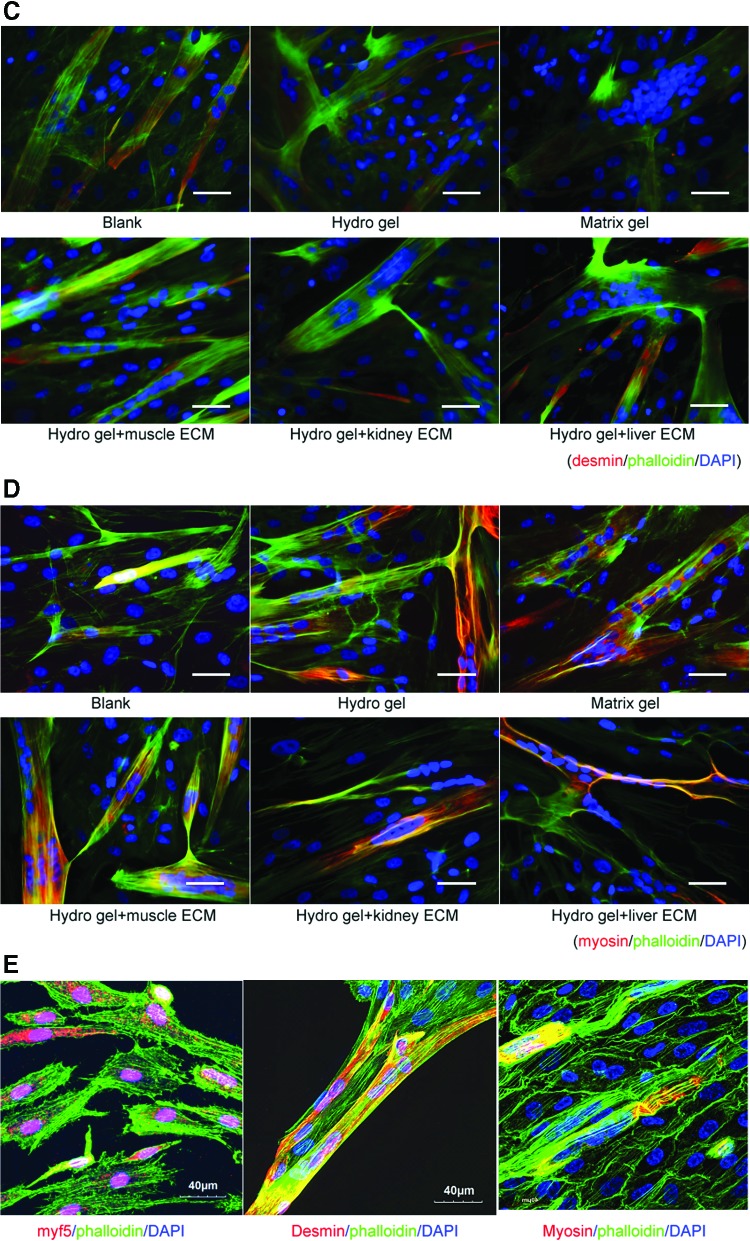
Immunofluorescent staining showed that numbers of cells expressing specific myogenic cell markers significantly increased when MPCs were cultured on mECM-HA-HP and liver ECM- HA-HP substrates (Fig. 6; Table 2) (p < 0.05). This immunofluorescent data were confirmed by quantification of positively stained nuclei by independent blinded scientists (Table 2). Myotube formation occurs when differentiated MPCs (Myf5 and MyoD positive) align and fuse together to form multinucleated myotubes as marked by expression of desmin and myosin. An increase in numbers and size of MyoD and desmin-expressing myotubes were detected in mECM-HA-HP gel than on other gels (Fig. 6), indicating that tissue-specific ECM proteins facilitated MPC viability and differentiation into skeletal myotubes expressing myogenin-specific phenotypes in vitro.
FIG. 6.
Cell numbers of hMPCs expressing skeleton muscle cell markers and myotube thickness on mECM-HA-HP and liver-ECM-HA-HP gel significantly increased on day 3, compared to other gel substrates (p < 0.05). (A) More myotube formation (green) with multinuclei expressing myf-5 maker (red) in mECM and liver-HA-HP gel, compared to other gels or blank. (B) Similarly, the thicker myotube generation with the multinuclei stained in red for MyD in mECM-HA-HA gel, compared to other gels. (C) Numbers of fully differentiated myoctyes expressing desmin (red) increased when cultured on mECM-HA-HP gel, (D) myodifferentiated MPCs expressing Myf5 maker (pink nuclear), desmin and myosin in yellow for cytofilaments. F-actin was labeled with phalloidin (green) to highlight cell skeleton; cell nuclei were stained by DAPI (blue), and myomarkers in nuclear (red). White arrows indicate myotubes with multinuclei. Scale bar = 50 μm. (E) Confocal image, scale bar = 40 μm. Color images available online at www.liebertpub.com/tea
Table 2.
Myogenic Differentiation of Human Muscle Progenitor Cells Cultured on Muscle-Extracellular Matrix-Hyaluronic Acid-Heparin Gels, Compared to Those on Other Gels
| Blank | Matrix gel | HA-HP gel | Muscle-ECM-HA-HP gel | Kidney-ECM-HA-HP gel | Liver-ECM-HA-HP gel | |
|---|---|---|---|---|---|---|
| Under phase contrast microscopya | ||||||
| Myofibrils | + | + | 2+ | 3+ | 3+ | 3+ |
| Myotubes | + | 2+ | 3+ | 4+ | 3+ | 3+ |
| Immunoflurorence stainingb | ||||||
| Myf5 | 2+ | 1+ | 1+ | 3+ | 3+ | 3+ |
| MyD | 1+ | 2+ | 2+ | 4+ | 3+ | 2+ |
| Desmin | 2+ | 1+ | 1+ | 4+ | 2+ | 3+ |
| Myosin | 1+ | 2+ | 2+ | 3+ | 2+ | 2+ |
The percentage of positive cells was scored as follows: 0 = no staining; 1+ = 0–25%; 2+ = 26–50%; 3+ = 51–75%; and 4+ = 75% above assessed immunofluorescent staining, triple samples. Both stained cells per section and the percentage of the area stained compared to the total area were calculated by computerized image analysis.
Myogenesis was evaluated by myofusion index in parent cells and thickness of myotubes 72 h after induced differentiation under phase contrast microscope.
The numbers of the nuclear expressing Myf5 and MyD per mm and the numbers of the cells expressing desmin and myosin were determined under immnuoflurorence microscope.
ECM, extracellular matrix; HA, hyaluronic acid; HP, heparin.
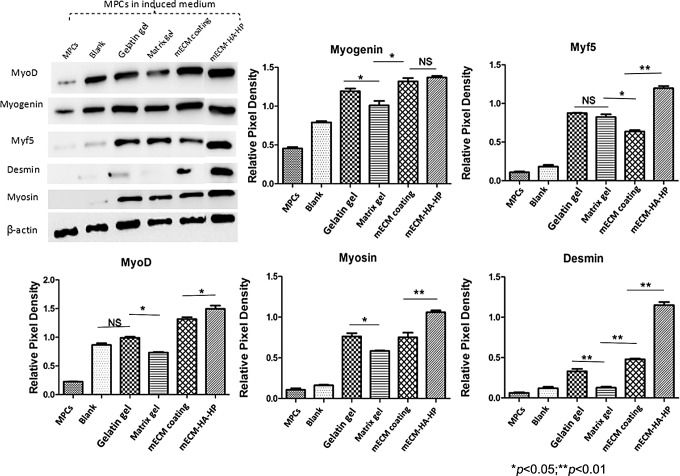
Correspondingly, Western blot analyses showed that MPCs possessed significantly higher levels of myogenic proteins when cultured on mECM-HA-HP gel compared to those on gelatin, mECM alone, and Matrigel coatings (Fig. 7) (p ≤ 0.05), after normalization to beta-actin. MPCs seeded on mECM had similar protein expression levels of MyoD and myogenin as in mECM-HA-HP gels in the early stages of myogenesis. However, Myf5, desmin, and myosin protein expression in mECM-coated gels were lower in the intermediate and maturation stages of myogenesis, indicating that mECM-HA-HP gels better facilitated the initiation to development of myogenesis.
FIG. 7.
Western blots of proteins related to myogenic differentiation of hMPC samples differentiated on multiple ECM components for 2 weeks. Quantification of Western blot band density of proteins related to beta-actin for myogenic differentiation of hMPC samples differentiated on different ECM component coatings.
Discussion
Tissue-specific ECM provides an optimal environment for cells that are derived from organs with different types and amounts of growth factors, cytokines, collagen, fibronectin, laminin, and glycosaminoglycans. Our previous studies showed that somatic skeletal myocytes, hepatocytes, or skin cells grow and retain their cellular phenotypes better on their own specific ECM than on other ECM substrates.10 Nevertheless, growth factors and proteins within these ECMs are rapidly degraded or washed away in culture conditions after the culture medium is changed. Thus, a system is needed for slow or controlled release of bioactive growth factors and cytokines from skeletal mECM compounds to promote cell proliferation, myogenic differentiation, and fusion of MPCs in vitro.
HA hydrogel as an immunoneutral polysaccharide is abundant in the human body, particularly in connective, neural, and epithelial tissue.13,14 It benefits joint support and soft tissue function (such as skin). An Food and Drug Administration-approved HA gel is already in clinical use.15 HA hydrogels have been used as a bioink for chondrogenesis with three-dimensional (3D) bioprinting.16,17
As a type of injectable biomaterial, unlike collagen, HA is able to penetrate soft tissues to improve and benefit the target tissue following local injection. Once infiltrated into the adjacent tissue, the implanted HA forms an air-permeable layer, thus boosting the elasticity and hydration of the engraftment. The protective barrier in the target tissue locks in moisture, improving grafted cell viability. Moreover, heparin linked with HA gel is capable of binding growth factors for sustained release. This study demonstrated that skeletal mECM components mixed with HA-HP hydrogel significantly improves cell expansion and the myogenic capacity of human MPCs, thus providing an alternative hydrogel for 3D printing with skeletal myocytes for muscle regeneration or an injectable biomaterial with stem cells for muscle tissue regeneration for the treatment of SUI.
Decellularization is the process used to remove cells from a tissue leaving an ECM scaffold of an allogeneic or xenogeneic host tissue, which is often further used in tissue repair. The ideal decellularization method can maximally remove all cellular components, while preserving bioactive compounds such as cytokines and growth factors.
Thus, cell-free ECM with bioactive compounds avoids potential immune reactions from the host, but can promote cell growth and differentiation of the host or grafted cells, and improves tissue development. Several decellularization approaches have been used, such as physical methods (temperature, force, pressure, and electrical disruption), chemical methods (acids, alkaline treatments, ionic detergents plus sodium dodecyl sulfate, and nonionic detergents such as Triton X-100 and zwitterionic detergents), and enzymatic treatments (lipases, thermolysin, galactosidase, nucleases, and trypsin) either singly or in combination.18
FBS has been successfully used in decellularization because nucleases in the serum play a key role in DNA/RNA degradation after cell lysis.19 In addition, serum maximally retains the proteins within the ECM compared to other decellularization reagents such as detergents (i.e., Triton X-100).19 Based on these experiments and our previous studies,11 we developed an optimal decellularization approach, including homogenization of the tissue into small particles for increased decellularization efficiency, rinsing with FBS for efficiently and gently washing cellular compounds away from the ECM, while retaining bioactive proteins, and lyophilization for the protection of retained growth factors and cytokines. In the clinical setting, the patient's own serum could be used in these processes, in replacement of animal-derived serum, to prevent the potential risks of contamination.
Bioactive growth factors, cytokines, and ECM proteins (collagen, fibronectin, and laminin), regulate cellular behavior and functions. Our previous study demonstrated that decellularized substrates from skeletal mECM retain a wide range of bioactive growth factors and cytokines, as well as macromolecules for its basal structure, including collagen, glycoproteins, and proteoglycans.20 In addition, mECM solutions contain several key myogenic growth factors (including hepatocyte growth factor, fibroblast growth factor, and insulin-like growth factor-binding protein) and angiogenic growth factors (including vascular endothelial growth factor and basic fibroblast growth factor) that are significantly different from other tissue ECM growth factors.20
Other potent cytokines are also present, including bone morphogenetic protein-5, stem cell factor (BMP-5, SCF) receptors, and transforming growth factor (3TGF-3).21 Furthermore, skeletal mECM solutions, in contrast to solutions containing ECM from other tissues, include different types and amounts of ECM proteins and glycosaminoglycans,11 collagen (types I, III, and IV), and elastin.21 Our decellularization method is simple and shortens the process of ECM fabrication to protect native growth factors, compared to other methods that commonly use DNase as a degradation method. Interestingly, we found that liver ECM substrate, but not kidney ECM substrate, achieved good outcomes in myogenesis. It is likely that liver ECM may contain the growth factors and cytokines required for myogenic differentiation and those proteins are retained using this decellularization method. A proper preservation of ECM fibers, growth factors, and other proteins is vital for the maintenance of the myogenic phenotype of MPCs during in vitro culture and expansion.
Our data demonstrated that skeletal mECM compounds promote cell proliferation and myogenic differentiation of MPCs when mECM alone is coated in a monolayer on culture dishes. Binding proteins with heparin in a HA gel can prolong the half-life of growth factors and alter their interaction with cell surface receptors. HA-HP hydrogel with liver tissue-specific ECM significantly enhanced cell survival and functional output of primary human hepatocytes in vitro, compared to other matrixes.11 This is because growth factors within the ECM can be combined with HA gel through heparin for slow or controlled release.
In this study, we used a similar strategy to combine mECM with HA-HP hydrogel and achieved optimal outcomes regarding proliferation and differentiation of MPCs, compared to kidney ECM, and HA-HP gel alone. Uniform mECM-HA-HP substrate-based coatings or surface modifications provide muscle tissue with an extracellular microenvironment to guide differentiation of MPCs in vitro. In contrast, although Matrigel™ promotes cell proliferation, it does not aid in myogenic differentiation in vitro. Moreover, Matrigel cannot be used in clinical applications due to its oncogenic potential.
Conclusions
In this initial study, we developed a skeletal muscle-specific ECM substrate mimicking the native extracellular environment that promotes cell survival, proliferation, myogenic differentiation, and myotube formation by fusion of MPCs in vitro. mECM combined with HA hydrogel-heparin gel could provide an alternative approach for cell replacement therapy by improving cell survival, myogenic differentiation, and integration cells or bioactive factors in the host tissue. Further studies of skeletal mECM with MPCs as an injectable HA-HP gel for skeletal muscle regeneration will be developed in vivo, which might provide an optimal alternative for skeletal muscle injuries with cell therapy or for urinary sphincter dysfunction in the treatment of SUI.
Acknowledgment
The authors thanks the National Institutes of Health grant R56 DK100669 (PI: Y.Z.).
Disclosure Statement
No biomedical financial interests exist.
References
- 1.Peters K.M., Dmochowski R.R., Carr L.K., Robert M., Kaufman M.R., Sirls L.T., Herschorn S., Birch C., Kultgen P.L., and Chancellor M.B. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol 192, 469, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Morgan D.M., Umek W., Guire K., Morgan H.K., Garabrant A., and DeLancey J.O. Urethral sphincter morphology and function with and without stress incontinence. J Urol 182, 203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sajadi K.P., Gill B.C., and Damaser M.S. Neurogenic aspects of stress urinary incontinence. Curr Opin Obstet Gynecol 22, 425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberli D., Soker S., Atala A., and Yoo J.J. Optimization of human skeletal muscle precursor cell culture and myofiber formation in vitro. Methods 47, 98, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Stangel-Wojcikiewicz K., Jarocha D., Piwowar M., Jach R., Uhl T., Basta A., and Majka M. Autologous muscle-derived cells for the treatment of female stress urinary incontinence: a 2-year follow-up of a Polish investigation. Neurourol Urodyn 33, 324, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Cheung D., Zhou Y., Han C., Fennelly C., Criswell T., and Soker S. An in vitro culture system that supports robust expansion and maintenance of in vivo engraftment capabilities for myogenic progenitor cells from adult mice. Biores Open Access 3, 79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A., Partridge T., and Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., and Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker M.H., Loretz C., Tyler A.E., Duddy W.J., Hall J.K., Olwin B.B., Bernstein I.D., Storb R., and Tapscott S.J. Activation of notch signaling during ex vivo expansion maintains donor muscle cell engraftment. Stem Cells 30, 2212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., He Y., Bharadwaj S., Hammam N., Carnagey K., Myers R., Atala A., and Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30, 4021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skardal A., Smith L., Bharadwaj S., Atala A., Soker S., and Zhang Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials 33, 4565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G.P.R., Wu R., Shi Y., Deng C., Zhou X., Atala A., Opara E., and Zhang Y. Skeletal myogenic differentiation of urine-derived stem cells, angiogenesis and innervation using hydrogel loaded with growth factors for potential in treatment of urinary incontinence. J Urol 193, e74, 2015 [Google Scholar]
- 13.Highley C.B., Prestwich G.D., and Burdick J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 40, 35, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Burdick J.A., and Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23, H41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birchall D., Ismail A.M., and Peat G. Clinical outcomes from a physiotherapist-led intra-articular hyaluronic acid injection clinic. Musculoskeletal Care 6, 135, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Pescosolido L., Schuurman W., Malda J., Matricardi P., Alhaique F., Coviello T., van Weeren P.R., Dhert W.J., Hennink W.E., and Vermonden T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 12, 1831, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Park J.Y., Choi J.C., Shim J.H., Lee J.S., Park H., Kim S.W., Doh J., and Cho D.W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 6, 035004, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Keane T.J., Swinehart I.T., and Badylak S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84, 25, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Gui L., Chan S.A., Breuer C.K., and Niklason L.E. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods 16, 173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long T., Yi H., Zhang D., Zhang Y., Hu Y., Wang Z., Yoo J., Atala A., and Zhang Y. Optimization of skeletal myocytes expansion for potential application in cell therapy for urinary incontinence. Tissue Eng Part A 22, S92, 2016 [Google Scholar]
- 21.Yi H., Forsythe S., Zhang Y., and Skardal A. Bio-functionalized alginate hydrogels for improved cell-matrix interactions and growth factor sequestration kinetics. Tissue Eng Part A 21, S187, 2015 [Google Scholar]