Abstract
Background & Aims
The paradox of selective hepatic insulin resistance, wherein the insulin-resistant liver fails to suppress glucose production but continues to produce lipids, has been central to the pathophysiology of hepatosteatosis and hyperglycemia. Our study was designed to investigate the mechanism(s) by which microRNA-206 alleviates the pathogenesis of hepatosteatosis and hyperglycemia.
Methods
Dietary obese mice induced by a high fat diet were used to study the role of microRNA-206 in the pathogenesis of hepatosteatosis and hyperglycemia. A mini-circle vector was used to deliver microRNA-206 into the livers of mice.
Results
Lipid accumulation impaired biogenesis of microRNA-206 in fatty livers of dietary obese mice and human hepatocytes (p <0.01). Delivery of microRNA-206 into the livers of dietary obese mice resulted in the strong therapeutic effects on hepatosteatosis and hyperglycemia. Mechanistically, miR-206 interacted with the 3′ untranslated region of PTPN1 (protein tyrosine phosphatase, non-receptor type 1) and induced its degradation. By inhibiting PTPN1 expression, microRNA-206 facilitated insulin signaling by promoting phosphorylation of INSR (insulin receptor) and impaired hepatic lipogenesis by inhibiting Srebp1c transcription. By simultaneously modulating lipogenesis and insulin signaling, microRNA-206 reduced lipid (p = 0.006) and glucose (p = 0.018) production in human hepatocytes and livers of dietary obese mice (p <0.001 and p <0.01 respectively). Re-introduction of Ptpn1 into livers offset the inhibitory effects of microRNA-206, indicating that PTPN1 mediates the inhibitory effects of microRNA-206 on both hepatosteatosis and hyperglycemia.
Conclusions
MicroRNA-206 is a potent inhibitor of lipid and glucose production by simultaneously facilitating insulin signaling and impairing hepatic lipogenesis. Our findings potentially provide a novel therapeutic agent for both hepatosteatosis and hyperglycemia.
Keywords: Insulin signaling, Lipogenesis, Hyperglycemia, NAFLD, MicroRNAs, Phosphorylation, Diet, high-fat
Introduction
Results from the American Heart Association showed that nearly 73% of U.S adults are overweight or obese. Obesity and its associated co-morbidities are among the most prevalent and challenging conditions confronting the medical profession in the past century. A major metabolic consequence of obesity is insulin resistance, which is a major risk factor of type 2 diabetes (T2D) and non-alcoholic fatty liver disease (NAFLD) [1]. NAFLD is the accumulation of excessive amounts of lipids within hepatocytes that is not caused by alcohol. It is estimated that 90% of obese patients have some form of fatty liver, ranging from hepatosteatosis to more severe forms of NASH (non-alcoholic steatohepatitis), which can give rise to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [2]. Until recently, T2D was a disease that primarily afflicted adults. However, there is now a growing number of children who are being diagnosed with obesity-related T2D [1]. Although the pathogenesis of NAFLD and T2D has been studied extensively, the molecular mechanisms underlying these two co-morbid disorders are still under investigation. A better understanding of their pathogenesis and the development of new therapeutic strategies will be required to combat the epidemic of both disorders [2].
It is widely accepted that insulin resistance is central to the pathogenesis of NAFLD and T2D [3]. The principal function of insulin in the liver is to suppress glucose production when blood glucose concentrations increase. This process is impaired in hepatic insulin resistance and contributes to hyperglycemia. In the normal state, insulin can potently induce de novo lipogenesis by modulating SREBPs (sterol regulatory element–binding protein) at multiple levels, including SREBP1c mRNA, the proteolytic processing of SREBP1c, and the stability and abundance of nuclear SREBP1c [4]. In the insulin-resistant state, compensatory hyper-insulinemia is postulated to activate SREBP1c transcription and cleavage, thereby increasing expression of lipogenic genes, enhancing fatty acid synthesis, and promoting triglyceride accumulation in the liver [5]. Based on the role of insulin in promoting lipogenesis, it is hard to use insulin resistance to explain hepatic lipid accumulation, and the molecular mechanisms regarding this observance have remained elusive.
MicroRNAs (miRNAs) are naturally-occurring small non-coding RNAs that function by binding to the 3′-untranslated regions (3′UTR) of specific mRNAs, which leads to either mRNA or translational pausing [6]. Given their critical role in lipid metabolism and carcinogenesis [7,8], miRNAs now represent novel therapeutic agents for human cancers and metabolic diseases. Many dysregulated miRNAs have been identified to modulate the pathogenesis of NAFLD, hyperlipidemia and T2D [7,9,10]. However, the specific miRNAs that have the capacity to prevent both NAFLD and hyperglycemia remain unknown. In this study, we investigated the underlying mechanisms by which miRNAs inhibit hepatic lipogenesis and glucose production, in part to explain the insulin resistance paradox.
Materials and methods
Establishment of dietary obese mice
Eight-week-old wild-type male C57Bl/6 mice (Jackson Laboratory, n = 6) were maintained on either a normal chow diet (Open Source D12450B: 10% kCal fat) or a high fat diet (HFD) (Open Source D12492: 60% kCal fat) for 8 weeks as described [11]. After 8 weeks of HFD administration, livers were collected for miRNA and gene expression analysis.
Preparation of mini-circle expression vectors for miR-206 and Ptpn1
We generated an in vivo expression vector of miR-206 by cloning human miR-206 precursor into mini-circle vectors purchased from System Biosciences (Cat. MN511A-1). A transthyretin gene (TTR) promoter was inserted into the upstream of miR-206 precursor to ensure liver-specific expression of miR-206 [12]. To rule out non-specific effects of the plasmid, we generated a miR-206 mismatched-expression vector by mutating the seed region of miR-206, termed MC-TTR-miR-206-MM. We inserted the coding region of mouse Ptpn1 into a mini-circle vector and the TTR promoter was used to ensure hepatic expression of Ptpn1. This vector was referred as to MC-TTR-Ptpn1. Parental MC-TTR-miR-206 or MC-TTR-Ptpn1 vector was transformed into a special host E. coli bacterial strain ZYCY10P3S2T (System Biosciences, Cat: MN900A-1). Mini-circles were generated based on the manufacturer’s instructions.
MC-TTR-miR-206 treatment of dietary obese mice
Two-month-old wild-type C57Bl/6 mice were kept on HFD for 8 weeks. At 16 weeks of age, mice were divided into two groups: one group (n = 10) treated with MC-TTR-miR-206 and the other with MC-TTR-miR-206-MM (control, n = 10). Mice received a dose of 1.5 μg/g MC-TTR-miR-206 or MC-TTR-miR-206-MM complexed with in vivo-jetPEI® (Polyplus Transfection, Strasbourg, France) weekly for eight weeks via tail vein injection. At that time point, the mice were anesthetized, and blood was collected by cardiac puncture. The livers were harvested and immediately frozen in liquid nitrogen for gene expression and histological analysis.
To examine whether Ptpn1 mediates the inhibitory effect of miR-206 on NAFLD and hyperglycemia, two-month old wild-type C57Bl/6 mice were maintained on the HFD (Open Source D12492: 60% kCal fat) for 8 weeks. At 16 weeks of age, mice were divided into three groups, in which group I (control group, n = 10) was injected with a combination of 1.5 μg/g MC-TTR-miR-206-MM; group II (n = 10) was injected with 1.5 μg/g MC-TTR-miR-206; and group III (n = 10) received a combination of 1.5 μg/g MC-TTR-miR-206 and 1.5 μg/g MC-TTR-Ptpn1. Group III was also used to determine whether additional treatment of Ptpn1 could reverse the inhibitory effects of miR-206 on the development of NAFLD and hyperglycemia. All mice were maintained on the HFD as described above. Mice were housed, fed, and monitored in accordance with protocols approved by the committee for animal research at the University of Minnesota.
Fatty acid treatment of HepG2 cells and primary human hepatocytes
Human hepatocytes were purchased from Invitrogen. Sodium oleate was obtained from Sigma-Aldrich and was dissolved in DMEM medium with 1% fatty acid free bovine serum albumin (BSA) (Sigma). Oleate treatment of HepG2 cells was carried out as previously described with minor revision [13,7]. Specifically, HepG2 cells or human hepatocytes were plated in 4-well chamber slides with DMEM medium supplemented with 10% FBS (Invitrogen). After 24 h, cells were treated with either control medium (DMEM supplemented with 1% fatty acid free BSA), or medium containing oleate (0.5 mM). The cells were cultured for another 24 h, after which lipid accumulation and miR-206 expression were determined by Oil-Red O staining (Sigma-Aldrich) and qRT-PCR, respectively.
To determine whether PTPN1 mediates the effect of miR-206 on lipogenesis, HepG2 cells cultured in the DMEM containing 0.5 mM oleate were transfected with MC-TTR-miR-206 (200 ng in 4-well chamber slides), or a combination of MC-TTR-miR-206 and PTPN1 Target Protector (TP) (20 nM in 4-well chamber slides). The PTPN1 TP and control TP were designed and generated by Exiqon. Lipofectamine 2000 was used for transfection of MC-TTR-miR-206 and PTPN1 TP. After another 24 h of culture, lipid accumulation was assessed by Oil-Red O staining, microfluorimetry or imaging.
Gluconeogenic assay
HepG2 cells were maintained in the minimum Eagle’s medium with low glucose (Invitrogen) in 24 wells plate overnight. 12 h later, cells were washed three times using cold PBS to remove glucose and incubated for 16 h in a 0.5 ml of glucose production medium (phenol and glucose free DMEM containing gluconeogenic substrates including 20 mM sodium lactate and 2 mM sodium pyruvate). Insulin was added into medium within the last 3 h; and glucose levels measured with an Amplex Red Glucose/Glucose Oxidase Assay Kit (Invitrogen). Glucose concentrations in the media were normalized with cellular protein concentrations [14].
Statistical analysis
Statistical analysis was performed using GraphPad Prism Software®. Data derived from cell-line experiments were presented as mean ± SEM and assessed by a two-tailed Student’s t test. Mann-Whitney U test was used to evaluate the statistical significance for mouse experiments. All experiments were repeated at least three times. p <0.05 was considered to be statistically significant.
Full details of these and other methods can be found in the Supplementary materials and methods.
Results
Lipid accumulation impaired biogenesis of miR-206 in livers of dietary obese mice and human hepatocytes
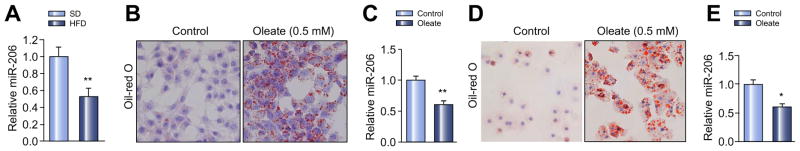
We measured expression of miR-206 in livers of wild-type mice on HFD, which led to increased hepatic lipid accumulation and blood glucose (Supplementary Fig. 1A–D). qRT-PCR revealed that miR-206 was significantly reduced in fatty livers of dietary obese mice compared to mice treated with standard diet (Fig. 1A). We then determined whether intracellular lipid accumulation can decrease expression of miR-206 in primary human hepatocytes and HepG2 cells [15]. Oleic acids are the most abundant fatty unsaturated acids in liver triglycerides in both normal subjects and patients with NAFLD. As expected, oleate treatment significantly increased intracellular lipids in HepG2 cells, which further reduced expression of miR-206 (Fig. 1B and C). In normal human hepatocytes, oleate treatment displayed the same phenotype as seen in HepG2 cells (Fig. 1D and E). Together, our studies indicated that miR-206 expression is reduced in fatty livers of dietary obese mice and human liver cells with accumulated lipid.
Fig. 1. miR-206 expression is reduced in livers of dietary obese mice and in human liver cells with accumulated lipid.
(A) Levels of miR-206 in livers of a high fat diet (HFD)-fed mice (n = 6) compared to standard diet-fed control mice (n = 6). (B and C) Oil-Red O staining reveals increased intracellular lipid accumulation in HepG2 cells treated with oleate (0.5 mM), which subsequently led to decreased expression of miR-206 as shown by qRT-PCR. (D and E) Oil-Red O staining reveals increased intracellular lipid accumulation in human hepatocytes treated with oleate (0.5 mM), which subsequently led to decreased expression of miR-206 as detected by qRT-PCR. Data represent mean ± SEM. Student’s t test was used for statistical analysis. *p <0.05; **p <0.01.
PTPN1 is a direct target of miR-206
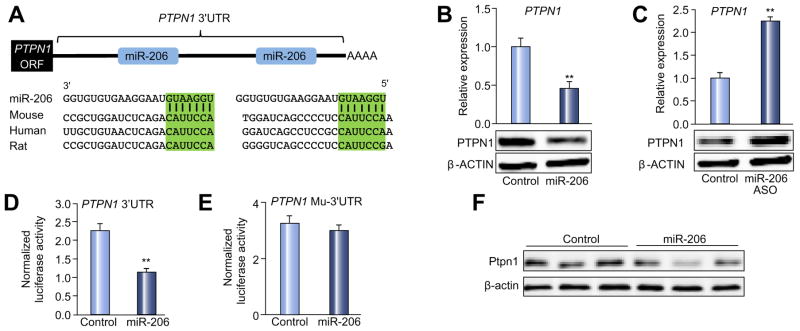
We firstly predicted target genes of miR-206 by combining several miRNA target prediction algorithms including TargetScan [16], Pictar [17], and Starbase [18]. To increase the prediction accuracy, only hits from TargetScan or PicTar algorithm that were confirmed by Ago HITS-CLIP (high-throughput sequencing of RNAs isolated by crosslinking immunoprecipitation from Argonaute protein complex) were selected as potential targets of miR-206. We identified putative binding sites for miR-206 within 3′UTRs of six genes that are important regulators of lipogenesis, insulin resistance, and carcinogenesis (Supplementary Table 1). Among six potential targets of miR-206, PTPN1 is a negative regulator of insulin signaling and a therapeutic target for T2D and obesity [19]. Liver-specific deletion of Ptpn1 improves insulin sensitivity and impairs lipogenesis by inhibiting Srebp1c transcription [20,21]. Notably, PTPN1 3′UTR contains two miR-206 binding sites that are conserved between human and mouse (Fig. 2A), suggesting that miR-206 should have strong inhibitory effect on expression of PTPN1. Indeed, overexpression of miR-206 in HepG2 cells robustly decreased both mRNA and protein levels of PTPN1 (Fig. 2B), while antagonizing miR-206 increased PTPN1 levels (Fig. 2C). To establish that miR-206 directly recognizes the predicted target sites within the 3′ UTR of PTPN1, its 3′ UTR was cloned into a luciferase reporter vector. Inclusion of the 3′UTR of PTPN1 into a luciferase reporter construct reduced luciferase activity upon co-transfection with miR-206 into Hepa1,6 cells (Fig. 2D). In contrast, mutation of the miR-206 binding sites was necessary to completely offset the inhibitory effects of miR-206 on luciferase activity (Fig. 2E). In mice, delivery of miR-206 into livers also reduced both protein and mRNA levels of Ptpn1 (Fig. 2F). Together, these experiments indicated that miR-206 is able to inhibit expression of PTPN1 by directly interacting with its 3′UTR.
Fig. 2. PTPN1 is a direct target of miR-206.
(A) Graphic representation of the conserved miR-206 binding motifs within the 3′UTR of PTPN1. Complimentary sequences to the seed regions of miR-206 within the 3′UTR mRNA are conserved among three species (highlighted in green). (B) qRT-PCR and immunoblot analysis of PTPN1 after MC-TTR-miR-206 or MC-TTR-miR-206-MM transfection into HepG2 cells. (C) qRT-PCR and Western blot analysis of PTPN1 after miR-206-ASO (anti-sense oligonucleotide) transfection into HepG2 cells (20 nM). The control HepG2 cells received scramble (20 nM). (D and E) Luciferase activity of the luciferase reporter constructs containing either wild-type or mutated 3′UTR of murine Ptpn1 after miR-206 mimics treatment. Luciferase activity was normalized to the activity of β-galactosidase. Hepa1,6 cells treated with scramble and the luciferase reporter constructs served as controls. PTPN1 mu-3′UTR: PTPN1 mutated 3′UTR. (F) Western blot and qRT-PCR revealing reduced protein and mRNA levels of Ptpn1 after MC-TTR-miR-206 injection into dietary obese mice (1.5 μg/g body weight). The control mice were treated with the same dose of MC-TTR-miR-206-MM. Data represent mean ± SEM. Student’s t test was used for statistical analysis. **p <0.01.
MiR-206 impairs lipogenesis and facilitates insulin signaling by modulating PTPN1-INSR/IRS and PTPN1-PP2A-SP1-Srebp1c pathways respectively
PTPN1 impairs insulin signaling by dephosphorylating INSR (insulin receptor) and IRS1 (insulin receptor substrate 1) but enhances lipogenesis by de-phosphorylating PP2A (phosphatase 2A). Dephosphorylated PP2A activates transcription of Srebp1c by dephosphorylating Sp1, a transcription factor of Srebp1c [19]. These findings led us to speculate that miR-206 has the capacity to simultaneously facilitate insulin signaling through a PTPN1-INSR/IRS axis and prevent lipogenesis via a PTPN1-PP2A-SP1-SREBP1C pathway. To test our hypothesis, HepG2 cells were incubated with oleate or gluconeogenic substrates and transfected with either miR-206 expression vector or a combination of miR-206 and PTPN1 TP morpholinos. The morpholino is complimentary to the miR-206 binding sites within the 3′UTR of PTPN1 mRNA and prevents miR-206 from binding to PTPN1 3′UTR [22]. This design allowed us to determine whether PTPN1 mediates the effects of miR-206 on inhibiting lipogenesis and facilitating insulin signaling. MiR-206 overexpression led to reduced PTPN1 and the additional treatment of PTPN1 TP recovered expression of PTPN1 (Supplementary Fig. 2A).
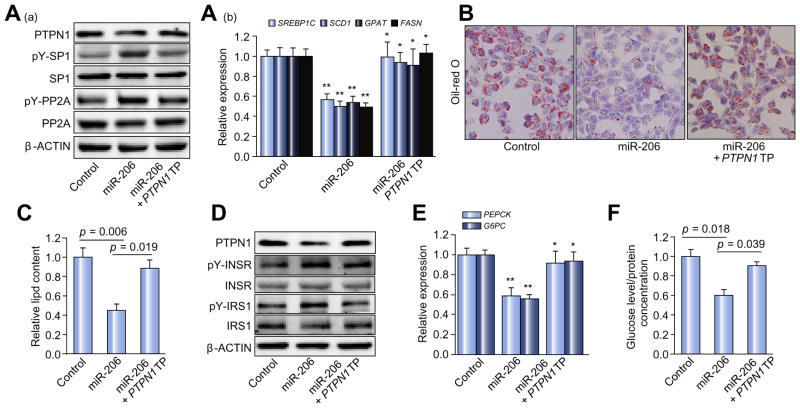
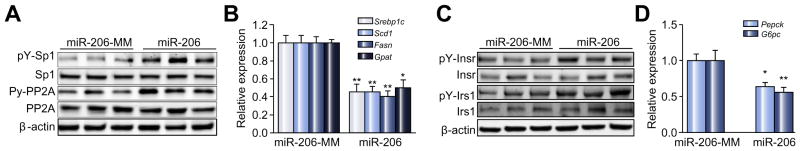
This observation indicated that PTPN1 TP was able to prevent miR-206 from binding to PTPN1 3′UTR. Transfection of miR-206 into HepG2 cells incubated with oleate led to reduced protein levels of PTPN1, increased phosphorylated PP2A and SP1 and subsequently reduced mRNA levels of SREBP1C and the lipogenic genes including SCD1 (stearoyl-CoA desaturase-1), FASN (fatty acid synthase) and GPAT (glycerol-3-phosphate acyltransferase) (Fig. 3A). The additional treatment of PTPN1 TP offset the inhibitory effects of miR-206 on PP2A-SP1-SREBP1C pathway and expression of the lipogenic genes (Fig. 3A). However, miR-206 treatment did not change protein levels of total SP1 and PP2A (Fig. 3A), indicating that alteration of phosphorylated PP2A and SP1 due to miR-206 overexpression accounts for reduced expression of lipogenic genes in HepG2 cells. Consistent with the impaired PP2A-SP1-SREBP1C pathway and the reduced expression of the lipogenic genes, miR-206 significantly reduced intracellular lipid content in HepG2 cells, and the prevention of the interaction of miR-206 with PTPN1 recovered lipogenesis (Fig. 3B and C). This observation indicated that PTPN1 potentially mediates the role of miR-206 in inhibiting lipogenesis through the PTPN1-PP2A-SP1-SREBP1C pathway.
Fig. 3. Ptpn1 mediates the roles of miR-206 in facilitating insulin signaling and inhibiting lipogenesis simultaneously.
(A) (a) Western blot analysis of PTPN1, phosphorylated SP1, total SP1, phosphorylated PP2A, and total PP2A; and (b) mRNA levels of SREBP1C and the lipogenic genes including SCD1, GPAT, and FASN in three groups of HepG2 cells transfected with either MC-TTR-miR-206 (miR-206), a combination of MC-TTR-miR-206 and PTPN1 TP or MC-TTR-miR-206-MM (control). HepG2 cell were maintained on DMEM medium containing 0.5 mM oleate. (B) Oil-Red O staining of three groups of HepG2 cells. (C) Intracellular lipid content in three groups of HepG2 cells. (D and E) Protein levels of phosphorylated INSR, total INSR, phosphorylated IRS1, and total IRS1 as well as mRNA levels of the gluconeogenic genes including PEPCK and G6PC in HepG2 cells transfected with MC-TTR-miR-206, a combination of MC-TTR-miR-206 and PTPN1 TP or MC-TTR-miR-206-MM (control). HepG2 cells were kept in medium containing gluconeogenic substrates, 20 mM sodium lactate and 2 mM sodium pyruvate in the presence of 1 nM insulin. (F) Glucose levels in HepG2 cells treated with MC-TTR-miR-206, a combination of MC-TTR-miR-206 and PTPN1 TP or MC-TTR-miR-206-MM (control). Glucose concentration was normalized with cellular protein concentration. Data represent mean ± SEM. Student’s t test was used for statistical analysis. *p <0.05; **p <0.01.
The same strategy as described above was used to evaluate the potential of miR-206 to facilitate insulin signaling and subsequently inhibit gluconeogenesis (Supplementary Fig. 2B). Transfection of miR-206 into the HepG2 cells maintained in medium containing gluconeogenic substrates led to reduced protein levels of PTPN1, increased levels of phosphorylated INSR and IRS and decreased mRNA levels of the gluconeogenic genes encoding rate-limiting enzymes including glucose-6-phosphatase (G6PC) and phosphoenolpyruvate carboxykinase (PEPCK), and the additional treatment of PTPN1 TP counteracted the effects of miR-206 (Fig. 3D and E). However, miR-206 treatment has no effect on protein levels of total INSR and IRS1 (Fig. 3D) All these observations indicated that miR-206 had the capacity to facilitate insulin signaling through PTPN1-mediated phosphorylation of INSR and IRS1. Phenotypically, miR-206 treatment reduced glucose content, and the combined treatment of miR-206 and PTPN1 TP recovered glucose levels in HepG2 cells (Fig. 3F).
Liver-specific expression of miR-206 exhibits the robust therapeutic effects on obesity, NAFLD and hyperglycemia
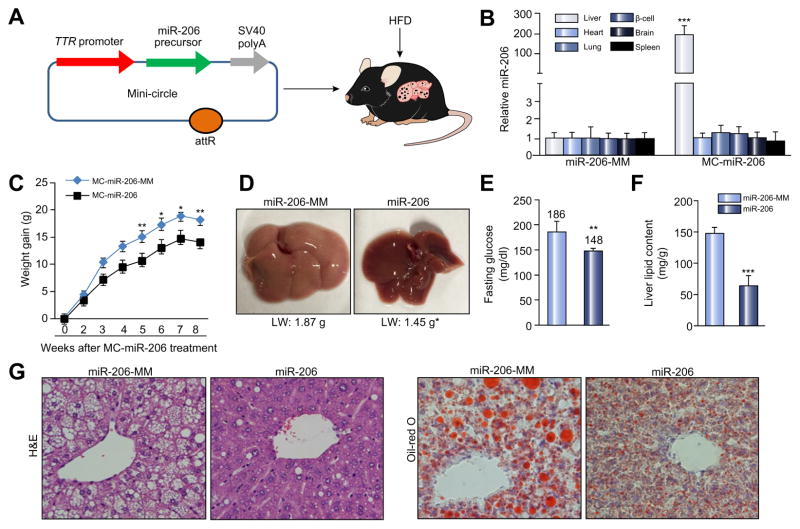
We next assessed the functional contribution of increased miR-206 expression to the development of NAFLD and hyperglycemia in dietary obese mice. For this purpose, we generated a miR-206 in vivo expression system using a mini-circle episomal DNA vector [23]. A TTR promoter was used to ensure hepatocyte-specific expression of miR-206 [7,24] (Fig. 4A), and the construct referred to as MC-TTR-miR-206 (Fig. 4A). Injection of MC-TTR-miR-206 into mice led to high levels of miR-206 in liver, but no significant change in other tissues (Fig. 4B). C57Bl/6 mice, which had been on a HFD for eight weeks to induce hepatosteatosis and hyperglycemia, were treated with either MC-TTR-miR-206 or MC-TTR-miR-206-MM for eight weeks (Supplementary Fig. 3A). Delivery of miR-206 into dietary obese mice led to a significant decrease in body and liver weight (Fig. 4C and D). Consistent with the ability of miR-206 to improve insulin sensitivity and inhibit hepatic lipogenesis, liver-specific expression of miR-206 reduced hepatic lipid content and blood glucose (Fig. 4E–G). In summary, miR-206 is a strong suppressor of both hepatosteatosis and hyperglycemia.
Fig. 4. Delivery of miR-206 into livers alleviates NAFLD and hyperglycemia.
(A) Diagram of liver-specific miR-206 expression vector construction. (B) miR-206 levels after intravenous injection of MC-TTR-miR-206 into HFD-fed mice (n = 6). The control mice received MC-TTR-miR-206-MM (n = 6). (C) Impaired weight gain of HFD-fed mice after MC-TTR-miR-206 administration. C57Bl/6 mice at 8 weeks of age were kept on HFD for 8 weeks. At 16 weeks of age, two groups of mice received a dose of 1.5 μg/g MC-TTR-miR-206 (n = 10) or MC-TTR-miR-206-MM (n = 10, control) weekly for eight weeks. (D) Liver weight of dietary obese mice after MC-TTR-miR-206 or MC-TTR-miR-206-MM treatment (n = 10). (E) Fasting glucose levels in two groups of dietary obese mice. (F) Hepatic lipid content in dietary obese mice treated with MC-TTR-miR-206 or MC-TTR-miR-206-MM. (G) Oil-Red O and H&E staining of livers excised from two groups of mice. Data represent mean ± SEM. Mann–Whitney U test was used for statistical analysis. *p <0.05; **p <0.01; and ***p <0.001. LW, liver weight.
MiR-206 facilitates insulin signaling and inhibits Srebp1c-mediated lipogenesis in dietary obese mice
The strong inhibitory effects of miR-206 on hepatosteatosis and hyperglycemia led us to hypothesize that miR-206 was able to simultaneously facilitate insulin signaling and reduce lipogenesis by interacting with Ptpn1 in livers of dietary obese mice. To test this hypothesis, we analyzed two pathways of Ptpn1-PP2A-Sp1-Srebp1c and Ptpn1-Insr/Irs1 as described above. As expected, MC-TTR-miR-206 treatment had no effects on total Sp1 and PP2A but led to increased phosphorylated Sp1 and PP2A (Fig. 5A), which subsequently reduced mRNA levels of Srebp1c and the lipogenic genes including Scd1, Fasn and Gpat (Fig. 5B). In addition, delivery of miR-206 into livers of dietary obese mice also led to increased phosphorylated Insr and Irs1 (Fig. 5C), which subsequently inhibited expression of two genes encoding ratelimiting enzymes of gluconeogenesis G6pc and Pepck1 (Fig. 5D). All these findings indicate that miR-206 has the capacity to facilitate insulin signaling and inhibit lipogenesis through modulating pathways of Ptpn1-PP2A-Sp1-Srebp1c and Ptpn1-Insr/Irs1 in vivo.
Fig. 5. Liver-specific expression of miR-206 facilitates insulin signaling and impairs Srebp1c-mediated lipogenesis.
(A) Western blot analysis of phosphorylated Sp1, total Sp1, phosphorylated PP2A and total PP2A; and (B) mRNA levels of Srebp1c and the lipogenic genes including Scd1, Fasn, and Gpat in livers of dietary obese mice treated with MC-TTR-miR-206 or MC-TTR-miR-206-MM. (C) Protein levels of phosphorylated and total Insr and Irs1; and (D) mRNAs of the gluconeogenic genes including Pepck and G6pc in livers of two groups of mice. Data represent mean ± SEM. Student’s t test was used for statistical analysis. *p <0.05; **p <0.01.
It is known that impaired fatty acid oxidation contributes to the pathogenesis of hepatosteatosis [25]. To further exclude the role of fatty acid oxidation in reduced NAFLD after miR-206 treatment, we measured mRNA levels of genes encoding critical enzymes and regulators that control fatty acid oxidation in livers of mice treated with or without miR-206. qRT-PCR revealed that miR-206 overexpression had no significant effect on expression of these key genes involved in fatty acid oxidation including peroxisome proliferator-activated receptor alpha (Pparα), acyl-coenzyme A dehydrogenase 1 (Acad1), carnitine palmitoyltransferase 1A (Cpt1), acyl-CoA dehydrogenase, very long chain (Vlcad), acyl-coenzyme A oxidase 1 (Acox1), and pyruvate dehydrogenase kinase, isozyme 4 (Pdk4) (Supplementary Fig. 4) [26], indicating that impaired lipogenesis rather than fatty acid oxidation is the major contributor in miR-206-inhibited NAFLD. Together, these findings indicate that miR-206 is a critical regulator to facilitate insulin signaling and inhibit Srebp1c-mediated lipogenesis in vivo.
PTPN1 mediates the inhibitory effect of miR-206 on the pathogenesis of NAFLD and hyperglycemia
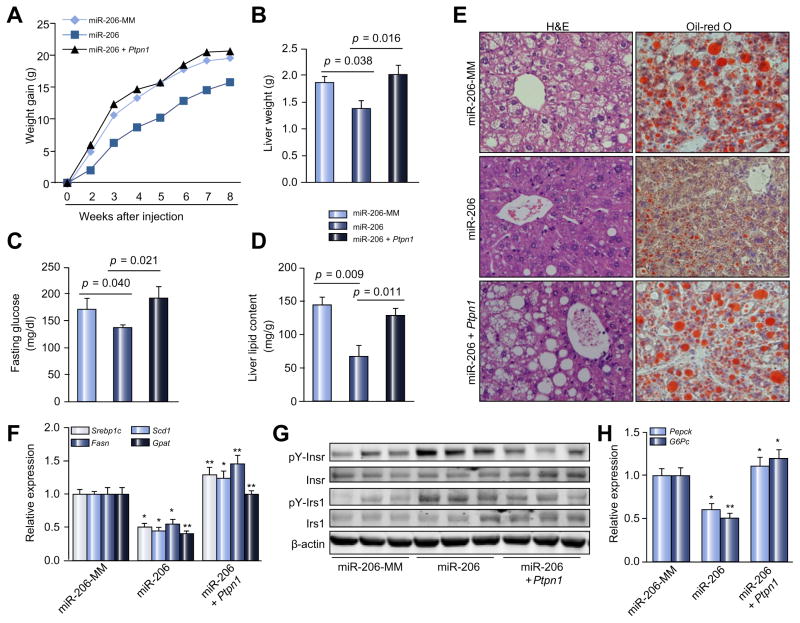
To investigate whether Ptpn1 mediates the inhibitory effects of miR-206 on both NAFLD and hyperglycemia, we re-introduced Ptpn1 into livers of dietary obese mice treated with MC-TTR-miR-206 (Supplementary Fig. 3B). Indeed, MC-TTR-miR-206 treatment led to reduced body and liver weight as well as decreased hepatic lipid content and blood glucose, and re-introduction of Ptpn1 offset the effects of miR-206 (Fig. 6A–E). This observation indicated that the interaction between miR-206 and Ptpn1 contributes to the pathogenesis of both hepatosteatosis and hyperglycemia. We then analyzed the components of the Ptpn1-PP2A-Sp1 and Ptpn1-Insr/Irs1 pathways in livers of three groups of mice treated with either MC-TTR-miR-206, a combination of MC-TTR-miR-206 and MC-TTR-Ptpn1, or MC-TTR-miR-206-MM (control). Livers of dietary obese mice treated with MC-TTR-miR-206 had significantly reduced mRNA levels of Srebp1c and the lipogenic genes, and the additional treatment of MC-TTR-Ptpn1 recovered expression of the lipogenic genes inhibited by miR-206 (Fig. 6F). MC-TTR-miR-206 treatment also facilitated phosphorylation of Insr and Irs1 and reduced mRNA levels of Pepck and G6pc controlling gluconeogenesis, while the re-introduction of Ptpn1 offset the effects of miR-206 (Fig. 6G and H). Together, miR-206 is a critical regulator that improves insulin sensitivity and reduces lipogenesis simultaneously by interacting with Pptn1, which subsequently alleviates highly-associated NAFLD and high blood glucose. Although the mechanism(s) on how miR-206 modulates NAFLD and hyperglycemia requires further investigation, our study reveals an important role of miR-206 in simultaneously facilitating insulin signaling via the PTPN1-INRS/IRS axis, inhibiting lipogenesis through the PTPN1-PP2A-Srebp1c axis (Fig. 7).
Fig. 6. Ptpn1 mediates the inhibitory effects of miR-206 on NAFLD and hyperglycemia.
(A) Body weight gain of HFD-treated mice received MC-TTR-miR-206 (n = 10), a combination of MC-TTR-miR-206 (n = 10) and MC-TTR-Ptpn1 or MC-TTR-miR-206-MM (n = 10, control). C57Bl/6 mice at 2-months of age were kept on HFD for 8 weeks. At 16 weeks of age, mice received a dose of 1.5 μg/g MC-TTR-miR-206, a combination of MC-TTR-miR-206 and MC-TTR-Ptpn1 or MC-TTR-miR-206-MM (control) weekly for eight weeks via tail vein injection. (B) Liver weight of dietary obese mice after treatment of MC-TTR-miR-206, a combination of MC-TTR-Ptpn1 or MC-TTR-miR-206. (C) Blood glucose levels in three groups of dietary obese mice. (D) Hepatic lipid content in three groups of dietary obese mice. (E) Oil-Red O and H&E staining of livers excised from three groups of mice. (F) mRNA levels of Srebp1c and lipogenic genes including Scd1, Fasn, and Gpat in livers of three groups of mice. (G) Protein levels of phosphorylated and total Insr and Irs1. (H) mRNAs of the gluconeogenic genes including Pepck. Mann-Whitney U test was used for statistical analysis. *p <0.05; **p <0.01.
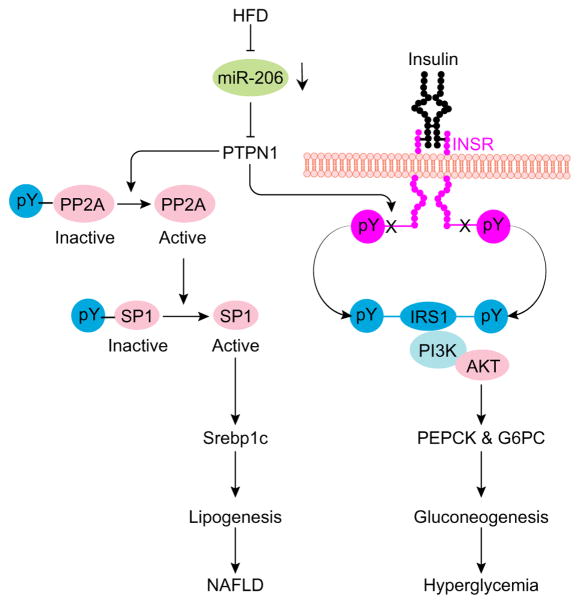
Fig. 7. A Proposed mechanism by which miR-206 impairs lipogenesis and facilitates insulin signaling.
HFD treatment impairs miR-206 biogenesis. Ptpn1 encodes a non-receptor phospho-tyrosine protein phosphatase (Ptp1b), which can dephosphorylate Insr, Irs and PP2A (phosphatase 2A). By targeting Ptpn1, miR-206 prevents dephosphorylation of Insr and Irs1, which subsequently improves insulin sensitivity and prevents gluconeogenesis. PP2A is able to activate Srebp1c transcription by dephosphorylating Sp1, a transcription factor of Srebp1c. Thus, miR-206 is able to inhibit lipogenesis via the Ptpn1-PP2A-Sp1-Srebp1c pathway. Thus, miR-206 can simultaneously improve insulin sensitivity through Ptpn1-Insr/Irs1 and inhibit lipogenesis via Ptpn1-Pp2a-Sp1-Srebp1c axis.
Discussion
It is widely considered that insulin resistance is central to the pathogenesis of T2D and NAFLD [3]. However, the phenomenon of selective hepatic insulin resistance, in which hepatic glucose metabolism becomes unresponsive to insulin but hepatic lipogenesis continues unabated, is a long-standing paradox. Since hepatic insulin resistance is considered to promote lipogenesis, the standard of care approach begs the question of whether treating T2D patients with insulin might in turn exacerbate NAFLD. MiR-206 represents the first reported miRNA that has strong therapeutic effects on both NAFLD and hyperglycemia. Notably, we also identified two unique pathways by which miR-206 simultaneously facilitates insulin signaling and prevents Srebp1c-mediated lipogenesis. These novel findings not only address the mechanisms of selective hepatic insulin resistance in the pathogenesis of NAFLD and hyperglycemia but also potentially provide a novel therapeutic agent for two distinct but co-morbid disorders.
Although miR-206 is widely considered a muscle-specific miRNA [27], it is also expressed in livers [28]. Importantly, miRNA profiling revealed that miR-206 was one of the most downregulated miRNAs in fatty livers of mice with ablated SHP (small heterodimer partner) [28]. SHP is a critical gate-keeper of hepatosteatosis and its knockout in livers promotes the pathogenesis of hepatosteatosis [29,30]. These observations indicated that miR-206 might play an important role in regulating lipid and glucose homeostasis. A drawback, however, is that the SHP knockout mouse model does not address the complexity of NAFLD and hyperglycemia initiation and progression and their interactions with the microenvironment. Therefore, in this study, we investigated the role of miR-206 in regulating NAFLD and hyperglycemia in a dietary obese mouse model.
With the development of HITS-CLIP technique [31,32], many targets of miR-206 have been identified [33–35]. MiRNAs can simultaneously target many genes, indicating the importance to elucidate which target mediates the physiological function(s) of a specific miRNA. In this study, we used TP to prevent the interaction of miR-206 with its targets [22], and validated Ptpn1 as a functional target of miR-206 in modulating insulin signaling and lipogenesis. Recently, an in vitro study identified LXRα as another target of miR-206 [36]. LXRα is a transcription activator of ATP-binding cassette transporter (ABCA1), ATP-binding cassette sub-family G member 1 (ABCG1), apolipoprotein E (ApoE), and SREPB1C – important regulators of cholesterol and triglyceride homeostasis [37]. As expected, miR-206 treatment reduced expression of Lxrα, Abca1, ApoE, Srebp1c and 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr) in livers of dietary obese mice (Supplementary Fig. 5A). Based on the role of LXRα in promoting lipogenesis and promoting reverse cholesterol transfer [38], delivery of miR-206 into livers should increase blood cholesterol levels by inhibiting Lxrα. In fact, our results showed that miR-206 significantly reduced levels of both hepatic and blood cholesterol in mice (data not shown). It is known that cholesterol transfer is mediated by macrophages; and both cholesterol and triglyceride synthesis occurs primarily in hepatocytes. In addition, miR-206 was able to induce expression of Lxrα, Abca1, and ApoE in macrophage cells (Supplementary Fig. 5B). These observations suggested that miR-206 inhibits synthesis of triglycerides and cholesterol in hepatocytes but promotes cholesterol transfer in macrophages. These findings led us to believe that the interaction between miR-206 and Lxrα might also contribute to reduced hepatosteatosis and hyperglycemia, which needs further investigation.
In this study, we used mini-circle vectors to generate a miR-206 in vivo expression system; and a TTR promoter was used to reduce off-target effects. A single injection of MC-TTR-miR-206 maintained a >2-fold expression of miR-206 in livers for two weeks compared to the control mice (Supplementary Fig. 6). Notably, miR-206 treatment had negligible effects on aspartate aminotransferase (AST) and dramatically reduced alanine aminotransferase (ALT) levels (Supplementary Fig. 7), suggesting low hepatic toxicity of miR-206. Although safety and off-target concerns have been taken into consideration in our study, further studies are required to increase delivery efficiency of miR-206 and further evaluate the toxicity and off-target effects of miR-206 before bringing these findings to the bedside.
In summary, our study reveals a novel and important mechanism by which miR-206 simultaneously facilitates insulin signaling via the PTPN1-INRS/IRS axis and inhibits lipogenesis via the PTPN1-PP2A-SP1-Srebp1c axis. Our findings also provide the strong evidence that miR-206 has therapeutic potential for both NAFLD and hyperglycemia. To provide a comprehensive and accurate explanation on the miR-206′s role in NAFLD and hyperglycemia, we next will use modified HITS-CLIP and biotin-labelled miR-206 mimics to identify the targetome of miR-206. The comprehensive study of miR-206 targets will not only provide an insight into the mechanisms underlying insulin resistance, NAFLD and hyperglycemia, but also obtain more evidence to bring miR-206 to the bedside.
Supplementary Material
Lay summary.
The epidemic of obesity is causing a sharp rise in the incidence of insulin resistance and its major complications, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). However, there are no effective treatments because the mechanisms underlying both disorders are not well described.We identified microRNA-206 as a novel and effective inhibitor for both glucose and lipid production in liver and potentially provide a unique therapeutic drug for both hepatosteatosis and hyperglycemia
Acknowledgments
Financial support
This work was in part supported by the National Institutes of Health (R01 DK102601, G.S. and R01 CA136606, XC), Research Scholar Grant (ISG-16-210-01-RMC) from the American Cancer Society, Gilead Sciences Liver Research Program (G.S), and the Minnesota Obesity Center (G.S).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2016.12.016.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
Acquisition of data: Wu H, Zhang T, Pan F, Li Z, Chen X and Steer C.J: Analysis and interpretation of data. Song G: Obtaining funding, study supervision, study concept and design and initial drafting of the manuscript.
References
- 1.Nath D, Heemels M-T, Anson L. Obesity and diabetes. Nature. 2006;444:839–888. [Google Scholar]
- 2.Postic C. Pathogenesis of fatty liver disease. Endocr Abstracts. 2012;29:S55. [Google Scholar]
- 3.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, Song B-L. SREBP: a novel therapeutic target. Acta Biochim Biophys Sin. 2013;45:2–10. doi: 10.1093/abbs/gms112. [DOI] [PubMed] [Google Scholar]
- 5.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12:83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Ng R, Wu H, Xiao H, Chen X, Willenbring H, Steer CJ, et al. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology. 2014;60:554–564. doi: 10.1002/hep.27153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferland-McCollough D, Ozanne SE, Siddle K, Willis AE, Bushell M. The involvement of microRNAs in type 2 diabetes. Biochem Soc Trans. 2010;38:1565–1570. doi: 10.1042/BST0381565. [DOI] [PubMed] [Google Scholar]
- 11.Vickers KC, Shoucri BM, Levin MG, Wu H, Pearson DS, Osei-Hwedieh D, et al. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2013;57:533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, Li B, et al. Optimized Adeno-Associated Virus (AAV)–protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum Gene Ther. 2010;21:271–283. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui W, Chen SL, Hu K-Q. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010;2:95–104. [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Y, Oumi S, Nagasawa T, Nishizawa N. Oxidative stress induces phosphoenolpyruvate carboxykinase expression in H4IIE cells. Biosci Biotechnol Biochem. 2006;70:2191–2198. doi: 10.1271/bbb.60135. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lechon MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor J-E. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Friedman R, Farh K, Burge C, Bartel D. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krek A, Grün D, Poy M, Wolf R, Rosenberg L, Epstein E, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 18.Yang J-H, Li J-H, Shao P, Zhou H, Chen Y-Q, Qu L-H. StarBase: a database for exploring microRNA–mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39:D202–D209. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu S, Ugi S, Maegawa H, Egawa K, Nishio Y, Yoshizaki T, et al. Protein-tyrosine phosphatase 1B as new activator for hepatic lipogenesis via sterol regulatory element-binding protein-1 gene expression. J Biol Chem. 2003;278:43095–43101. doi: 10.1074/jbc.M306880200. [DOI] [PubMed] [Google Scholar]
- 20.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong E-G, Cho Y-R, et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Rodríguez Á, Gutierrez JAM, Sanz-González S, Ros M, Burks DJ, Valverde ÁM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes. 2010;59:588–599. doi: 10.2337/db09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staton AA, Giraldez AJ. Use of target protector morpholinos to analyze the physiological roles of specific miRNA-mRNA pairs in vivo. Nat Protoc. 2011;6:2035–2049. doi: 10.1038/nprot.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayrhofer P, Schleef M, Jechlinger W. Gene therapy of cancer. Springer; 2009. Use of minicircle plasmids for gene therapy; pp. 87–104. [DOI] [PubMed] [Google Scholar]
- 24.Tao J, Ji J, Li X, Ding N, Wu H, Liu Y, et al. Distinct anti-oncogenic effect of various microRNAs in different mouse models of liver cancer. Oncotarget. 2015;6:6977–6988. doi: 10.18632/oncotarget.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, et al. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26:628–638. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song G, Wang L. Nuclear receptor SHP activates miR-206 expression via a cascade dual inhibitory mechanism. PLoS One. 2009;4:e6880. doi: 10.1371/journal.pone.0006880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–157. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 30.Tseng H-T, Park YJ, Lee YK, Moore DD. The orphan nuclear receptor small heterodimer partner is required for thiazolidinedione effects in leptin-deficient mice. J Biomed Sci. 2015;22:1. doi: 10.1186/s12929-015-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore MJ, Zhang C, Gantman EC, Mele A, Darnell JC, Darnell RB. Erratum: Mapping Argonaute and conventional RNA-binding protein interactions with RNA at single-nucleotide resolution using HITS-CLIP and CIMS analysis. Nat Protoc. 2016;11:616. doi: 10.1038/nprot0316-616c. [DOI] [PubMed] [Google Scholar]
- 32.Dutka T, Sarshad AA, Hafner M. Field guidelines for genetic experimental designs in high-throughput sequencing. Springer; 2016. PAR-CLIP: a genomic technique to dissect RNA-protein interactions; pp. 261–289. [Google Scholar]
- 33.Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. MiR-206 expression is down-regulated in estrogen receptor α–positive human breast cancer. Cancer Res. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- 34.Yan D, Dong XDE, Chen X, Wang L, Lu C, Wang J, et al. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem. 2009;284:29596–29604. doi: 10.1074/jbc.M109.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Liu M, Wang C, Lin C, Sun Y, Jin D. Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression. Anticancer Res. 2011;31:3859–3863. [PubMed] [Google Scholar]
- 36.Zhong D, Huang G, Zhang Y, Zeng Y, Xu Z, Zhao Y, et al. MicroRNA-1 and microRNA-206 suppress LXRalpha-induced lipogenesis in hepatocytes. Cell Signal. 2013;25:1429–1437. doi: 10.1016/j.cellsig.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.