Abstract
Objectives: The aim of this study is to identify predictors of pill placebo response and to characterize the temporal course of pill placebo response in anxious youth.
Methods: Data from placebo-treated patients (N = 76) in the Child/Adolescent Anxiety Multimodal Study (CAMS), a multisite, randomized controlled trial that examined the efficacy of cognitive-behavioral therapy, sertraline, their combination, and placebo for the treatment of separation, generalized, and social anxiety disorders, were evaluated. Multiple linear regression models identified features associated with placebo response and models were confirmed with leave-one-out cross-validation. The likelihood of improvement in patients receiving pill placebo—over time—relative to improvement associated with active treatment was determined using probabilistic Bayesian analyses.
Results: Based on a categorical definition of response (Clinical Global Impressions-Improvement Scale score ≤2), nonresponders (n = 48), and pill placebo responders (n = 18) did not differ in age (p = 0.217), sex (p = 0.980), race (p = 0.743), or primary diagnosis (all ps > 0.659). In terms of change in anxiety symptoms, separation anxiety disorder and treatment expectation were associated with the degree of pill placebo response. Greater probability of placebo-related anxiety symptom improvement was observed early in the course of treatment (baseline to week 4, p < 0.0001). No significant change in the probability of placebo-related improvement was observed after week 4 (weeks 4–8, p = 0.07; weeks 8–12, p = 0.85), whereas the probability of improvement, in general, significantly increased week over week with active treatment.
Conclusions: Pill placebo-related improvement occurs early in the course of treatment and both clinical factors and expectation predict this improvement. Additionally, probabilistic approaches may refine our understanding and prediction of pill placebo response.
Keywords: : antidepressant, anxiety, clinical trial, Bayesian, response
Introduction
The randomized controlled trial represents the gold standard for establishing the efficacy of treatments across a spectrum of illnesses/disorders, including affective and anxiety disorders in youth. However, investigators and clinicians routinely encounter certain individuals who respond to placebo treatments (Lasagna et al. 1954) and some contend that placebo represents an active intervention in and of itself (Kaptchuk and Miller, 2015). More recently, predicting and understanding the determinants of pill placebo response have emerged as important aspects of both clinical research and clinical practice (Newcorn et al. 2009; Cohen et al. 2010; Iovieno and Papakostas 2012; Nakonezny et al. 2015). Ultimately, a better understanding of placebo response, and the ability to predict its magnitude, may increase the likelihood of detecting treatment effects in randomized controlled trials, particularly those involving children and adolescents as they may inform study design and the conduct of clinical trials.
The response to pill placebo (henceforth referred to as PBO) (i.e., the degree of symptomatic improvement in patients receiving PBO relative to those treated with the active medication) appears to be increasing over time for methodological reasons and, in some pediatric antidepressant trials, PBO response rates have reached 40%–50% (Bridge et al. 2009; Emslie et al. 2014). PBO response rates in pediatric patients with anxiety disorders vary significantly across studies (Beidel et al. 2007; da Costa et al. 2013), which may be a function of the disorder under study (e.g., anxiety disorder vs. depressive disorder) or other key clinical and demographic features of the study sample (Cohen et al. 2010; Dobson and Strawn 2016). PBO response rates have been shown to be higher in non-Federally funded studies and in studies conducted outside of the United States (Dobson and Strawn 2016). However, few studies have evaluated PBO response specifically in pediatric patients (Bridge et al. 2009; Cohen et al. 2010; Dobson and Strawn 2016) and most of these evaluations have focused on trial-level factors (e.g., number of study sites, total number of subjects, randomization pattern, funding source, and trial duration).
The two meta-analyses that evaluated PBO response (Cohen et al. 2010; Dobson and Strawn 2016) in pediatric patients with anxiety disorders suggest several important determinants. In the first, which included youth with depressive disorders, anxiety disorders, and obsessive compulsive disorder (OCD) from 23 studies, it showed that children diagnosed with an anxiety disorders had a higher PBO response rate than those diagnosed with OCD and a lower PBO response rate than those diagnosed with major depressive disorder (MDD) (Cohen et al. 2010). Factors found to predict PBO response included ethnicity (with Caucasian participants being lower), gender (with males being lower), and the presence of a study washout period (with longer washout periods associated with a lower PBO response). The second meta-analysis evaluating PBO response rates included 14 trials of 9 medications in pediatric patients with non-OCD anxiety disorders (N = 2,230 patients) (Dobson and Strawn 2016). In this sample, higher PBO response rates were associated with a greater number of study sites and fewer patients per site. Lower PBO response rates were associated with federally funded studies, studies conducted in the United States, and increased the likelihood of demonstrating efficacy.
To date, no studies have evaluated patient-level factors that might be associated with pill PBO response in pediatric anxiety disorders. With this in mind, we examined patient-level PBO response data from pediatric patients who were randomized to PBO in the Child/Adolescent Anxiety Multimodal Study (CAMS), a multisite, randomized controlled trial that examined the efficacy of cognitive-behavioral therapy (CBT), antidepressant (SRT; sertraline), their combination, and pill PBO for the treatment of separation, generalized, and social anxiety disorders (Walkup et al. 2008). We hypothesized that based on studies of anxious adults (Rutherford et al. 2015) and results from a recent meta-analysis (Dobson and Strawn, 2016), lower symptom severity, younger age, and high expectations for treatment outcome would be associated with an increased pill placebo response.
Methods
Subjects
The CAMS methods have been extensively described in prior publications (Compton et al. 2010) as have baseline characteristics of the patients (Kendall et al. 2010) and acute (Walkup et al. 2008) and long-term outcomes (Piacentini et al. 2014). In short, patients aged 7–17 years of age (mean age: 10.7 years) who met DSM-IV criteria for ≥1 pediatric anxiety triad disorders (GAD, SAD, or social anxiety disorder/social phobia) were randomized (2:2:2:1) to the following: CBT (n = 139), SRT (n = 133), SRT plus CBT (n = 140), or pill placebo (PBO; n = 76). The study protocol was approved and monitored by institutional review boards at each site. Parents/guardians and patients provided informed consent and assent, respectively, before any study-related procedures.
Assessment of anxiety symptoms and response
The primary response was measured by using both a categorical measure and a dimensional measure. As described in the initial efficacy study (Walkup et al. 2008), categorical response was defined by a score of 1 (very much improved) or 2 (much improved) on the Clinical Global Impressions-Improvement Scale (Guy 1976), which ranges from 1 to 7 (lower scores reflect greater improvement compared with baseline), whereas the Pediatric Anxiety Rating Scale score (PARS) was used as the primary dimensional outcome measure for anxiety symptom severity. This instrument includes a 50-item symptom checklist as well as a second section consisting of specific severity/impairment items that are rated on a 6-point Likert scale (RUPP 2002) and is administered by an independent rater. For patients who dropped out before week 12 (endpoint), a last observation carried forward (LOCF) approach was utilized for missing PARS scores.
Self-report assessments of anxiety
The State-Trait Anxiety Inventory comprises 40 items, which assesses both state anxiety (transient, present moment feelings) and trait anxiety (persistent, ongoing feelings) (Spielberger 1983), which was administered to the parent as a proxy for parental anxiety. (Birmaher et al. 1997, 1999) Anxious self-talk was assessed with the Negative Affectivity Self-Statement Questionnaire (NASSQ) (Ronan et al. 1994), which consisted of a 70-item self-report that characterizes the frequency of negative effect-related thoughts using a 1–5 Likert scale and has been well described in anxious youth (Chansky and Kendall 1997). The SCARED consists of 41 items, which are divided among five factors and reflect DSM-IV anxiety disorders, as well as significant school avoidance, and has been validated in youth aged 8–18 years (Birmaher et al. 1997, 1999). The Multidimensional Anxiety Scale for Children (MASC), a 50-item self-report, assesses anxiety and related symptoms and has been validated in youth aged 8–19 years. Both the SCARED and the MASC were administered to the child and parent and capture self-reported and parent-reported measures of the child's anxiety symptoms.
Treatment expectation was determined by eliciting the child patient and parents' agreement with a series of three statements: (1) “I expect to get control over my anxiety through this treatment,” (2) “I expect to get better or become less anxious through this treatment,” and (3) “I expect my life to get better in some ways through this treatment.” Responses to each statement were measured on a 5-point Likert scale with the anchors being strongly disagree and strongly agree and a score of 3 being somewhat agree. The average of these three scores was taken to create a single variable: treatment expectation.
Statistical methods
Demographic and clinical variables (e.g., age, sex, race, socioeconomic status [SES], selected diagnoses) were compared for responders and nonresponders using χ2 tests, Fisher's Exact tests, and Student's t-tests, as appropriate. These variables were incorporated into a multiple regression model. This model was refined based on the estimated parameter p-values, evidence of omitted variable bias, the Bayesian information criterion, and Akaike's information criterion (Mills and Prasad 1992). As a confirmatory model selection approach, leave-one-out cross-validation was employed to evaluate the out-of-sample predictive performance of each possible set of explanatory variables (Picard and Cook 1984; Gelman et al. 2014; Cornwall and Mills 2017). The model predictions from each set of explanatory variables were compared using root mean squared prediction error (RMSPE) and the posterior density for RMSPE based on the predictive density of each model.
Finally, the temporal course of PBO response was evaluated using a probabilistic approach in which the posterior density of mean PARS score at each time point was determined, and the shift in this density over time was evaluated with regard to the posterior probability for each set of PARS scores across PBO-treated patients and those who received active treatments. To compare PARS scores at each time point (and with regard to comparison treatments), the evidence in favor of the null hypothesis of equality in means (H0) was evaluated and expressed using the difference in the means. The posterior density for the difference in the means was obtained by simulating a large random sample from each of the Student-t posteriors. The maximum posterior odds against the null hypothesis of equal means were then the ratio of the posterior density of difference in means at 0 and at the mode of the density. This ratio was computed from the simulated posterior density (Lancaster 2004; Greenberg 2008). The posterior odds for each regression coefficient were computed as the maximum odds against the null hypothesis of no effect; there is a one-to-one correspondence with the posterior odds ratios and the p-value in this case (Mills 2007). Statistical analyses were performed using R (version 3.1.2) and p-values <0.05 were considered statistically significant.
Results
Characteristics of PBO-treated patients and PBO responders
Seventy-six patients aged 11 ± 3 years (49% female) were randomized to PBO. The majority (n = 60, 78.9%) were white and the most frequent primary anxiety disorder was GAD (n = 42, 55.3%), while 40 (52.6%) had a primary diagnosis of social anxiety disorder, and 26 (34%) had a primary diagnosis of separation anxiety disorder. PBO-treated patients had a baseline PARS score of 19.4 ± 4.4 and, of these patients, 66 (87%) had postbaseline CGI-I scores and were thus included in the categorical analysis of response.
Associations between PBO response and demographic and clinical features
The multiple linear regression model revealed that change in PARS score over the course of 12 weeks of treatment was best predicted by a diagnosis of ADHD or separation anxiety disorder, parent and child expectations for treatment, and SES (p = 0.00016). Additionally, the model explained nearly 30% of the variance in PARS score (multiple R2 = 0.292). In this model, separation anxiety disorder was the strongest predictor of PBO response (p = 0.003). Additionally, in the multiple linear regression model, parental expectations for treatment success predicted PBO response (p = 0.043), while the patient's expectations trended toward an association with treatment response (p = 0.061). ADHD and higher SES did not reach statistical significance in terms of their prediction of PBO response (ps = 0.078 and 0.081, respectively).
Categorical PBO response
Based on the categorical definition of response (CGI-I score ≤2), 18 patients (27%) were classified as responders and 48 patients (72%) were classified as nonresponders. No statistically significant differences were observed between responders and nonresponders for age (p = 0.217), sex (p = 0.979), race (p = 0.743), or SES (p = 0.748) or in the number of individuals who continued in open-label treatment following the acute treatment phase (p = 0.673). As shown in Table 1, primary diagnosis, baseline symptom severity, or other clinical features did not differ between groups. Regarding cognitive factors, the amount of anxious self-talk (before treatment)—as reflected by NASSQ score—did not differ between PBO responders and nonresponders (p = 0.500). However, expectation of treatment efficacy was significantly higher in PBO responders compared with nonresponders (4.0 ± 0.7 vs. 3.3 ± 0.8, p < 0.001), while expectation of treatment efficacy as reported by the patients' parents was also higher in PBO responders compared with nonresponders (3.9 ± 0.7 vs. 3.6 ± 0.8), but was not statistically significant (p = 0.07).
Table 1.
Clinical and Demographic Characteristics of Patients Randomized to Placebo, Including Responders and Nonresponders, Based on a Clinical Global Impressions-Improvement Scale Score ≤2
| Total (N = 66) | Responders (n = 18) | Nonresponders (n = 48) | p value | |
|---|---|---|---|---|
| Female sex (%) | 31 (47.0) | 9 (50.0) | 22 (45.8) | 0.9799 |
| Age (year ± SD) | 11.14 ± 2.89 | 10.42 ± 2.98 | 11.41 ± 2.85 | 0.2168 |
| Age ≥13 years (%) | 30 (30.3) | 5 (27.8) | 15 (31.3) | 1.0000 |
| White (%) | 53 (80.3) | 15 (83.3) | 38 (79.2) | 0.7430 |
| SES score (±SD) | 48.1 ± 11.6 | 48.8 ± 11.0 | 47.8 (11.9) | 0.7484 |
| Any externalizing (%) | 14 (21.2) | 5 (27.8) | 9 (18.8) | 0.7827 |
| ADHD (%) | 6 (9.1) | 2 (11.1) | 4 (8.3) | 0.6608 |
| Pediatric triad diagnoses | ||||
| 1 (%) | 6 (9.1) | 1 (5.6) | 5 (10.4) | 0.4654 |
| 2 (%) | 31 (47.0) | 11 (61.1) | 20 (41.7) | 0.3038 |
| 3 (%) | 29 (44.0) | 6 (33.3) | 23 (48.0) | 0.6608 |
| Primary diagnosis | ||||
| GAD (%) | 32 (48.5) | 8 (44.4) | 24 (50.0) | 0.9000 |
| Social anxiety disorder (%) | 29 (44.0) | 7 (38.9) | 22 (45.8) | 0.8198 |
| SAD (%) | 24 (36.4) | 7 (38.9) | 17 (35.4) | 1.0000 |
| Time since earliest diagnosis (months) | 77.6 ± 37.5 | 85.7 ± 36.6 | 74.5 ± 37.8 | 0.2815 |
| Baseline severity | ||||
| PARS | 19.8 ± 4.3 | 19.4 ± 4.4 | 20.0 ± 4.3 | 0.6327 |
| PARS impairment (items 5–7) | 7.6 ± 3.8 | 7.4 ± 3.7 | 7.6 ± 3.8 | 0.8488 |
| SCARED-Child | 23.4 ± 15.7 | 23.4 ± 19.8 | 23.4 ± 14.0 | 0.9987 |
| SCARED-Parent | 31.1 ± 12.8 | 31.9 ± 12.8 | 30.8 ± 11.4 | 0.7622 |
| MASC-Child | 50.2 ± 13.3 | 48.8 ± 17.1 | 50.8 ± 11.8 | 0.5961 |
| MASC-Parent | 63.8 ± 15.9 | 62.2 ± 19.6 | 64.4 ± 14.4 | 0.6171 |
| NASSQ | 64.6 ± 24.5 | 71.9 ± 23.9 | 61.8 ± 21.5 | 0.1058 |
| STAI | 38.1 ± 10.0 | 37.1 ± 10.9 | 38.5 ± 9.7 | 0.6305 |
| Treatment expectancy | ||||
| Child | 3.6 ± 0.9 | 3.9 ± 0.8 | 3.5 ± 0.8 | 0.0859 |
| Parent | 3.7 ± 0.8 | 4.0 ± 0.9 | 3.6 ± 0.8 | 0.0934 |
| Continued in extension phase (%) | 58 (87.9) | 15 (83.3) | 43 (89.6) | 0.6731 |
MASC, Multidimensional Anxiety Scale for Children; NASSQ, Negative Affectivity Self-Statement Questionnaire; PARS, Pediatric Anxiety Rating Scale Score; SCARED, Screen for Child Anxiety-Related Emotional Disorders; STAI, State-Trait Anxiety Inventory.
Temporal course of PBO response for anxiety symptoms
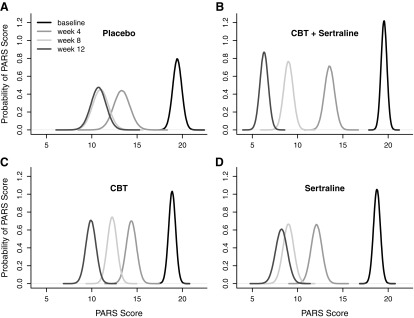
The probability of PBO-related improvement in anxiety symptoms on a week-by-week basis decreased over time and plateaued at week 4. In this regard, the probability of improvement associated with PBO from week 4 to week 8 only trended toward significance (p = 0.071) and there was no statistically significant change in anxiety symptoms in PBO-treated youth from week 8 to week 12 (p = 0.85) (Fig. 1A). By comparison, using the same Bayesian analysis, CBT ± SRT (Fig. 1B, C) were associated with a statistically significant increase in the probability of response across the course of the 12 weeks of treatment. Finally, sertraline monotherapy demonstrated a significant probability of response from baseline to week 4 (p < 0.0001) and from week 4 to week 8 (p < 0.0004), but the probability of additional improvement in anxiety symptoms became nonsignificant from week 8 to week 12 (p = 0.39) (Fig. 1D).
FIG. 1.
Distribution of response in children and adolescents with anxiety disorders. Posterior densities for the mean PARS score—at baseline, and week 4, week 8, and week 12—for placebo (A), sertraline (B), CBT (C), CBT + sertraline (D). CBT, cognitive-behavioral therapy; PARS, Pediatric Anxiety Rating Scale.
Discussion
To our knowledge, this study is the first to evaluate pill PBO response in pediatric patients with anxiety disorders and one of the first to examine symptomatic improvement, using a Bayesian approach, in a pediatric clinical trial. Consistent with our hypothesis, high expectations for treatment outcome were associated with an increased pill placebo response; however, our hypothesis that lower symptom severity and younger age would be associated with higher pill PBO response was not supported by these data. The findings of this study reveal that the patient's expectation of improvement, the presence of ADHD, and the presence of separation anxiety disorder increase the likelihood of a PBO-related decrease in anxiety symptoms, whereas more severe or impairing anxiety, gender, age, and duration of illness do not appear to be associated with pill PBO-related improvement in anxiety symptoms. Additionally, the present study reveals that the week-over-week probability of improvement in anxiety symptoms plateaus at week 4 for PBO-treated youth.
In the current analysis, separation anxiety disorder was associated with higher rates of pill PBO response with regard to anxiety symptoms. This finding is not surprising, in that the clinical trial represents a structured assessment of symptomatology and invariably results in patients and/or families (1) recognizing specific symptoms; (2) attending to fluctuation in these symptoms over time and in response to precipitating factors; and (3) receiving a modicum of psychoeducation related to the disorder under study through the assessment process. Additionally, there are often nonspecific aspects of the clinical trial visit that—regardless of efforts to minimize their impact—affect improvement trajectory and exist ubiquitously in psychiatry. These aspects or common factors have been extensively evaluated in psychotherapy literature and include the patient's experience of the therapeutic relationship, the collaborative development of treatment goals, and the patient–family–therapist alliance. Interestingly, in recent clinical trials of adults with major depressive disorder, these factors, as they pertain to the treating psychiatrist, accounted for nearly 10% of the variance in treatment response (triple the variance associated with the medication) (McKay et al. 2006). Furthermore, some of the pill PBO-related improvement in the youth with separation anxiety disorder may also relate to the common factors and to increased attention to symptoms and to the tracking of these symptoms. With regard to separation anxiety disorder, pill PBO response may result from changes in family-related factors such as accommodation (i.e., the degree to which a family system shifts family or parental behaviors to reassure the child or to assist a patient with the avoidance of symptom triggers) that are recognized as part of the assessment and symptom tracking processes. In this regard, family overaccommodation is high among youth with separation anxiety disorder (Lebowitz et al. 2013; Storch et al. 2015) compared with youth with other anxiety disorders and uniquely contributes to symptom severity and symptom burden (Settipani and Kendall 2015). Thus, a reduction in accommodation through parental recognition could contribute to pill PBO response in patients with separation anxiety disorder.
Our finding that the expectation of treatment response strongly predicts pill PBO response is important both with regard to (1) clinical trial design and interpretation and (2) clinical implications. The expectation of therapeutic benefit has been a focus of prior evaluations of PBO response and has been considered a key factor in PBO response (Benedetti et al. 2011; Rutherford et al. 2017). Such expectations have been associated with a number of cognitive factors, which may be germane to the anxious pediatric patient, including cognitive reassessment with positive expectation, which may result in adaptation of behavior or symptom expression (Benedetti et al. 2011). Additionally, the expectation of a positive effect of treatment in and of itself may decrease anxiety (Benedetti et al. 2011) and thus patients' expectations for treatment success may represent a cognitive trait, which may reflect the patient's greater ability to be impacted by the therapeutic structure of a clinical trial. As such, from a clinical standpoint, additional studies are needed to assess and understand the potential of augmenting treatment expectation as part of routine care for anxious youth. From a clinical standpoint, it is noteworthy that negative expectations, or threat bias, are often present in anxious youth (Roy et al. 2008; Britton et al. 2011) and may be associated with poorer response to anxiety-focused interventions. In this regard, the neural response to this bias, as assessed with functional MRI, was recently observed in a randomized clinical trial of youth with generalized, social, and/or separation anxiety disorders to predict positive response to both CBT and sertraline (Kujawa et al. 2016).
From a clinical trial standpoint, several double-blind PBO-controlled studies have attempted to decrease PBO response (Strawn et al. 2015, 2017). These attempts have included the requirement of high levels of anxiety (e.g., PARS score ≥15), the use of hierarchical disorder-related severity for the primary disorder, strict exclusion of comorbid disorders, and exclusion of patients with chronic disorders. These nonevidence-based approaches have been criticized, in that they limit the generalizability of findings and degrade statistical power through eo ipso reductions in sample size (Dobson and Strawn 2016). Additionally, the data presented herein regarding decreases in anxiety symptoms in pill PBO-treated patients do not support exclusion of patients in clinical trials based on disorder severity, primary disorder, age, or any demographic features. Furthermore, with regard to the design of clinical trials, given that the probability of pill PBO-related improvement occurs early (i.e., within the first 4 weeks) and then decreases, placebo washouts (i.e., PBO lead-ins) could be helpful in identifying those patients who demonstrate pill PBO-related improvement, particularly during the initial period of a study. However, while PBO lead-ins have been associated with a decreased PBO response in clinical trials of youth with MDD, it is noteworthy that in a recent meta-analysis of PBO response in adults with anxiety disorders, single-blind lead-in periods failed to account for significant variability in standardized mean change (Rutherford et al. 2015). Furthermore, in this meta-analysis of adults with anxiety disorders, no lead-in × treatment assignment interactions were detected (Rutherford et al. 2015), suggesting that the PBO lead-in may require double-blinding rather than single-blinding.
Regarding the time course of pill PBO response in terms of anxiety symptoms, the use of a Bayesian approach to modeling the probability of pill PBO-related improvement revealed that this phenomenon occurs early in the course of treatment and significantly decreased over time. The probability of improvement in PARS score associated with pill PBO appears to plateau and fails to significantly separate from week 4 to week 8 or week 8 to week 12, whereas the probability of improvement, in general, significantly increased week over week in active treatment arms. This finding is of interest, in that it parallels other findings in clinical trials of children and adolescents with major depressive disorder (March et al. 2009) and may parallel the child's perception of the strength of the therapeutic relationship (Kagan et al. 2016). In this regard, it remains possible that early in the process of double-blind PBO-controlled treatment, the effects of nonspecific psychosocial interventions (e.g., symptom recognition, awareness of interdependence of symptoms and fluctuation in symptoms, and psychoeducation) on anxiety symptoms emerge quickly, but are ephemeral relative to the effects of active psychotherapy or medication (or their combination). Moreover, the finding that pill PBO response emerges early and plateaus, whereas the probability of week-over-week improvement increases for all other treatments (with the exception of week 8 to week 12 probability of improvement for sertraline), has implications for the evaluation of novel or sequenced treatments in youth with anxiety disorders, particularly as there has been interest in decreasing (or in some cases eliminating) PBO exposure, and in some trials, particularly in conditions where PBO might increase mortality or [result in] irreversible morbidity (Durrleman et al. 2003). To address this, the possibility of putative placebo has been proposed given concerns that uncontrolled trials may be uninformative as they can neither demonstrate the effectiveness of a new agent nor provide a valid comparison with control therapy unless assay sensitivity can be assured, which often cannot be accomplished without inclusion of a concurrent placebo group (Durrleman et al. 2003). Bayesian approaches—similar to ours—have been successfully utilized to circumvent these concerns and such a characterization of the probability and course of PBO response in pediatric anxiety disorders as described herein could allow such a putative placebo group for future studies of psychopharmacologic treatments in youth with anxiety disorders.
The present evaluation does have several limitations. First, the number of patients is small (N = 76) relative to similar studies in adults with anxiety disorders; thus, there are implicit limitations with regard to the generalizability of these findings. Second, this analysis of pill PBO response in pediatric anxiety disorders is limited in its ability to resolve multicollinearity among variables, as has been the case with other studies of PBO response. As such, the influence of multiple variables that may be inter-related is difficult to control for in this small sample. However, our Bayesian approach minimizes the linear combination of type I and type II errors and our model of out-of-sample response, which has heretofore never been used in the analysis of pediatric clinical trial data, confirms the relative strength of this model of pill PBO response in pediatric anxiety disorders. Third, for primary analyses, we used a probabilistic approach rather than a more frequentist approach, as has been used in prior analyses of the data from studies (Walkup et al. 2008; Compton et al. 2014; Piacentini et al. 2014; Rynn et al. 2015). However, given that the former approach decreases the statistical burden of multiple assumptions that may be inherent in a frequentist approach and permits more robust evaluation of the model in question, we believe that this approach is best suited to the data at hand. Fourth, the instrument utilized to assess treatment expectations has not been systematically evaluated and thus its construct validity represents a potential limitation. Finally, characteristics other than those evaluated in the present study may influence response to pill PBO, including a number of psychological factors and alliance, as well as genetic factors, and biological features (e.g., sympathetic tone) may affect PBO response, but could not be evaluated in the present sample.
Conclusion
Taken together, the analyses presented herein raise the possibility that the expectation of improvement and the presence of separation anxiety disorder increase the likelihood of a PBO-related decrease in anxiety symptoms. By contrast, more severe or impairing anxiety, gender, age, and duration of illness do not appear to be associated with pill PBO-related improvement in anxiety symptoms. Finally, the week-over-week probability of improvement in anxiety symptoms plateaus at week 4 for PBO-treated youth, whereas the probability of improvement associated with active treatment, in general, increases week over week during the 12-week trial.
Clinical Significance
The present study has clear implications for the practicing child and adolescent psychiatrist who may implicitly wish to increase the PBO-related components of psychopharmacologic treatment. The relationship between patients' expectations of clinical improvement, in particular, has direct clinical implications that have recently been systematically evaluated (Rutherford et al. 2017) and discussed (Linden 2017) in adults with depressive disorders. Assessing patients' expectations of treatment response may prove helpful, in that psychoeducational or psychotherapeutic interventions that address negative expectations and threat bias, as well as those that increase a patient's positive expectation with regard to treatment, may bolster the placebo response that is part of medication response. Recently, some have suggested strategies to capture the placebo response with an active psychopharmacologic treatment and to maximize the effect of a moderator of placebo response—expectation (Linden 2017). Accordingly, clinicians might present treatments in such a way that enhances patient expectancy, which may involve educating patients about the effectiveness of the prescribed medication and utilizing a confident interpersonal style (Linden 2017). Moreover, given that multiple factors subtend expectation (e.g., optimism and tolerance of uncertainty), clinicians could actively address these factors. For example, clinicians may (1) help patients to recognize positive changes; (2) work to induce and bolster hope and optimism (Linden 2017); and (3) actively explore the patient's expectation of therapeutic outcome as well as expectations of the patient's family.
Acknowledgments
Dr. Strawn receives support from the National Institute of Mental Health (NIMH, K23MH106037). Mr. Dobson received support from the American Academy of Child and Adolescent Psychiatry Campaign for America's Kids and the Child and Adolescent Multimodal Study was supported by NIMH grants U01 MH064089 (JTW), U01 MH64092 (AMA), U01 MH64003 (BB, SNC), U01 MH63747 (PCK), U01 MH64107 (JM), and U01 MH64088 (JP).
Disclosures
Dr. Strawn has received research support from Edgemont, Eli Lilly, Shire, Forest Research Institute, Lundbeck, Neuronetics, and the National Institute of Mental Health (NIMH, K23MH106037). He receives royalties from Springer Publishing for two texts and has received material support from Assurex. Mr. Dobson has received support from the American Academy of Child & Adolescent Psychiatry (AACAP) (Campaign for America's Kids). Dr. Rynn has received support from NIH, NIMH, the National Institute of Child Health and Human Development (NICHD), Eli Lilly and Co., Pfizer, Merck, and Shire. She has served as a consultant to Shire and has received royalties from American Psychiatric Publishing and a writing fee from Oxford University Press. She has received honoraria from AACAP and the American Society of Clinical Psychopharmacology in addition to fees for academic lectures. Dr. Walkup has received grant support from the Hartwell Foundation and the Tourette Syndrome Association. He has served as a consultant to Shire. He has received free medication and placebo from Eli Lilly and Co., Pfizer, and Abbott for NIMH-funded studies. He has served on the advisory board and speakers' bureau of the Tourette Syndrome Association. He has received royalties from Guilford Press and Oxford University Press for books on Tourette syndrome. He has received an honorarium and travel support for an educational meeting from the Tourette Syndrome Association. He also has received travel support for an unpaid position on the Medical Advisory Board of the Tourette Syndrome Association. He is an unpaid member of the Scientific Advisory Board of the Trichotillomania Learning Center, the Scientific Council of the Anxiety and Depression Association of America, and a Scientific Advisor to the American Foundation of Suicide Prevention. Dr. Compton has received research support from NIMH, NARSAD, Shire Pharmaceuticals, and Mursion, Inc. He has received honoraria from the Nordic Long-Term Obsessive-Compulsive Disorder (OCD) Treatment Study Research Group and serves as an associated editor for Journal of Consulting and Clinical Psychology (until mid 2016), Journal of Child and Adolescent Psychopharmacology, and BMC Psychiatry. He has provided expert testimony at Duke Forensic Group. Dr. Sakolsky has received research support from NIMH and the National Alliance for Research on Schizophrenia and Depression (NARSAD). She has received honoraria from AACAP for teaching at the 2012 Annual Review Course. She has served as an editorial board member of Child and Adolescent Psychopharmacology News and is a specialty consultant for the Prescriber's Letter. Dr. Kendall has received grant support from NIMH and NICHD. He has received royalties from publication of anxiety treatment materials and books on child mental health from Guilford Press, Ericsson, Workbook Publishing, and Oxford University Press. He has received honoraria from lectures on the topic of anxiety in youth. Dr. McCracken has received research support from NIMH, NICHD, Maternal and Child Health Bureau, Seaside Therapeutics, and Roche. He has served as a consultant to BioMarin and Roche. He has received speaker honoraria from the Tourette Syndrome Association Speaker's Bureau, the Nevada Psychiatric Association, and AACAP. He has received study drug and placebo from Shire. Dr. Ginsburg has received grant support from NIMH and book royalties from Oxford University Press. Dr. Albano has received research grant support from NIMH, Duke University Research Institute, and private donors. She has received royalties from Oxford University Press, Lynn Sonberg Books, and Penguin/Avery Press. She has received honoraria from the American Psychological Association and consultant fees from Brackett Global. She is an unpaid member of the data safety monitoring board for IMPACT, a study through Cambridge University, United Kingdom. She is also an unpaid board of directors and scientific advisory board member of the Anxiety and Depression Association of America. Dr. Piacentini has received grant or research support from NIMH, Pfizer Pharmaceuticals through the Duke University Child and Adolescent Psychiatry Trials Network (CAPTN), the Tourette Syndrome Association, and private donors. He has served on the advisory boards (unpaid) and received speaking honoraria and travel reimbursement from the International Obsessive-Compulsive Disorder Foundation and Trichotillomania Learning Center. He has served on the advisory boards (unpaid) and received speaking honoraria from the Anxiety Depression Association of America. He has received speaking honoraria and travel reimbursement from the Tourette Syndrome Association. He has received book royalties from Guilford Press and Oxford University Press. He is a coauthor of several assessment tools, all of which are in the public domain, and therefore no royalties are received. Dr. Birmaher has received grant support from NIMH and book royalties from Random House, Inc., Lippincott Williams and Wilkins, and UpToDate. Finally, views expressed within this article represent those of the authors and are not intended to represent the position of NIMH, the National Institutes of Health (NIH), or the Department of Health and Human Services.
References
- Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S: SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry 46:1622–1632, 2007 [DOI] [PubMed] [Google Scholar]
- Benedetti F, Carlino E, Pollo A: How placebos change the patient's brain. Neuropsychopharmacology 36:339–354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M: Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry 38:1230–1236, 1999 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM: The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36:545–553, 1997 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS: Development of anxiety: The role of threat appraisal and fear learning. Depress Anxiety 28:5–17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansky TE, Kendall PC: Social expectancies and self-perceptions in anxiety-disordered children. J Anxiety Disord 11:347–363, 1997 [DOI] [PubMed] [Google Scholar]
- Cohen D, Consoli A, Bodeau N, Purper-Ouakil D, Deniau E, Guile J-M, Donnelly C: Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol 20:39–47, 2010 [DOI] [PubMed] [Google Scholar]
- Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, March JS, Gosch E a, Ginsburg GS, Rynn M a, Piacentini JC, McCracken JT, Keeton CP, Suveg CM, Aschenbrand SG, Sakolsky D, Iyengar S, Walkup JT, Albano AM: Predictors and moderators of treatment response in childhood anxiety disorders: Results from the CAMS trial. J Consult Clin Psychol 82:212–224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, Ginsburg GS, Rynn MA, McCracken JT, Waslick BD, Iyengar S, Kendall PC, March JS: Child/Adolescent Anxiety Multimodal Study (CAMS): Rationale, design, and methods. Child Adolesc Psychiatry Ment Health 4:1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall GC, Mills JA: Prediction based model selection criteria. University of Cincinnati, Department of Economics: University of Cincinnati Working Papers Series, 2017 [Google Scholar]
- da Costa CZ, de Morais RM, Zanetta DM, Turkiewicz G, Lotufo Neto F, Morikawa M, Rodrigues CL, Labbadia EM, Asbahr FR: Comparison among clomipramine, fluoxetine, and placebo for the treatment of anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol 23:687–692, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ET, Strawn JR: Placebo response in pediatric anxiety disorders: Implications for clinical trial design and interpretation. J Child Adolesc Psychopharmocol 26:686–693, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S, Chaikin P, Goodman SN: The use of putative placebo in active control trials: Two applications in a regulatory setting. Ann Intern Med 22:941–952, 2003 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Prakash A, Zhang Q, Pangallo Ba, Bangs ME, March JS: A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:170–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hwang J, Vehtari A: Understanding predictive information criteria for Bayesian models. Stat Comput 24:997–1016, 2014 [Google Scholar]
- Greenberg E: Introduction to Bayesian Econometrics. New York: NY, Cambridge University Press, 2008 [Google Scholar]
- Guy W: CGI Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976, pp 217–222 [Google Scholar]
- Iovieno N, Papakostas GI: Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: A meta-analysis. J ClinPsychiatry 73:1300–1306, 2012 [DOI] [PubMed] [Google Scholar]
- Kagan ER, Peterman JS, Carper MM, Kendall PC: Accomodation and treatment of anxious youth. Depress Anxiety 33:840–847, 2016 [DOI] [PubMed] [Google Scholar]
- Kendall PC, Compton SN, Walkup JT, Birmaher B, Albano AM, Sherrill J, Ginsburg G, Rynn M, McCracken J, Gosch E, Keeton C, Bergman L, Sakolsky D, Suveg C, Iyengar S, March J, Piacentini J: Clinical characteristics of anxiety disordered youth. J Anxiety Disord 24:360–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Fitzgerald KD, Monk CS, Phan KL: Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology 41:1983–1990, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster T: Introduction to Modern Bayesian Econometrics. Oxford, Blackwell, 2004 [Google Scholar]
- Lasagna L, Mosteller F, Von Felsinger JM, Beecher HK: A study of the placebo response. Am J Med 16:770–779, 1954 [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Woolston J, Bar-Haim Y, Calvocoressi L, Dauser C, Warnick E, Scahill L, Chakir AR, Shechner T, Hermes H, Vitulano LA., King RA., Leckman JF: Family accommodation in pediatric anxiety disorders. Depress Anxiety 30:47–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M. Placebo: unsolved problems for science, and simple conclusions for clinical practice. Am J Psychiatry 174:91–92, 2017 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Curry J, Wells K, Fairbank J, Burns B, Domino M, Vitiello B, Severe J, Riedal K, Goldman M, Feeny N, Findling R, Stull S, Baab S, Weller EB, Robbins M, Weller RA, Jessani N, Waslick B, Sweeney M, Dublin R, Walkup J, Ginsburg G, Kastelic E, Koo H, Kratochvil C, May D, LaGrone R, Vaughan B, Albano AM, Hirsch GS, Podniesinki E, Chu A, Reincecke M, Leventhal B, Rogers G, Jacobs R, Pathak S, Wells J, Lavanier SA, Danielyan A, Rohde P, Simons A, Grimm J, Frank S, Emslie G, Kennard B, Hughes C, Mayes TL, Rosenberg D, Benazon N, Butkus M, Bartoi M: The Treatment for Adolescents With Depression Study (TADS): Outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 166:1141–1149, 2009 [DOI] [PubMed] [Google Scholar]
- McKay KM, Imel ZE, Wampold BE: Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Disord 92:287–290, 2006 [DOI] [PubMed] [Google Scholar]
- Mills JA: Objective Bayes factors for precise hypothesis testing. National Science Foundation Seminar in Bayesian Interference in Economics and Statistics. St. Louis: Washington University, 2007 [Google Scholar]
- Mills JA, Prasad K: A comparison of model selection criteria. Econom Rev 11:201–233, 1992 [Google Scholar]
- Nakonezny PA, Mayes TL, Byerly MJ, Emslie GJ: Predicting placebo response in adolescents with major depressive disorder: The Adolescent Placebo Impact Composite Score (APICS). J Psychiatr Res 68:346–353, 2015 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Sutton VK, Zhang S, Wilens T, Kratochvil C, Emslie GJ, D'Souza DN, Schuh LM, Allen AJ: Characteristics of placebo responders in pediatric clinical trials of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 48:1165–1172, 2009 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bennett S, Compton SN, Kendall PC, Birmaher B, Albano AM, March J, Sherrill J, Sakolsky D, Ginsburg G, Rynn M, Bergman RL, Gosch E, Waslick B, Iyengar S, McCracken J, Walkup J: 24- and 36-week outcomes for the child/adolescent anxiety multimodal study (CAMS). J Am Acad Child Adolesc Psychiatry 53:297–310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard RR, Cook R: Cross-validation of regression models. J Am Stat Assoc 79:575–583, 1984 [Google Scholar]
- Ronan KR, Kendall PC, Rowe M: Negative affectivity in children: Development and validation of a self-statement questionnaire. Cognit Ther Res 18:509–528, 1994 [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, Bergman RL, McClure-Tone EB, Pine DS: Attention bias toward threat in pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry 47:1189–1196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Bailey VS, Schneier FR, Pott E, Brown PJ, Roose SP: Influence of study design on treatment response in anxiety disorder clinical trials. Depress Anxiety 32:944–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Wall MM, Brown PJ, Choo TH, Wagner TD, Peterson BS, Chung S, Kirsch I, Roose SP: Patient expectancy as a mediator of placebo effects in antidepressant clinical trials. Am J Psychiatry 174:135–142, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynn MA, Walkup JT, Compton SN, Sakolsky DJ, Sherrill JT, Shen S, Kendall PC, Mccracken J, Albano AM, Piacentini J, Riddle MA, Keeton C, Waslick B, Chrisman A, Iyengar S: Child/Adolescent Anxiety Multimodal Study: evaluating safety. J Am Acad Child Adolesc Psychiatry 54:180–190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settipani CA, Kendall PC: The effect of child distress on accommodation of anxiety: relations with maternal beliefs, empathy, and anxiety. J Clin Child Adolesc Psychol 16:1–14, 2015 [DOI] [PubMed] [Google Scholar]
- Spielberger CD: State-Trait Anxiety Inventory (STAI). Mind Gard 94061:261–3500, 1983 [Google Scholar]
- Storch EA, Salloum A, Johnco C, Dane BF, Crawford EA, King MA, McBride NM, Lewin AB: Phenomenology and clinical correlates of family accommodation in pediatric anxiety disorders. J Anxiety Disord 35:75–81, 2015 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Compton SN, Robertson B, McMorn A, Hamdani M, Rynn MA: A randomized, placebo-controlled study of extended-release guanfacine for the treatment of children and adolescents with generalized, separation or social anxiety disorder. J Child Adolesc Psychopharmacol 27:29–37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, Findling RL: A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 54:283–293, 2015 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPP: The pediatric anxiety rating scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069, 2002 [DOI] [PubMed] [Google Scholar]