Summary
Lysine acetylation is involved in various biological processes and is considered a key reversible post-translational modification in the regulation of gene expression, enzyme activity, and subcellular localization. This post-translational modification is therefore highly relevant in the context of circadian biology, but its characterization on the proteome-wide scale and its circadian clock dependence are still poorly described. Here, we provide a comprehensive and rhythmic acetylome map of the mouse liver. Rhythmic acetylated proteins showed subcellular localization-specific phases that correlated with the related metabolites in the regulated pathways. Mitochondrial proteins were over-represented among the rhythmically acetylated proteins and were highly correlated with SIRT3-dependent deacetylation. SIRT3 activity being nicotinamide adenine dinucleotide (NAD)+ level-dependent, we show that NAD+ is orchestrated by both feeding rhythms and the circadian clock through the NAD+ salvage pathway but also via the nicotinamide riboside pathway. Hence, the diurnal acetylome relies on a functional circadian clock and affects important diurnal metabolic pathways in the mouse liver.
Keywords: circadian clock, acetylation, liver metabolism, SIRT3, NAD+, SILAC proteomics
Graphical Abstract
Highlights
-
•
Phase of daily acetylated proteins is subcellular localization-dependent
-
•
Mitochondrial proteins are over-represented among the rhythmically acetylated proteins
-
•
Acetylated mitochondrial protein are enriched in SIRT3 targets
-
•
Circadian clock regulates the NAD+-dependent SIRT3 activity through the NR pathway
Mauvoisin et al. provide a rhythmic acetylome map of the mouse liver. Rhythmic acetylated proteins showed subcellular localization-specific phases with an over-representation of SIRT3 targets. Feeding rhythms and the circadian clock regulate NAD+ synthesis through the salvage and nicotinamide riboside pathways, affecting metabolite accumulation.
Introduction
Lysine acetylation on proteins has been shown to broadly regulate diverse sets of cellular functions (Choudhary et al., 2009). Understanding the dynamics of protein acetylation in regulating cellular pathways is therefore key to deciphering its biological functions (Smith and Workman, 2009). However, comprehensive analysis of lysine acetylation is technologically achievable only recently, using antibodies recognizing acetylated lysine residues (Kim et al., 2006). To date, only a few studies have described further acetylome maps in mammals. Early studies identified hundreds of acetylated peptides in the liver, with a strong enrichment in mitochondrial proteins (Kim et al., 2006, Schwer et al., 2009, Zhao et al., 2010). More recently, multi-organ studies identified thousands of acetylated peptides in rodents, opening new avenues regarding the regulation of acetylation and its effect on cellular functions, including metabolism (Lundby et al., 2012, Yang et al., 2011). Descriptions of mechanisms involved in regulating such acetylation, mainly through enzymatically controlled deacetylation, are rising and strongly linked to metabolism and physiological conditions (Menzies et al., 2016). However, the dynamics of protein acetylation are still poorly described, despite the currently known regulation by food-related metabolites and nutrient availability.
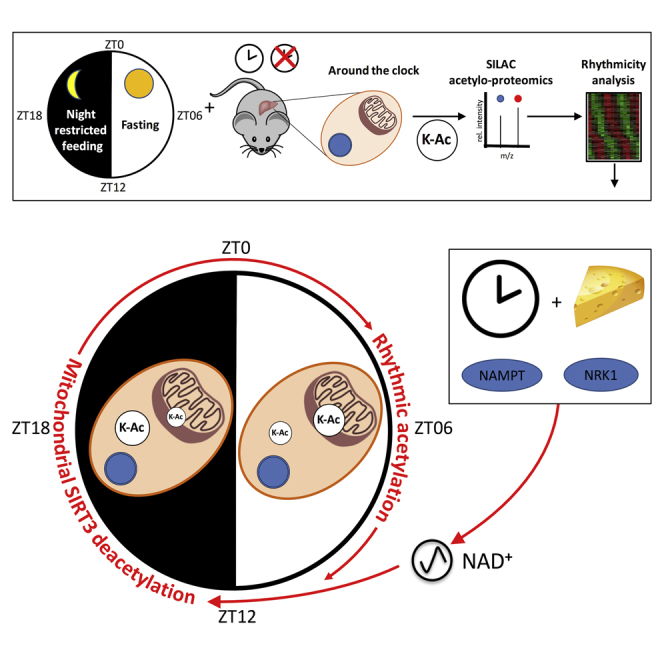
Through the regulation of diurnal feeding rhythms and nutrient metabolism, the circadian clock is likely an important modulator of protein acetylation. The circadian clock, an endogenous and autonomous oscillator with a nearly 24-hr period, coordinates most aspects of physiology and behavior in mammals (Gerhart-Hines and Lazar, 2015). This oscillatory clockwork is organized in a hierarchical manner, with a master clock in the suprachiasmatic nuclei of the hypothalamus that communicates timing cues to downstream oscillators in peripheral tissues. At the molecular level, rhythms in gene expression are generated by interconnected transcriptional and translational feedback loops in which multiple layers of control, including temporal, transcriptional, post-transcriptional, and post-translational regulation, play important roles (Crane and Young, 2014). Although efforts have been conducted in the last decade to comprehensively study transcriptional regulation orchestrated by the circadian clock, regulation at the proteome level is still largely unexplored territory, despite recent advances in the field (Mauvoisin et al., 2014, Neufeld-Cohen et al., 2016, Robles et al., 2014, Wang et al., 2017). Evidence also indicates that the circadian clock is a key regulator of deacetylase activity, affecting mitochondrial function (Peek et al., 2013). However, little is known about the global diurnal acetylome, despite the recent description of 19 diurnally acetylated sites in wild-type (WT) mouse liver (Masri et al., 2013). Using optimized sample preparation, including antibody-based enrichment protocols combined with sensitive nano-reverse-phase liquid chromatography (RP-LC) tandem MS (MS/MS) applied to total (TE) and nuclear (NE) mouse liver protein extracts harvested during two diurnal cycles, we were able to detect 1,831 and quantified 1,050 acetylated sites, of which around 13% presented a diurnal acetylation pattern. These acetylated proteins are characterized by a bimodal distribution of peak times. Proteins acetylated during the night are enriched in cytoplasmic and nuclear proteins, whereas proteins acetylated during the day are enriched in mitochondrial proteins. This rhythm in mitochondria is associated with the activity of the nicotinamide adenine dinucleotide (NAD)+-dependent SIRT3 deacetylase. Rhythmic acetylated proteins are involved in several key metabolic pathways, and metabolite levels within these pathways correlated with acetylation levels. Of interest is that the diurnal distribution of acetylation and metabolites is highly disturbed in clock-disrupted animals such as Bmal1 knockout (KO) and Cry1/2 double knockout (DKO) mice, mainly through the differential regulation of NAD+ synthesis, supporting a crucial role of the circadian and feeding rhythms in finely tuning the diurnal liver acetylome.
Results
The Acetylome of the Mouse Liver
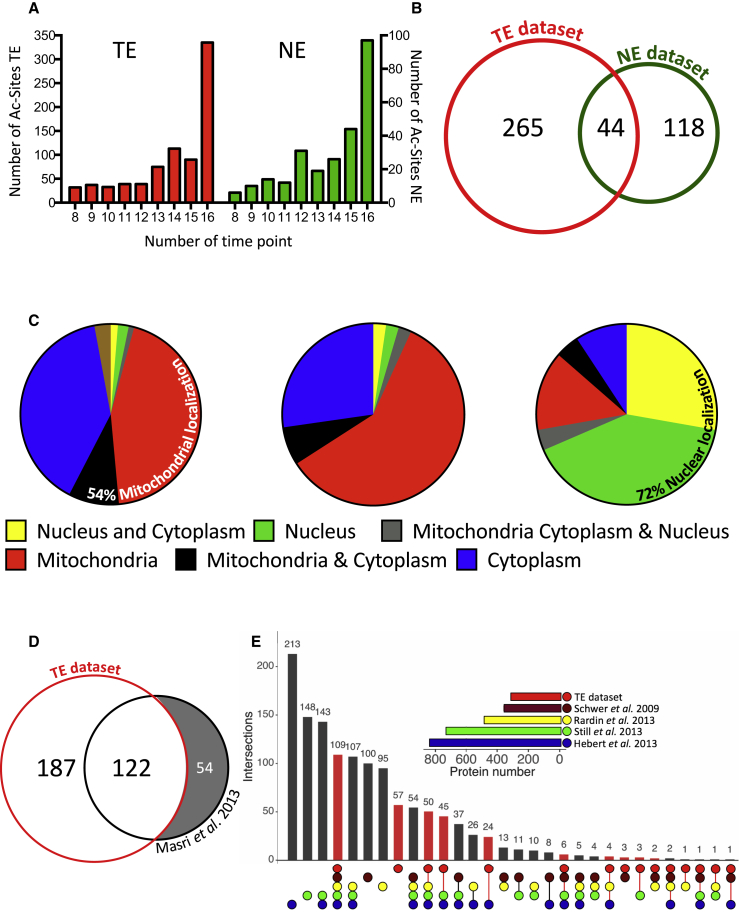
To obtain further insights into the diurnal acetylation in mouse liver, we performed an in vivo stable isotope labeling by amino acids (SILAC) MS-based proteomic experiment in which TE and NE protein extracts were collected every 3 hr during two consecutive days under light-dark conditions and night-restricted feeding. After trypsin digestion, these extracts were enriched for acetylated peptides (Experimental Procedures). Relative acetylated peptide abundance in the resulting 16 TE and 16 NE samples was quantified using a common SILAC total or nuclear reference extract, respectively (Figure S1A), whose labeling efficiency has already been reported (Mauvoisin et al., 2014). This experimental setup allowed the identification of 1,298 lysine acetylation sites in TE and 533 in NE, of which 236 unique sites were commonly detected in both datasets (Figure S1B; Table S1). The reproducibility of our experiment was high because the correlation between biological replicates was 76% on average (Figure S1C). Moreover, as a proxy of nuclear enrichment quality, 75% of the obtained raw MS signal in NE originated from acetylated peptides of nuclear proteins (Figure S1D). For further analysis, only acetylation sites yielding relative quantitative measurements in at least 8 of the 16 samples were considered. Additionally, only sites with a previously quantified accumulation of the corresponding protein in TE (Mauvoisin et al., 2014) and NE (Wang et al., 2017) were retained (Figure 1A; Table S2). Using such criteria, 797 acetylation sites within 309 proteins in TE and 253 acetylation sites within 162 proteins in NE were quantified (Figure 1B; Table S2). In TE, the majority of identified and quantified acetylation sites originated from cytoplasmic and mitochondrial proteins, whereas the NE dataset was enriched in acetylation sites from nuclear proteins and proteins shuttling between the cytoplasmic and nuclear compartments (Figure 1C; Figure S1D). In total, 44 proteins were found acetylated in both datasets, likely proteins shuttling between different organelles or dually targeted proteins that can be present in different compartments, as reported for mitochondrial/nuclear proteins (Figure 1C; Monaghan and Whitmarsh, 2015). Comparison of our datasets (Table S2) with the previously published diurnal acetylome performed on whole liver extracts under ad libitum feeding (Masri et al., 2013) showed that, besides our increased coverage, which is more than doubled, our quantified TE acetylome includes 70% of the aforementioned data (Figure 1D). Additional comparisons of this dataset with published mitochondrial liver acetylomes (Hebert et al., 2013, Rardin et al., 2013a, Schwer et al., 2009, Still et al., 2013) showed that 70% of our acetylated proteins were present in at least two of these datasets, corroborating the enrichments for mitochondrial proteins in our liver acetylome (Figure 1E). The remaining non-overlapping proteins are likely cytoplasmic or nuclear proteins that are not present in theses mitochondrial datasets.
Figure 1.
Characterization of the Liver Acetylome
(A) Distribution of sample number for the quantified acetylation sites in TE (red bars plotted on the left y axis) and NE (green bars plotted on the right y axis).
(B) Venn diagram showing the number of acetylated proteins quantified in TE (red circle, 309 acetylated proteins identified) and NE (green circle, 162 acetylated proteins identified).
(C) Distribution of cellular localization of proteins with quantified acetylation sites in TE (left), TE and NE (center), and NE (right). Data are expressed in percent.
(D) Venn diagram comparing the TE dataset of acetylated proteins with the previous circadian liver acetylome (Masri et al., 2013) (176 unique acetylated proteins identified).
(E) Matrix layout for all intersections (vertical bars), sorted by size, of our TE dataset, with the data from studies performed using liver mitochondrial protein extract: Schwer et al. (2009) (356 acetylated proteins), Rardin et al. (2013b) (483 acetylated proteins), Still et al. (2013) (729 acetylated proteins), and Hebert et al. (2013) (834 acetylated proteins). Color-coded circles in the matrix indicate sets that are part of the intersection and, the top horizontal bar graph displays the protein number for each study.
The Rhythmic Acetylome of the Mouse Liver
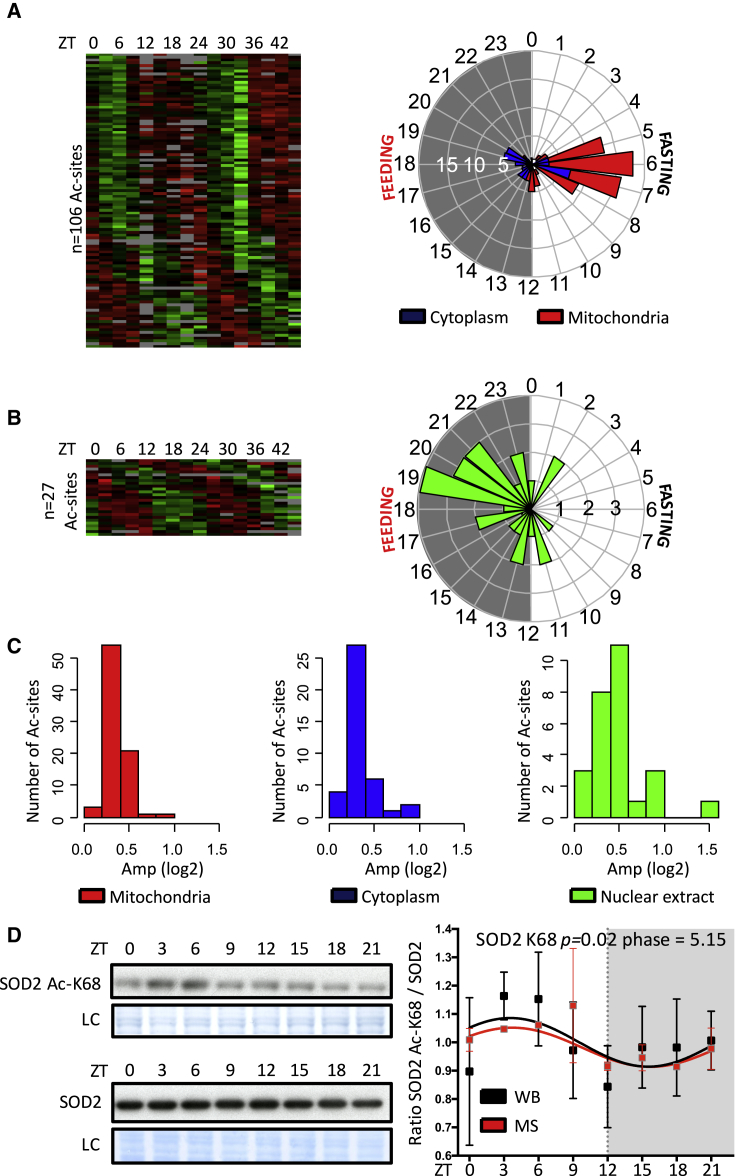
We next identified actively acetylated lysine by analyzing the rhythmicity of the acetylation signal normalized by its corresponding protein signal (Mauvoisin et al., 2015; Experimental Procedures; Table S2). Using this relative acetylation signal, we first observed that most of the rhythmic acetylated peptides are found on non-rhythmic proteins, which characterize an active acetylation process. Hence, we found 133 rhythmic acetylated lysines (p < 0.05), 106 in TE and 27 in NE and 3 of them found in both datasets (Figure S2A; Table S2). These rhythmic sites were found in 73 unique proteins in TE (including 8 rhythmic at the protein level; Mauvoisin et al., 2014), 23 in TE (including 13 rhythmic at the protein level in the nucleus; Wang et al., 2017), and 2 proteins common between TE and NE. The proportion of about 13% of rhythmic acetylated sites is higher compared with the previously published liver diurnal acetylome (19 rhythmic acetylation sites in the WT, about 6% of the dataset; Masri et al., 2013), probably because of the higher coverage and time resolution as well as the feeding schedule in the present study. Comparing the intersection between the two datasets revealed a minor overlap; only 5 acetylation sites were found in common in both studies (Figure S2A). We additionally identified acetylation sites with a 12-hr period: 34 sites within 30 proteins in TE and 17 sites within 14 proteins in NE (Figures S2B and S2C; Table S2).
Using protein localization databases, we annotated the cellular localization of all the rhythmic acetylated sites. Surprisingly, clear localization-specific phases were observed. Although mitochondrial proteins were acetylated during the day, when nutrient and energy are depleted, cytoplasmic and nuclear proteins were preferentially acetylated during the night, when nutrients are available at high levels (Figures 2A and 2B). Compared with other post-translational modifications, such as rhythmic phosphorylation (Robles et al., 2017, Wang et al., 2017), despite a few high amplitudes of acetylation sites observed in the nucleus and mitochondria, we found that the amplitudes of rhythmic acetylation were relatively low. This could reflect the fact that acetylation/deacetylation are low-stoichiometry reactions (Weinert et al., 2015) compared with phosphorylation (Wu et al., 2011). Moreover, only a small fraction of the pool of proteins is subjected to acetylation on lysine, leading to the observed global low change on acetylation levels (Figure 2C). Validation of rhythmic acetylation of mitochondrial superoxide dismutase 2 (SOD2) at lysine 68 (Chen et al., 2011) was performed by western blot and presented similar diurnal variation as found by MS (Figure 2D).
Figure 2.
Rhythmicity Analysis of the Liver Acetylome
(A–C) Heatmaps showing rhythmic acetylation sites normalized by their corresponding total protein amount in TE (A) and NE (B) under light/dark and night-restricted feeding conditions. Data were standardized by rows, and gray blocks indicate missing protein data. The polar plots on the right of each heatmap display peak phase distribution of the rhythmic acetylation (Ac) sites, and (C) shows amplitude. The colors of the polar plots (A and B) and histograms (C) indicate Ac sites that have a corresponding total protein with a defined mitochondrial (n = 80 Ac sites, red) or cytoplasmic (n = 40 Ac sites, blue) localization or Ac sites that are identified in NE (n = 27 Ac sites, green).
(D) Western blot (WB) analysis of total protein extracts using acetyl-K68 SOD2 (top blot) or total SOD2 (bottom blot). MS data in red, mean ± SEM, are from Table S2. WB data in black, mean ± SEM, are from three independent biological samples and represent the ratio of the acetyl-K68 SOD2 signal on the total SOD2 signal. Data (MS and WB) are normalized to the temporal mean. Naphtol blue black staining of the membranes was used as a loading control (LC) and served as a reference for normalization of the quantified values.
Acetylation is acknowledged in general as a passive process mainly regulated by the availability of acetyl-coenzyme A (CoA) and counteracted by the regulated orchestration of deacetylation (Choudhary et al., 2014). In mammalian cells, acetyl-CoA concentration is not homogeneous. Indeed, its synthesis and accumulation are cell compartment-specific (Siess et al., 1978) and regulated by feeding/fasting cycles (Shi and Tu, 2015). For instance, during the fasting period, a 10-fold higher concentration of acetyl-CoA was observed in the mitochondria, where it is synthetized from acetate by acetyl-CoA synthetase 2 (ACECS2, encoded mainly by the Acss3 gene in the mouse liver) (Verdin et al., 2010). In contrast, lower concentrations were found in the cytoplasm and the nuclear compartments, where acetyl-CoA is produced from mitochondrially derived citrate by ATP citrate lyase (ACLY) or from acetate by acetyl-CoA synthetase 1 (ACECS1, encoded by the Acss2 gene in the mouse) during feeding time (Wellen and Thompson, 2012). Remarkably, the observed compartment-specific rhythmic acetylation is well correlated with this food-driven acetyl-CoA synthesis in the respective compartment.
Although ACSS3 is not rhythmically expressed in TE or liver mitochondria (Neufeld-Cohen et al., 2016), ACSS2 and ACLY (Figures S2D and S2E), with the latter being highly expressed compared with other ACSS isoforms (Figures S2F and S2G), presented a synchronized diurnal accumulation in the mouse liver in both TE and NE. The maximum of expression of these acetyl-CoA-producing enzymes occurred during the light period in TE and at the night-day transition in NE, when cytoplasmic and nuclear acetylation levels were low (Figures 2A and 2B; Figures S2D and S2E). Proteomic data from circadian clock-disrupted mice in TE showed a minor disturbance of ACLY and ACSS2 expression (Figure S2H). Although ACSS3 expression was increased in Bmal1 KO mice, it was previously shown that acetyl-CoA concentration is not affected in this model (Peek et al., 2013).
Together, these data are in line with the fact that lysine acetylation is mainly driven by passive acetylation caused by food-driven fluctuation of acetyl-CoA availability in their respective cell compartments; i.e., day in mitochondria and night in the cytoplasmic and nuclear compartments (Shi and Tu, 2015). The expression levels of acetyl-CoA-producing enzymes as well as the circadian clock components appear to weakly affect this diurnal compartment-specific regulation of acetyl-CoA level in a normal feeding regimen, as already shown (Chavan et al., 2016, Peek et al., 2013). However, the mitochondrial acetylation peak phase is likely followed by sirtuin-dependent deacetylation events in the opposite phase. Sirtuin-dependent deacetylation has been shown to be clock-regulated (Peek et al., 2013) and could be involved in the observed rhythmic acetylation.
The Rhythmic Acetylome Is Modulated by Circadian Clock-Orchestrated Rhythmic Deacetylation
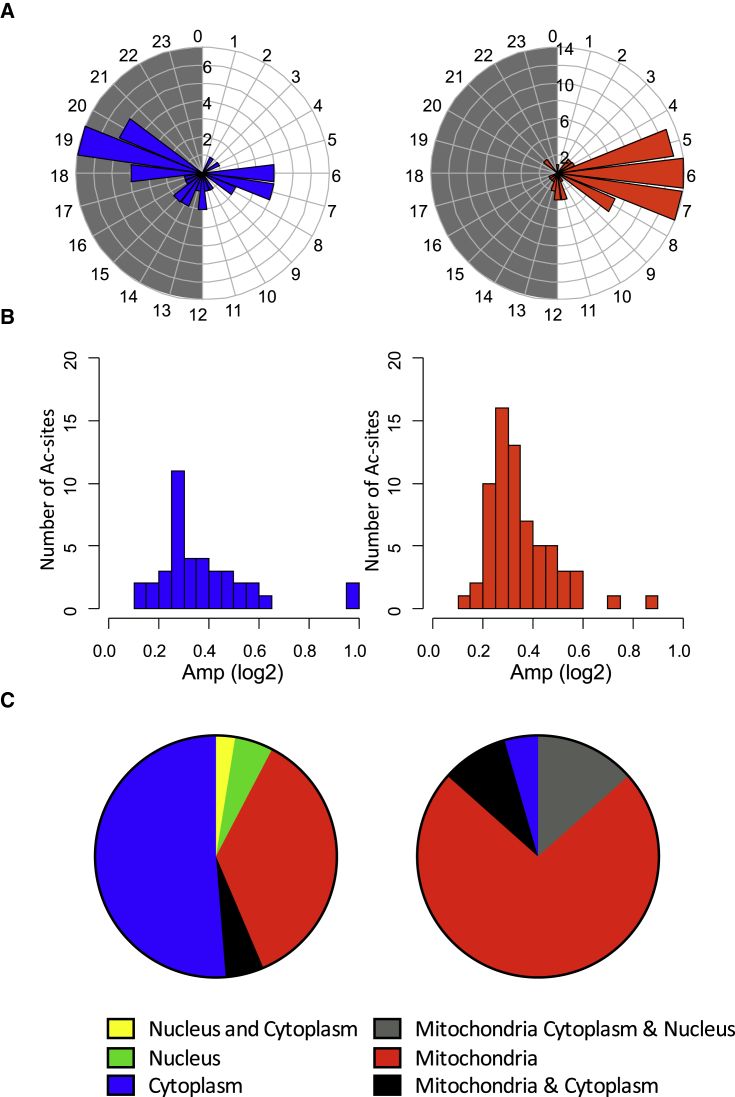
Previously published results suggest that rhythmic deacetylation by sirtuins is likely involved in establishment of the rhythmic acetylome (Peek et al., 2013). Among sirtuins, SIRT2 is present mainly in the cytosol, whereas SIRT6 and SIRT7 are located in the nucleus. SIRT1 shuttles between the cytosol and the nucleus and is therefore involved in deacetylation within these two organelles (Menzies et al., 2016). Although SIRT4 (Mathias et al., 2014) and SIRT5 (Du et al., 2011) have very little deacetylase activity, SIRT3 is likely the main mitochondrial deacetylase. Of interest is that SIRT3-deacetylated proteins have been extensively studied in different mouse tissues and, notably, in the liver (Dittenhafer-Reed et al., 2015, Hebert et al., 2013, Lombard et al., 2007, Rardin et al., 2013b, Weinert et al., 2015). Many characterized targets of SIRT3 presented a rhythmic acetylation in our dataset, with a clear phase preference during the day, synchronized with rhythmically acetylated proteins in the mitochondria (Figures 3A and 3B; Table S3). In contrast, non-SIRT3 targets displayed a bimodal maximum of acetylation during the middle of the day and night, mostly synchronized with cytoplasmic and nuclear proteins. This observation was confirmed by the fact that most rhythmic SIRT3 targets are mitochondrial proteins, whereas the majority of rhythmic non-SIRT3 targets are localized in the cytoplasm, where they can be deacetylated by another unidentified deacetylase (Figure 3C). The 12-hr acetylation sites we identified were not enriched in SIRT3 targets compared with 24-hr acetylation rhythms (Figure S3A), suggesting that SIRT3 is not implicated in implementing 12-hr rhythms of acetylation. Remarkably, as described previously (Asher et al., 2008, Wang et al., 2017), SIRT1 and its activator RPS19BP1 (Kim et al., 2007), as well as SIRT2 and SIRT7, are rhythmic and synchronized in the nuclear compartment, with a maximum of accumulation during the night-day transition, after the peak of acetylation in this organelle (Figures S3B–S3D), and could therefore also be involved in this deacetylation process.
Figure 3.
Rhythmic Behavior of Non-SIRT3 and SIRT3 Targets
(A–C) Peak phase (A), amplitude (B), and cellular localization (C) of rhythmic acetylation sites non-targeted (left) or targeted (right) by the SIRT3 deacetylase. Acetylation sites were defined as targeted by SIRT3 when their levels were significantly upregulated in Sirt3 KO mice in Rardin et al., 2013b, Hebert et al., 2013, or Dittenhafer-Reed et al. (2015) (Table S3).
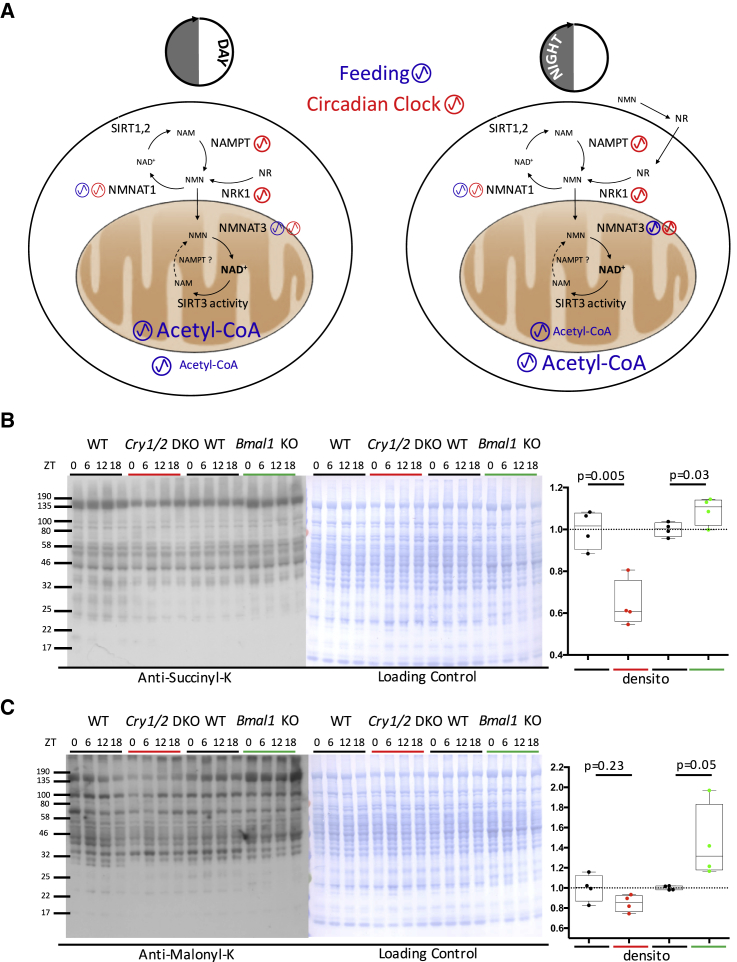
NAD+ Synthesis and the Diurnal Acetylome Are Regulated by the Circadian Clock and the Feeding Schedule
As recently shown, the diurnal activity of SIRT3 is regulated by the circadian clock-regulated NAD+ salvage pathway through the transcriptional control of nicotinamide phosphoribosyltransferase (Nampt), whose expression level peaks at the day-night transition (Nakahata et al., 2009, Peek et al., 2013, Ramsey et al., 2009). However, the level of NAD+ appeared to be only partially related to Nampt expression, with the feeding regimen playing an important role in rhythmic NAD+ accumulation. Under fasting conditions, liver rhythmic NAD+ levels are maximal during the day (Peek et al., 2013), whereas they are biphasic under ad libitum feeding (Ramsey et al., 2009). The strong influence of feeding schedule on NAD+ accumulation was additionally confirmed by mathematical modeling (Woller et al., 2016). Hence, in addition to clock disruption, the feeding schedule also appears to be a key player that fine-tunes NAD+ diurnal oscillations. Hepatic NAD+ production originates mainly from the amidated route (Mori et al., 2014), which includes the aforementioned NAD+ salvage pathway involving NAMPT but also the nicotinamide riboside (NR) pathway. This NR pathway synthesizes NAD+ from the dietary supplies of NR and nicotinamide mononucleotide (NMN) and involves nicotinamide riboside kinase 1 (NRK1), encoded by the Nmrk1 gene (Ratajczak et al., 2016). In addition, the existence of organelle-specific NAD+ biosynthesis enzymes also suggests that the regulation of NAD+ pools is organelle-specific. Indeed, in the cytosol and the nucleus, NAD+ is likely the product of NMN conversion by NMNAT1 and 2, whereas synthesis of the mitochondrial pool involves NMNAT3 (Cambronne et al., 2016). This suggests that the availability of NAD+ precursors during the feeding period may affect the rhythmic deacetylation through rhythmic activation of sirtuins, and not only the circadian clock via the regulation of NAMPT expression.
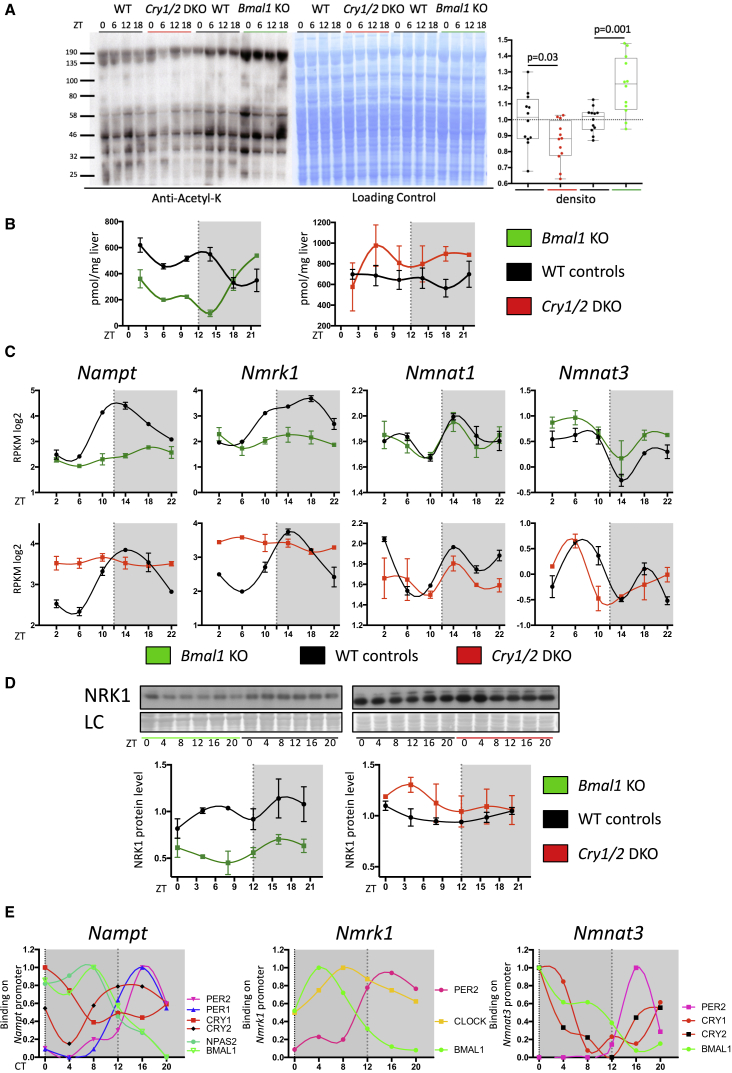
To test this hypothesis, we first investigated the effect of the circadian clock on rhythmic global acetylation by studying lysine acetylation in liver extracts from Cry1/2 and Bmal1 KO mice that present an inactivated circadian clock (Storch et al., 2007, van der Horst et al., 1999). As shown in Figure 4A, global acetylation was slightly decreased in Cry1/2 DKO mice but increased in Bmal1 KO mice. This change in global acetylation could therefore reflect the regulation of NAD+ synthesis by the circadian clock and its associated regulation of sirtuin activity. Accordingly, we observed higher and lower NAD+ levels in the livers of Cry1/2 DKO and Bmal1 KO mice, respectively, showing that the clock indeed controlled NAD+ synthesis (Figure 4B). As mentioned before, although the circadian clock regulation of NAD+ synthesis through the NAD+ salvage pathway seems to be active under fasting conditions (Peek et al., 2013) or for cell-autonomous synthesis of NAD+ (Nakahata et al., 2009), feeding-related pathways likely affect NAD+ synthesis. We therefore speculated about the regulation of NAD+ synthesis by the clock through the nutrient-dependent NR pathway. The genes encoding NAMPT and NRK1 as well as NMNAT3 presented a rhythmic expression at the mRNA level (Figure 4C). Although Nampt expression was, as described, perturbed in clock-disrupted mice (Figure 4C), this was also the case for Nmrk1 at both the mRNA and protein levels (Figures 4C and 4D). However, Nmnat3 expression as well as that of the non-rhythmic Nmnat1 were not significantly affected in clock-disrupted mice (Figure 4C), whereas Nmnat2 is not expressed in mouse liver.
Figure 4.
Regulation of Global Acetylation and NAD+ Metabolism by the Circadian Clock
(A) Western blot analysis of global lysine acetylation performed on total protein extract from Cry1/2 DKO, Bmal1 KO, and their corresponding WT control mice (left). Naphtol blue black staining of the membrane was used as a loading control (center) and served as a reference for normalization of the quantified values displayed at the right. The average acetylation level was compared between clock-disrupted mice and their controls using Student’s t test (n = 12/genotype). Error bars represent min to max.
(B) NAD+ liver concentrations in Bmal1 KO (n = 2/time point) and Cry1/2 DKO mice (n = 2/time point) and their corresponding WT controls (mean ± SEM).
(C) Top: mRNA expression levels of Nampt, Nmrk1, Nmnat1, and Nmnat3 in Bmal1 KO mice (green) and their WT littermates (black) under night-restricted feeding conditions. Bottom: the same results in Cry1/2 DKO (red) mice and their WT controls (black). The data are from Atger et al. (2015); n = 2 for each time point and each genotype). Error bars represent ±SEM.
(D) WB analysis in TE extracts of NRK1 in Bmal1 KO and Cry1/2 DKO mice. Quantifications of the blots are displayed below. Naphtol blue black staining of the membranes was used as a loading control and served as a reference for normalization of the quantified values. Error bars represent ±SEM.
(E) Temporal binding of circadian clock core regulators on the Nampt, Nmrk1, and Nmnat3 gene promoters. At each time point, for each factor, the averages of all rhythmic bindings are presented. The data are from Koike et al. (2012).
To test whether the circadian clock directly regulates these genes, we took advantage of the recently published report regarding rhythmic genome-wide binding of circadian clock regulators (Koike et al., 2012) to study their binding on these specific genes. Interestingly, similar to the rhythmic binding of BMAL1 and NPAS2 around CT8 and the PER and CRY proteins at CT16 on the Nampt promoter, the promoter of Nmrk1 was bound by BMAL1, CLOCK around CT6, and PER2 around CT16. Although BMAL1, CRY1, and CRY2 also bound the promoter of Nmnat3 around CT0 and PER2 bound at CT16, they have apparently minor effect on its expression (Figure 4E), which is likely regulated by additional transcription factors regulated by food-derived signals, potentially contributing to its dysregulation observed in obesity (Drew et al., 2016, Jukarainen et al., 2016). Taken together, these results highlighted the fact that not only the NAD+ salvage pathway but also the feeding-related NR pathway are influenced by the circadian clock and that both play an important role in clock-regulated NAD+ synthesis.
Pathways Affected by Circadian Clock-Regulated Acetylation and Links with Metabolism
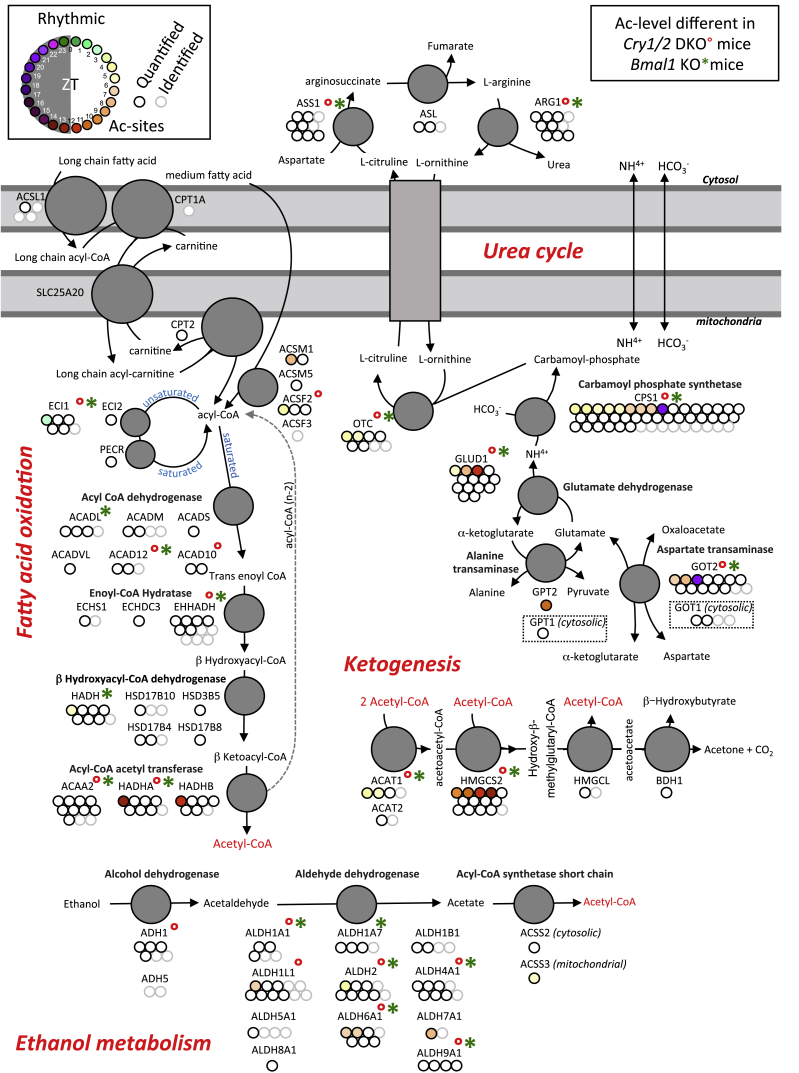
A search for metabolic pathways enriched in rhythmically acetylated proteins revealed a significant involvement in amino acid and lipid metabolism in addition to citrate (tricarboxylic acid cycle [TCA]) and urea cycles. Moreover, many proteins involved in mitochondrial oxidative phosphorylation, ketogenesis, and ethanol metabolism were also rhythmically acetylated (Figure 5; Figure S4; Table S4). Interestingly, many proteins in these pathways showed deregulated acetylation levels in Cry1/2 and Bmal1 KO mice (Figure S5). More specifically, we found that many proteins involved in these pathways presented an increased acetylation level in both KO mice, mainly at ZT18, when rhythmically acetylated mitochondrial proteins showed a minimum level of acetylation in WT mice. Interestingly, acetylation controls several proteins of the TCA cycle. Succinate dehydrogenase A (SDHA) (Cimen et al., 2010, Finley et al., 2011), malate dehydrogenase 2 (MDH2) (Kim et al., 2013), pyruvate dehydrogenase alpha 1 (PDHA) (Fan et al., 2014), and isocitrate dehydrogenase 2 (IDH2) (Yu et al., 2012) are all characterized by reversible acetylation regulated through deacetylation by the deacetylase SIRT3. Except for MDH2, this acetylation leads to enzyme activity inhibition. The global increased acetylation of TCA cycle enzymes in Cry1/2 and Bmal1 KO mice (Figure S5) could be a factor explaining the decreased TCA cycle activity that occurs in Bmal1 KO mice (Peek et al., 2013).
Figure 5.
Metabolic Pathways Affected by Rhythmic Acetylation
Rhythmic protein acetylations and their regulation by the circadian clock for fatty acid oxydation, urea cycle, ketogenesis, and ethanol metabolism. Each dot represents a unique acetylation site within the protein of interest. Grey and black dots represent non-rhythmic identified and quantified acetylation sites, respectively, whereas phases of maximum acetylation levels for rhythmic acetylation sites are color-coded. Superscripted red dots and green asterisks indicate protein acetylation levels significantly different in Cry1/2 DKO and Bmal1 KO mice, respectively.
Similar observations were made for the urea cycle, where carbamoyl phosphate synthetase (CPS1) (Ogura et al., 2010), glutamate oxaloacetate transaminase 2 (GOT2) (Yang et al., 2015), and ornithine carbamoyltransferase (OTC) (Hallows et al., 2011, Yu et al., 2009) also possess reversible acetylation as an enzymatic activity regulator. These enzymes also displayed increased acetylation levels in Cry1/2 and at ZT18 in Bmal1 KO mice. Most of the enzymes involved in ketogenesis are prone to be regulated by SIRT3 (Dittenhafer-Reed et al., 2015), notably 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) (Shimazu et al., 2010), showing circadian clock-regulated acetylation. The acetyl-CoA synthetase enzymes in the ethanol metabolism pathway are also regulated by SIRT3-dependent deacetylation, which activates their enzymatic activity (Hallows et al., 2006, Schwer et al., 2006). In the same pathway, numerous aldehyde dehydrogenases are known to be deacetylated in a sirtuin-dependent fashion (Lu et al., 2011, Zhao et al., 2014) and show circadian clock-regulated acetylation. Regarding fatty acid oxidation, the enzymes acyl-CoA synthetase I (ACSL1) (Frahm et al., 2011), long-chain acyl CoA dehydrogenase (ACADL) (Hirschey et al., 2010), and enoyl-CoA hydratase/3-hydroxyacyl-CoA (EHHADH) (Zhao et al., 2010) have also been shown to be regulated by acetylation and present altered acetylation in clock-deficient mice. Mitochondrial oxidative phosphorylation protein complexes I to V have been shown to be acetylated (Kim et al., 2006); this acetylation is at least partly under the control of SIRT3 (Rahman et al., 2014). As previously shown for several of these (Cela et al., 2016), rhythmic acetylation of these proteins was found to be subjected to circadian clock regulation. Altogether, these observations suggest that clock-controlled diurnal acetylation could have an effect on global liver metabolism via the regulation of enzymatic activity.
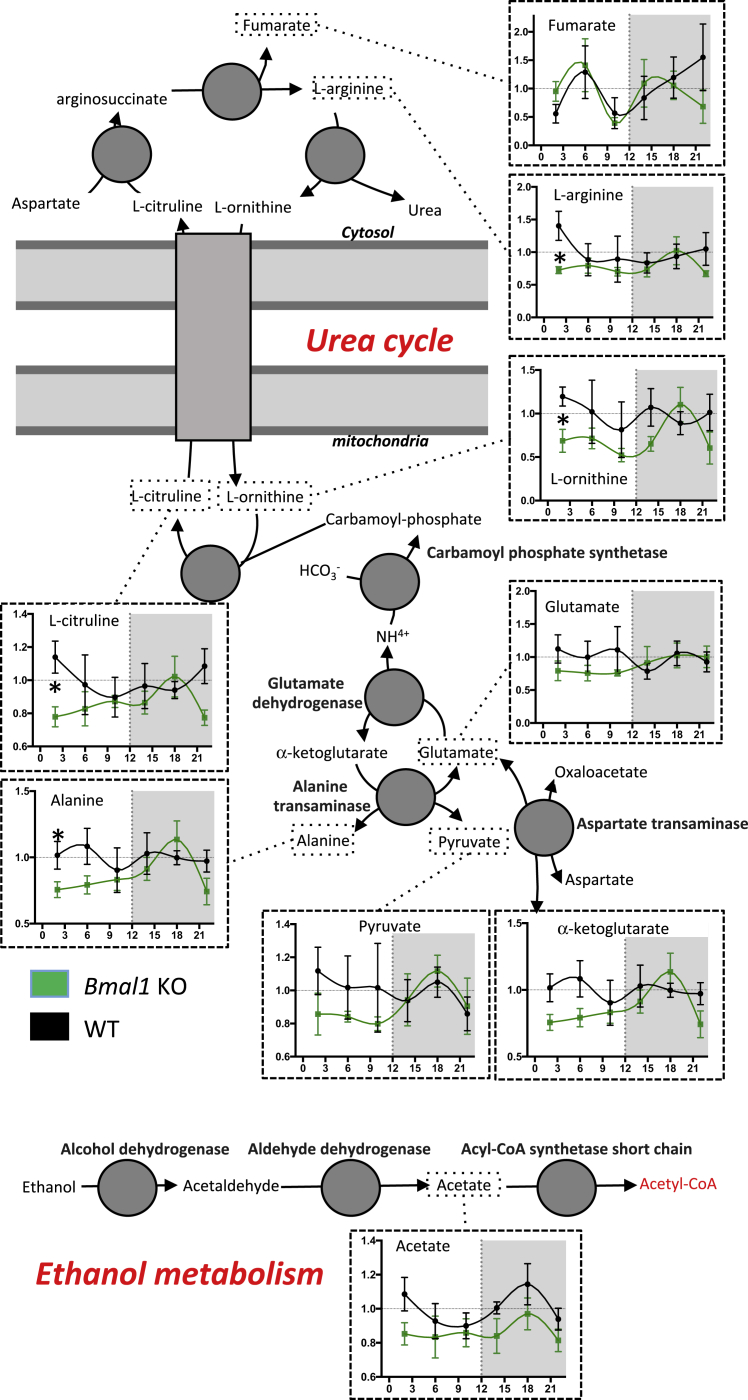
To identify potential effects of the described circadian clock-regulated rhythmic acetylation on metabolism, in addition to the proteomic approach we also performed proton nuclear magnetic resonance (1H-NMR) spectroscopy-based metabolomics on hydrophilic liver tissue extracts from WT and Bmal1 KO mice. As described in Figures 6 and S6, several reciprocities between levels of metabolites and differential acetylation levels in Bmal1 KO mice could be found. For instance, in the urea cycle, low levels of arginine and ornithine during the day in Bmal1 KO animals paralleled the decreased acetylation of ASS1 and ARG1 during this period and the increased acetylation of OTC at ZT18. Although the function of ASS1 and ARG1 acetylation has not yet been described, we could speculate that a decrease in OTC activity because higher acetylation at ZT18 also contributes to this process. In the same pathway, matched low levels of glutamate, α-ketoglutarate, pyruvate, and alanine were also observed during the day, preceded by increased acetylation and inhibition of GLUD1, CPS1, and GOT2 at the end of the night. Several of these metabolites are also part of the imbricate TCA cycle and tie up with the decreased succinate level at day-night transition, when many enzymes presented an increased acetylation level (Figures S5 and S6). In addition to these pathways, we also observed a strong correspondence between low levels of lysine at night-day transition and acetylation of aminoadipate-semialdehyde synthase (AASS), suggesting an uncharacterized link between the acetylation and activity of this enzyme. Finally, several aldehyde dehydrogenases of the ethanol pathway displayed disturbed acetylation in KO mouse liver that could be put alongside a general decrease in acetate levels. We also noted in many cases that quantitative profiles of metabolites are correlated with the profile of NAD+ level in Bmal1 KO mice, suggesting a potential role of NAD+-dependent deacetylase in the regulation of enzyme activities in the aforementioned metabolic pathways or at least a strong correlation between the two processes (Figures 4B and 6).
Figure 6.
Impact of Rhythmic Acetylation on Metabolite Levels
Liver temporal profiles of metabolites in Bmal1 KO (green lines) and WT littermates (black lines). Data (mean ± SEM) are expressed relative to the temporal mean of WT values. The analysis is related to key metabolites of the urea cycle, ketogenesis, and ethanol metabolism. Student’s t test was performed to compare the average level of metabolite at every time point between different genotypes (n = 4). ∗p < 0.05 between Bmal1 KO and WT mice.
Discussion
Although phosphorylation has been considered the major modifier of protein activity and function for a long time, many additional modifications, including acetylation, have emerged as important post-translational modifications (Hirschey and Zhao, 2015). The high-coverage acetylome described here allowed us to gain more insights about diurnal protein acetylation in mouse liver compared with previous work (Masri et al., 2013). In particular, we showed that acetylation of mitochondrial proteins is in the opposite phase compared with the extra-mitochondrial compartments, probably because of the different sirtuins locally expressed within these cell compartments. These rhythmic sirtuin activities, and particularly SIRT3 in mitochondria, are likely the consequence of rhythmic accumulation of NAD+ in the different cell compartments (Peek et al., 2013). Although regulation of the NAD+ salvage pathway by the circadian clock through the regulation of Nampt expression appeared to be critical during fasting or for cell-autonomous NAD+ synthesis (Nakahata et al., 2009, Ramsey et al., 2009), this pathway seems to be less critical under normal conditions, where NAD+ seems to originate mostly from food metabolism. Indeed, the circadian clock controls NAMPT, involved in the synthesis of NAD+ through the salvage pathway or from the food-contained nicotinamide (NAM), but also NRK1, involved in the synthesis of NAD+ from NR and NMN (Ratajczak et al., 2016; Figure 7A). The circadian clock therefore regulates most aspects of NAD+ synthesis in the mouse liver and, by this, enlarges the landscape of interconnection between the circadian clock and sirtuins (Masri and Sassone-Corsi, 2014).
Figure 7.
Interconnection between Feeding and Circadian Rhythms in Regulating NAD+ Synthesis and Circadian Clock Disruption Effects on Lysine Succinylation and Malonylation
(A) Rhythmic feeding effect (blue) versus the circadian clock (red) on key components implicated in acetyl-CoA-dependent acetylation and the regulation NAD+ synthesis during the day (fasting phase) and night (feeding phase).
(B and C) Western blot analysis of global lysine succinylation (B) and malonylation (C) performed on total protein extract from Cry1/2 DKO, Bmal1 KO, and their corresponding WT control mice (left). Naphtol blue black staining of the membrane was used as a loading control (center) and served as a reference for normalization of the quantified values displayed at the right. Average acetylation levels were compared between clock-disrupted mice and their controls using Student’s t test (n = 4). Error bars represent min to max.
In contrast to the observed maximum acetylation of mitochondrial proteins during the night under fasting conditions because of the more active SIRT3 activity during this period (Peek et al., 2013), we observed, under our night feeding conditions, maximal acetylation of these proteins during the day, mostly as a consequence of the food-dependent increase in acetyl-CoA synthesis (Shi and Tu, 2015). Under these two conditions, this maximal acetylation is correlated with the minimal mitochondrial activity (Neufeld-Cohen et al., 2016, Peek et al., 2013), deacetylation of mitochondrial proteins being key to maintaining proper mitochondrial activity (Hirschey et al., 2010). In addition, feeding rhythms also appear to be important in this pathway because restricted night feeding is able to restore normal rhythmic mitochondrial activity in Per2 KO animals (Neufeld-Cohen et al., 2016), highlighting the strong interconnection between feeding and the circadian clock in regulating mitochondrial activity. In addition, supplementation strategies designed to improve NAD+ concentration protect against metabolic diseases, neurodegenerative disorders, and age-related physiological decline in mammals (Cantó et al., 2015). The key role of the circadian clock in regulating NAD+ synthesis suggests that timing of supplementation should also be taken into account as an important aspect of this strategy.
Our study also shows the key role of the circadian clock in the metabolism of vitamin B3, nicotinic acid (NA) constituting another source of NAD+. Interestingly, circulating levels of several vitamins, like vitamins A, B9, B12, D, E, and K, showed a diurnal rhythm in mammals (Hutchins and Ball, 1983, Piccione et al., 2004), but the role of the circadian clock in these rhythms has not yet been established. One exception is vitamin B6, for which a clear role of the clock has been demonstrated through the regulation of pyridoxal kinase (Pdxk) by the circadian clock-regulated proline acidic amino acid-rich basic leucine zipper (PARbZip) transcription factors (Gachon et al., 2004). All of these elements suggest that the circadian clock, in addition to its role in macronutrient metabolism, also plays a crucial role in micronutrient and vitamin metabolism. Given the broad effect of these circadian clock-regulated vitamins in general metabolism, in particular neurotransmitter synthesis for vitamin B6 (Gachon et al., 2004) and mitochondrial activity for vitamin B3 (Menzies et al., 2016), this pathway likely contributes to the metabolic deficiency observed in circadian clock-deficient animals.
Although acetylation is currently the most described modification of lysine residues by acylation, lysine can also alternatively be formylated, methylated, propionylated, butyrylated, crotonylated, malonylated, succinylated, glutarylated, or myristoylated (Choudhary et al., 2014). Sirtuins are also involved in the deacylation of these lysine modifications, playing an important role in the regulation of metabolism. SIRT5, for instance, has recently been shown to regulate glycolysis through reversible malonylation (Nishida et al., 2015), the urea cycle through deglutarylation (Tan et al., 2014), and ketogenesis as well as other metabolic pathways through desuccinylation (Park et al., 2013, Rardin et al., 2013a). Interestingly, these activities are also NAD+-dependent and, as a result, potentially subjected to circadian clock regulation. Using antibodies specific to malonylated and succinylated lysine, we also showed that malonylation was increased in the liver of Bmal1 KO mice, whereas succinylation was decreased in Cry1/2 DKO mice and increased in Bmal1 KO mice (Figures 7B and 7C), in accordance with NAD+ levels in these animals. These additional findings suggest that the circadian clock, in addition to its key role in the regulation of transcription, is also able to regulate key metabolic function through post-translational regulation events, including acetylation, and probably other metabolism-related lysine acylations.
Experimental Procedures
Animal Experiments
Animal studies were conducted in accordance with the regulations of the veterinary office of the Canton of Vaud. Cry1/2 DKO mice in the C57BL/6J genetic background (Bur et al., 2009) and Bmal1 KO mice (Jouffe et al., 2013) have been described previously. Mice had free access to food and water in 12-hr light/12-hr dark cycles under standard animal housing conditions. However, unless indicated otherwise, in all experiments animals were fed only at night, starting 4 days before the experiment. SILAC mice, generated according to a standard procedure (Krüger et al., 2008), have been described previously (Mauvoisin et al., 2014).
Total and Nuclear Protein Extraction
TE and NE protein extracts were generated as described previously (Wang et al., 2017). The detailed procedure can be found in the Supplemental Experimental Procedures.
Analysis of Acetylation by RP-LC MS/MS
The detailed procedure for the RP-LC MS/MS analysis of lysine-acetylated enriched peptides can be found in the Supplemental Experimental Procedures. The accession numbers for the mass spectrometry proteomic data reported in this paper were deposited in the ProteomeXchange Consortium via the PRIDE partner repository: PXD005317 and PXD005310 for TE and NE, respectively.
Annotation of Protein Localization
We used UNIPROT (UniProt Consortium, 2015) and COMPARTMENT (Binder et al., 2014) to annotate protein localization (i.e., nuclear, shuttling, cytoplasmic, or mitochondrial), for TE and NE.
Analysis of Rhythmicity
We assessed the rhythmicity in temporal accumulations of acetylated sites normalized by corresponding protein levels using harmonic regression as described previously (Mauvoisin et al., 2014).
KEGG Pathway Enrichment Analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment for quantified proteins was performed using the Database of Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources 6.8. at https://david.ncifcrf.gov/home.jsp (Huang et al., 2009).
Western Blotting
20 μg of total liver protein was used for western blotting according to standard procedures. Densitometry analyses of the blots were performed using the ImageJ software. Naphtol blue black staining of the membranes was used as a loading control and served as a reference for normalization of the quantified values. References for antibodies used in this paper can be found in the Supplemental Experimental Procedures.
NAD+ Concentration
Liver NAD+ concentration was assessed with the EnzyChrom NAD/NADH Assay Kit (BioAssay Systems) on ∼25 mg of liver tissue and according to the manufacturer’s instructions.
1H NMR Spectroscopic Analysis of Liver Metabonome
The detailed procedure for 1H NMR metabonomic analysis of liver extracts can be found in the Supplemental Experimental Procedures.
Author Contributions
Conceptualization, D.M. and F.G.; Formal Analysis, D.M., J.W., F.N., and F.G.; Investigation, D.M., F.A., E.M., J.R., and C.C.; Proteomics Data Acquisition and Curation, L.D., A.N.G., and M.K.; Metabolomics Data Acquisition and Curation, L.D.S., I.M., S.C., and F.P.M.; Visualization, D.M. and J.W.; Writing – Original Draft, D.M. and F.G.; Writing – Review & Editing, D.M., F.A., L.D., A.N.G., J.W., E.M., L.D.S., I.M., S.C., F.P.M., J.R., C.C., M.K., F.N., and F.G.; Supervision, F.N. and F.G.; Funding Acquisition, F.N. and F.G.
Acknowledgments
This research was supported by the Swiss National Science Foundation (through individual research grants 31003A-153340 and 310030-173079 to F.N.), the Ecole Polytechnique Fédérale de Lausanne, the Novartis Foundation for Medical-Biological Research (16C213 to F.N.), the European Research Council (through individual starting grant ERC-2010-StG-260988 to F.G.), and the Leenaards Foundation (to F.G. and F.N.). We thank Philipp Gut for positive insightful discussions and comments. The authors thank Prof. Gijsbertus van der Horst (Erasmus University Medical Center, Rotterdam, the Netherlands) for providing Cry1/2 DKO mice. D.M., F.A., L.D., A.N.G., E.M., L.D.S., I.M., F.P.M., J.R., C.C., M.K., and F.G. are employees of the Nestlé Institute of Health Sciences S.A.
Published: August 15, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.07.065.
Accession Numbers
The accession numbers for the mass spectrometry proteomic data for TE and NE, respectively, reported in this paper are ProteomeXchange Consortium via the PRIDE partner repository: PXD005317 and PXD005310.
Supplemental Information
References
- Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F.W., Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Atger F., Gobet C., Marquis J., Martin E., Wang J., Weger B., Lefebvre G., Descombes P., Naef F., Gachon F. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc. Natl. Acad. Sci. USA. 2015;112:E6579–E6588. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.X., Pletscher-Frankild S., Tsafou K., Stolte C., O’Donoghue S.I., Schneider R., Jensen L.J. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database (Oxford) 2014;2014:bau012. doi: 10.1093/database/bau012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bur I.M., Cohen-Solal A.M., Carmignac D., Abecassis P.-Y., Chauvet N., Martin A.O., van der Horst G.T.J., Robinson I.C.A.F., Maurel P., Mollard P., Bonnefont X. The circadian clock components CRY1 and CRY2 are necessary to sustain sex dimorphism in mouse liver metabolism. J. Biol. Chem. 2009;284:9066–9073. doi: 10.1074/jbc.M808360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne X.A., Stewart M.L., Kim D., Jones-Brunette A.M., Morgan R.K., Farrens D.L., Cohen M.S., Goodman R.H. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., Menzies K.J., Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela O., Scrima R., Pazienza V., Merla G., Benegiamo G., Augello B., Fugetto S., Menga M., Rubino R., Fuhr L. Clock genes-dependent acetylation of complex I sets rhythmic activity of mitochondrial OxPhos. Biochim. Biophys. Acta. 2016;1863:596–606. doi: 10.1016/j.bbamcr.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Chavan R., Feillet C., Costa S.S.F., Delorme J.E., Okabe T., Ripperger J.A., Albrecht U. Liver-derived ketone bodies are necessary for food anticipation. Nat. Commun. 2016;7:10580. doi: 10.1038/ncomms10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S., Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Choudhary C., Weinert B.T., Nishida Y., Verdin E., Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- Cimen H., Han M.-J., Yang Y., Tong Q., Koc H., Koc E.C. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane B.R., Young M.W. Interactive features of proteins composing eukaryotic circadian clocks. Annu. Rev. Biochem. 2014;83:191–219. doi: 10.1146/annurev-biochem-060713-035644. [DOI] [PubMed] [Google Scholar]
- Dittenhafer-Reed K.E., Richards A.L., Fan J., Smallegan M.J., Fotuhi Siahpirani A., Kemmerer Z.A., Prolla T.A., Roy S., Coon J.J., Denu J.M. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015;21:637–646. doi: 10.1016/j.cmet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew J.E., Farquharson A.J., Horgan G.W., Williams L.M. Tissue-specific regulation of sirtuin and nicotinamide adenine dinucleotide biosynthetic pathways identified in C57Bl/6 mice in response to high-fat feeding. J. Nutr. Biochem. 2016;37:20–29. doi: 10.1016/j.jnutbio.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Du J., Zhou Y., Su X., Yu J.J., Khan S., Jiang H., Kim J., Woo J., Kim J.H., Choi B.H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Shan C., Kang H.-B., Elf S., Xie J., Tucker M., Gu T.-L., Aguiar M., Lonning S., Chen H. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol. Cell. 2014;53:534–548. doi: 10.1016/j.molcel.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley L.W.S., Haas W., Desquiret-Dumas V., Wallace D.C., Procaccio V., Gygi S.P., Haigis M.C. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J.L., Li L.O., Grevengoed T.J., Coleman R.A. Phosphorylation and Acetylation of Acyl-CoA Synthetase- I. J. Proteomics Bioinform. 2011;4:129–137. doi: 10.4172/jpb.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F., Fonjallaz P., Damiola F., Gos P., Kodama T., Zakany J., Duboule D., Petit B., Tafti M., Schibler U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004;18:1397–1412. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z., Lazar M.A. Circadian metabolism in the light of evolution. Endocr. Rev. 2015;36:289–304. doi: 10.1210/er.2015-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows W.C., Lee S., Denu J.M. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. USA. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows W.C., Yu W., Smith B.C., Devries M.K., Ellinger J.J., Someya S., Shortreed M.R., Prolla T., Markley J.L., Smith L.M. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert A.S., Dittenhafer-Reed K.E., Yu W., Bailey D.J., Selen E.S., Boersma M.D., Carson J.J., Tonelli M., Balloon A.J., Higbee A.J. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey M.D., Zhao Y. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol. Cell. Proteomics. 2015;14:2308–2315. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey M.D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D.B., Grueter C.A., Harris C., Biddinger S., Ilkayeva O.R. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hutchins D.A., Ball P.E. Circadian rhythms of folate and vitamin B(12) concentration in relation to convulsive thresholds of mice. Neurochem. Int. 1983;5:421–427. doi: 10.1016/0197-0186(83)90071-2. [DOI] [PubMed] [Google Scholar]
- Jouffe C., Cretenet G., Symul L., Martin E., Atger F., Naef F., Gachon F. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013;11:e1001455. doi: 10.1371/journal.pbio.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukarainen S., Heinonen S., Rämö J.T., Rinnankoski-Tuikka R., Rappou E., Tummers M., Muniandy M., Hakkarainen A., Lundbom J., Lundbom N. Obesity Is Associated With Low NAD(+)/SIRT Pathway Expression in Adipose Tissue of BMI-Discordant Monozygotic Twins. J. Clin. Endocrinol. Metab. 2016;101:275–283. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- Kim S.C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kim E.-J., Kho J.-H., Kang M.-R., Um S.-J. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol. Cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Han B.S., Kim W.K., Lee S.C., Bae K.-H. Acceleration of adipogenic differentiation via acetylation of malate dehydrogenase 2. Biochem. Biophys. Res. Commun. 2013;441:77–82. doi: 10.1016/j.bbrc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Koike N., Yoo S.-H., Huang H.-C., Kumar V., Lee C., Kim T.-K., Takahashi J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M., Moser M., Ussar S., Thievessen I., Luber C.A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Lombard D.B., Alt F.W., Cheng H.-L., Bunkenborg J., Streeper R.S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Bourdi M., Li J.H., Aponte A.M., Chen Y., Lombard D.B., Gucek M., Pohl L.R., Sack M.N. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity. EMBO Rep. 2011;12:840–846. doi: 10.1038/embor.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A., Lage K., Weinert B.T., Bekker-Jensen D.B., Secher A., Skovgaard T., Kelstrup C.D., Dmytriyev A., Choudhary C., Lundby C., Olsen J.V. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S., Sassone-Corsi P. Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci. Signal. 2014;7:re6. doi: 10.1126/scisignal.2005685. [DOI] [PubMed] [Google Scholar]
- Masri S., Patel V.R., Eckel-Mahan K.L., Peleg S., Forne I., Ladurner A.G., Baldi P., Imhof A., Sassone-Corsi P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. USA. 2013;110:3339–3344. doi: 10.1073/pnas.1217632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R.A., Greco T.M., Oberstein A., Budayeva H.G., Chakrabarti R., Rowland E.A., Kang Y., Shenk T., Cristea I.M. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159:1615–1625. doi: 10.1016/j.cell.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvoisin D., Wang J., Jouffe C., Martin E., Atger F., Waridel P., Quadroni M., Gachon F., Naef F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvoisin D., Dayon L., Gachon F., Kussmann M. Proteomics and circadian rhythms: it’s all about signaling! Proteomics. 2015;15:310–317. doi: 10.1002/pmic.201400187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies K.J., Zhang H., Katsyuba E., Auwerx J. Protein acetylation in metabolism - metabolites and cofactors. Nat. Rev. Endocrinol. 2016;12:43–60. doi: 10.1038/nrendo.2015.181. [DOI] [PubMed] [Google Scholar]
- Monaghan R.M., Whitmarsh A.J. Mitochondrial Proteins Moonlighting in the Nucleus. Trends Biochem. Sci. 2015;40:728–735. doi: 10.1016/j.tibs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Mori V., Amici A., Mazzola F., Di Stefano M., Conforti L., Magni G., Ruggieri S., Raffaelli N., Orsomando G. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE. 2014;9:e113939. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld-Cohen A., Robles M.S., Aviram R., Manella G., Adamovich Y., Ladeuix B., Nir D., Rousso-Noori L., Kuperman Y., Golik M. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl. Acad. Sci. USA. 2016;113:E1673–E1682. doi: 10.1073/pnas.1519650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Rardin M.J., Carrico C., He W., Sahu A.K., Gut P., Najjar R., Fitch M., Hellerstein M., Gibson B.W., Verdin E. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell. 2015;59:321–332. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura M., Nakamura Y., Tanaka D., Zhuang X., Fujita Y., Obara A., Hamasaki A., Hosokawa M., Inagaki N. Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem. Biophys. Res. Commun. 2010;393:73–78. doi: 10.1016/j.bbrc.2010.01.081. [DOI] [PubMed] [Google Scholar]
- Park J., Chen Y., Tishkoff D.X., Peng C., Tan M., Dai L., Xie Z., Zhang Y., Zwaans B.M.M., Skinner M.E. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek C.B., Affinati A.H., Ramsey K.M., Kuo H.-Y., Yu W., Sena L.A., Ilkayeva O., Marcheva B., Kobayashi Y., Omura C. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione G., Assenza A., Grasso F., Caola G. Daily rhythm of circulating fat soluble vitamin concentration (A, D, E and K) in the horse. J. Circadian Rhythms. 2004;2:3. doi: 10.1186/1740-3391-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Nirala N.K., Singh A., Zhu L.J., Taguchi K., Bamba T., Fukusaki E., Shaw L.M., Lambright D.G., Acharya J.K., Acharya U.R. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase β and regulates complex V activity. J. Cell Biol. 2014;206:289–305. doi: 10.1083/jcb.201404118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K.M., Yoshino J., Brace C.S., Abrassart D., Kobayashi Y., Marcheva B., Hong H.-K., Chong J.L., Buhr E.D., Lee C. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardin M.J., He W., Nishida Y., Newman J.C., Carrico C., Danielson S.R., Guo A., Gut P., Sahu A.K., Li B. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardin M.J., Newman J.C., Held J.M., Cusack M.P., Sorensen D.J., Li B., Schilling B., Mooney S.D., Kahn C.R., Verdin E., Gibson B.W. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc. Natl. Acad. Sci. USA. 2013;110:6601–6606. doi: 10.1073/pnas.1302961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J., Joffraud M., Trammell S.A.J., Ras R., Canela N., Boutant M., Kulkarni S.S., Rodrigues M., Redpath P., Migaud M.E. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles M.S., Cox J., Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10:e1004047. doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles M.S., Humphrey S.J., Mann M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017;25:118–127. doi: 10.1016/j.cmet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Schwer B., Bunkenborg J., Verdin R.O., Andersen J.S., Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. USA. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Eckersdorff M., Li Y., Silva J.C., Fermin D., Kurtev M.V., Giallourakis C., Comb M.J., Alt F.W., Lombard D.B. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Tu B.P. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T., Hirschey M.D., Hua L., Dittenhafer-Reed K.E., Schwer B., Lombard D.B., Li Y., Bunkenborg J., Alt F.W., Denu J.M. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E.A., Brocks D.G., Wieland O.H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z. Physiol. Chem. 1978;359:785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Smith K.T., Workman J.L. Introducing the acetylome. Nat. Biotechnol. 2009;27:917–919. doi: 10.1038/nbt1009-917. [DOI] [PubMed] [Google Scholar]
- Still A.J., Floyd B.J., Hebert A.S., Bingman C.A., Carson J.J., Gunderson D.R., Dolan B.K., Grimsrud P.A., Dittenhafer-Reed K.E., Stapleton D.S. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. J. Biol. Chem. 2013;288:26209–26219. doi: 10.1074/jbc.M113.483396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch K.-F., Paz C., Signorovitch J., Raviola E., Pawlyk B., Li T., Weitz C.J. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M., Peng C., Anderson K.A., Chhoy P., Xie Z., Dai L., Park J., Chen Y., Huang H., Zhang Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G.T.J., Muijtjens M., Kobayashi K., Takano R., Kanno S., Takao M., de Wit J., Verkerk A., Eker A.P.M., van Leenen D. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Verdin E., Hirschey M.D., Finley L.W.S., Haigis M.C. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Mauvoisin D., Martin E., Atger F., Galindo A.N., Dayon L., Sizzano F., Palini A., Kussmann M., Waridel P. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017;25:102–117. doi: 10.1016/j.cmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert B.T., Moustafa T., Iesmantavicius V., Zechner R., Choudhary C. Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 2015;34:2620–2632. doi: 10.15252/embj.201591271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E., Thompson C.B. A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- Woller A., Duez H., Staels B., Lefranc M. A Mathematical Model of the Liver Circadian Clock Linking Feeding and Fasting Cycles to Clock Function. Cell Rep. 2016;17:1087–1097. doi: 10.1016/j.celrep.2016.09.060. [DOI] [PubMed] [Google Scholar]
- Wu R., Haas W., Dephoure N., Huttlin E.L., Zhai B., Sowa M.E., Gygi S.P. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat. Methods. 2011;8:677–683. doi: 10.1038/nmeth.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Vaitheesvaran B., Hartil K., Robinson A.J., Hoopmann M.R., Eng J.K., Kurland I.J., Bruce J.E. The fasted/fed mouse metabolic acetylome: N6-acetylation differences suggest acetylation coordinates organ-specific fuel switching. J. Proteome Res. 2011;10:4134–4149. doi: 10.1021/pr200313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zhou L., Shi Q., Zhao Y., Lin H., Zhang M., Zhao S., Yang Y., Ling Z.-Q., Guan K.-L. SIRT3-dependent GOT2 acetylation status affects the malate-aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 2015;34:1110–1125. doi: 10.15252/embj.201591041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Lin Y., Yao J., Huang W., Lei Q., Xiong Y., Zhao S., Guan K.-L. Lysine 88 acetylation negatively regulates ornithine carbamoyltransferase activity in response to nutrient signals. J. Biol. Chem. 2009;284:13669–13675. doi: 10.1074/jbc.M901921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Dittenhafer-Reed K.E., Denu J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Mo Y., Li M.-T., Zou S.-W., Cheng Z.-L., Sun Y.-P., Xiong Y., Guan K.-L., Lei Q.-Y. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J. Clin. Invest. 2014;124:5453–5465. doi: 10.1172/JCI76611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.