Abstract
A common practice when analyzing multi-site epidemiological data is to include a term for ‘site’ to account for unmeasured effects at each location. This practice should be carefully considered when site can have complex relationships with important demographic and exposure variables. We leverage data from three longitudinal North American pregnancy cohorts to demonstrate a novel method to assess study heterogeneity and potential combinability of studies for pooled analyses in order to better understand how to consider site in analyses. Results from linear regression and fixed effects meta-regression models run both prior to and following the proposed combinability analyses were compared. In order to exemplify this approach, we examined associations between prenatal exposure to particulate matter and birth weight. Analyses included mother-child dyads (N=1966) from the Asthma Coalition on Community Environment and Social Stress (ACCESS) Project and the PRogramming of Intergenerational Stress Mechanisms (PRISM) study in the northeastern United States, and the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study in Mexico City. Mothers’ daily third trimester exposure to particulate matter ≤ 2.5 microns in diameter (PM2.5) was estimated using a validated satellite-based spatio-temporally resolved model in all studies. Fenton birth weight for gestational age z-scores were calculated. Linear regression analyses within each cohort separately did not find significant associations between PM2.5 averaged over the third trimester and Fenton z-scores. The initial meta-regression model also did not find significant associations between prenatal PM2.5 and birthweight. Next, propensity scores and log linear models were used to assess higher order interactions and determine if sites were comparable with regard to sociodemographics and other covariates; these analyses demonstrated that PROGRESS and ACCESS were combinable. Adjusted linear regression models including a 2-level site variable according to the pooling indicated by the log linear models (ACCESS and PROGRESS as one level and PRISM as another) revealed that a 5 μg/m3 increase in PM2.5 was associated with a 0.075 decrease in Fenton z-score (p<0.0001); linear models including a 3-level site variable did not reveal significant associations. By assessing the combinability of heterogeneous populations prior to combining data using a method that more optimally accounts for underlying cohort differences, we were able to identify significant associations between prenatal PM2.5 exposure and birthweight that were not detected using standard methods.
Keywords: air pollution, birth weight, propensity scores
1. Introduction
Ambient air pollution ranks among the top ten risk factors for global burden of disease (Lim et al. 2012). The fetus may be particularly susceptible to ambient air pollution exposure effects due to rapid development and immature detoxifying enzyme systems (Wells et al. 2009). Emerging evidence has linked prenatal exposure to both gaseous and particulate air pollution to a number of adverse fetal outcomes, including reduced growth and term low birth weight (LBW), typically defined as birthweight <2500 g for newborns born at ≥37 completed weeks of gestation) (Fleisch et al. 2015; Lakshmanan et al. 2015; Morello-Frosch et al. 2010); however, an association between ambient pollution and lower birth weight has not been consistently demonstrated across studies. In a study in Japan that examined national health survey data, higher suspended particulate matter (SPM) and sulfur dioxide (SO2) during pregnancy were associated with higher odds of term LBW (Yorifuji et al. 2015). A study of full term births in Massachusetts examined birth weight as a continuous outcome and reported that a 10 μg/m3 increase in particulate matter less than or equal 2.5 microns in diameter (PM2.5) levels during pregnancy was associated with a 13.80 g decrease in birth weight (Kloog et al. 2012). An analysis of birth records data in Canada found associations between PM2.5 exposure during pregnancy and greater odds of small for gestational age (SGA) and greater reductions in term birth weight, but not LBW (Stieb et al. 2016). In a pooled analysis of 14 European birth cohorts across 12 countries, a 5 μg/m3 average increase in PM2.5 during pregnancy, estimated using land use regression (LUR), was associated with higher odds of term LBW (Pedersen et al. 2013). Notably, previous reports on individual cohorts included in the pooled analysis in Pedersen et al. (2013) did not find statistically significant associations between PM2.5 and term birth weight (Gehring et al. 2011). In a meta-analysis of published literature on prenatal exposure to PM2.5 and PM10 and adverse birth outcomes, Sapkota et al reported increased odds of LBW with higher PM2.5 exposure, but the results were not statistically significant and deemed inconclusive (Sapkota et al., 2012). In a study of singleton live births in California limited to full-term infants, only prenatal exposure to ozone, not PM2.5 or NO2, was associated with higher odds of term LBW (Laurent et al. 2016). In another study that examined spatial variation in the association between ambient air pollutants and birth weight of full-term singletons in the state of Georgia, ozone was positively associated with higher birth weight, whereas PM2.5 was not associated with birth weight (Tu et al. 2016).
Discrepancies in these findings and methodological differences in studies (Dadvand et al. 2013; Woodruff et al. 2009) have limited the ability to synthesize the evidence and translate it into policy. These discrepancies may be attributable to differences in exposure assignment (e.g., land use regression vs. dispersion models vs. monitor assignment), sample size, patterns of exposure dependent on geographic location, or the range of exposure and/or outcome within each population which limits the ability to find significant associations within a given study.
Increasingly, epidemiologic studies are combining data among multiple cohorts not originally designed as a consortium, such as the newly launched NIH Environmental influences on Child Health Outcomes (ECHO). Methods that can rigorously pool data in these scenarios are clearly needed. The integration of data from multiple pediatric environmental health studies has the potential to provide enhanced power and exposure contrast to examine associations between ambient air pollution and fetal outcomes. A common practice when analyzing multi-site epidemiological data is to include a term for ‘site’ to account for unmeasured effects at each location. However, this practice should be carefully considered when site can have complex relationships with important demographic and exposure variables. For example, in order to properly integrate data, social factors that might influence exposure, such as racial/ethnic makeup and socioeconomic compositions of the cohorts of interest, should be taken into account more formally. Traditional meta-analysis do not deal with differences across cohorts, rather they compare effect estimates across studies. Covariates might be adjusted for within each study but differences in covariates are not adjusted for across studies. Developing tools that enhance our ability to combine multi-site data while accounting for study heterogeneity could be a significant methodological advancement.
The current study leveraged existing data from three established population-based birth cohorts, two in the northeastern United States (U.S.) and one in Mexico, to examine their potential combinability and to test associations between prenatal PM2.5 exposure and infant birthweight in the integrated sample. We utilize estimates of air pollution during the third trimester of pregnancy in these analyses to illustrate a novel approach to assessing combinability of cohort data accounting for potential sociodemographic confounders and covariates.
2. Methods
2.1 Study cohorts
We included three prenatally enrolled cohorts based in the U.S. or Mexico with similarly derived air pollution measures. Here we provide details on enrollment procedures.
Asthma Coalition on Community Environment and Social Stress (ACCESS) Project
Project ACCESS is a prospective cohort of mother-child dyads designed to study the effects of early life stress and other environmental factors on urban childhood asthma risk (Wright et al. 2008). Briefly, N=660 English- or Spanish-speaking pregnant women (≥18 years of age) with singleton pregnancies receiving care at Brigham & Women’s Hospital (BWH), Boston Medical Center (BMC), and affiliated community health centers were enrolled between August 2002 and January 2007. Data on sociodemographics, maternal health, and prenatal exposures included in these analyses were obtained within 2 weeks of enrollment. Procedures were approved by human studies committees at the Brigham and Women’s Hospital and Boston Medical Center; written informed consent in the mother’s primary language was obtained from all mothers.
PRogramming of Intergenerational Stress Mechanisms (PRISM) study
The PRISM study is a prospective pregnancy cohort of mother-child dyads originally designed to study how perinatal stress influences children’s stress response systems over early development and subsequent respiratory health while controlling for environmental exposures (e.g., ambient air pollution, nutrition) (Brunst et al. 2014). Between March 2011 and August 2012, N=365 women were recruited from prenatal clinics at the Beth Israel Deaconess Medical Center (BIDMC) and the East Boston Neighborhood Health Center in Boston, Massachusetts, U.S.A. English- or Spanish-speaking women with singleton pregnancies were eligible if they were age ≥ 18 years at enrollment and endorsed drinking <7 alcoholic drinks/week prior to pregnancy and none since pregnancy recognition. Procedures were approved by human studies committees at the Brigham & Women’s Hospital, Icahn School of Medicine at Mount Sinai (ISMMS), and BIDMC; written consent was obtained in the subject’s primary language.
Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study
PROGRESS is a prospective birth cohort originally designed to study the modifying effects of stress on lead toxicity and on the neurotoxicity of metal mixtures. Pregnant women receiving prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social –IMSS) between July 2007 and February 2011 were recruited into the PROGRESS study (Burris et al., 2013). Women were eligible to participate if they were less than 20 weeks pregnant, at least 18 years old, planned to stay in Mexico City for the next 3 years, had access to a telephone, had no medical history of heart or kidney disease, did not consume alcohol daily, and did not use any steroid or anti-epilepsy medications. Procedures were approved by institutional review boards at the Harvard School of Public Health, the Mexican National Institute of Public Health, and the ISMMS. Mothers provided written informed consent.
2.2 Ambient air pollution exposure estimates
Individuals’ prenatal exposure to PM2.5, an index of ambient pollution from traffic and other sources, was estimated based on residence during pregnancy (i.e., at enrollment and updated if they moved) using a validated hybrid satellite based spatio-temporal prediction model for all cohorts. For ACCESS and PRISM, the model incorporated Moderate Resolution Imaging Spectroradiometer (MODIS) derived aerosol optical depth (AOD) measurements at a 1×1 km spatial resolution, using day-specific AOD data calibrated against ground monitor-based PM2.5 measurements derived from 78 monitoring stations covering New England (Kloog et al. 2014; Kloog et al. 2011; Nwanaji-Enwerem et al. 2016). The model incorporated traditional land use regression (LUR) and meteorological variables. For locations on days without AOD data (due to cloud coverage, snow, etc.), predictions were based on a regression of predicted PM2.5 in that 1km cell on days when AOD was present against the mean of PM2.5 monitors within 60 km of the cell, and a thin plate spline of latitude and longitude and a random intercept for each cell to impute predictions at these missing locations. The out of sample ten-fold cross validation R2 for daily values was 0.88.
For PROGRESS, daily PM2.5 levels across Mexico City were estimated at a 1× 1 km spatial resolution at the participant’s reported residence during pregnancy (Just et al. 2015). Similar to the model used for ACCESS and PRISM, the model was run using day-specific calibrations of AOD data calibrated against ground PM2.5 measurements from 12 monitoring stations covering Mexico City and LUR and meteorological variables (roadway density, temperature, relative humidity, planetary boundary layer, and daily precipitation). As in previous studies, mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5−AOD relationship. For days without AOD data, the model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring. The out of sample ten-fold cross validation R2 of 0.724. We use PM2.5 exposure averaged over the third trimester of pregnancy to demonstrate our approach to assess combinability, to help reduce variability and increase precision of the estimates.
2.3 Birth weight z-score outcome
In ACCESS and PRISM, gestational age was calculated based on maternal report of last menstrual period (LMP) and updated based on obstetrical estimates based on ultrasound data from medical record review at delivery if discrepant by more than 3 weeks (Hoffman et al. 2008). In PROGRESS, ultrasounds were not routinely performed as standard of care; thus, gestational age was based on LMP and by a standardized physical examination at birth to determine gestational age (Capurro et al. 1978). If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the estimate of gestational age based on physical exam was used. The Capurro and ultrasound methods have been found to have good concordance (Pereira et al., 2013). Birth weight data were extracted from labor and delivery records for all studies. Given that birth weight is tightly tied to length of gestation, that each may have different predictors, and that birth weight rises in a non-linear pattern with increasing gestational age (Oken et al. 2003), traditional approaches that use raw birth weight data adjusted for gestational age in a linear regression model may add bias (Oken et al. 2003). Use of z-scores allows adjustment for gestational age more precisely, as z-scores factor in non-linear growth, which reduces both bias and residual confounding (Oken et al. 2003). Therefore, we derived Fenton birth weight for gestational age z-scores for all cohorts (Fenton et al. 2013). These reference curves were derived from a growth curve modeling meta-analysis of preterm birth and validated with the World Health Organization (WHO) growth curves for postnatal growth. Z-scores facilitate harmonization across different cohorts, including those from different countries (Fenton et al. 2013).
2.4 Covariates
Previously identified covariates related to air pollution exposure and birth weight were considered For all cohorts, maternal age, race/ethnicity, and maternal education as an indicator of individual-level socioeconomic status (SES) were ascertained by questionnaire Maternal pre-pregnancy height and weight were determined via self-report at enrollment; body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Sensitivity analyses were also carried out with the inclusion of report smoking during pregnancy ascertained through questionnaire.
2.5 Statistical Analysis
The overarching goal of the analysis was to compare results to fixed effects meta-regression and linear regression with adjustment for site to the proposed combinability approach. Thus we approached the analyses in the following stepwise manner. First in the standard approach, linear models were used to evaluate the association between third trimester PM2.5 levels and birth weight for gestational age z-scores in each study separately. Models were adjusted for maternal education, race, age, and pre-pregnancy BMI. To address the possibility of unmeasured differences among the sites, a fixed effects meta-regression analysis was employed by extracting the beta coefficients and standard errors from the linear model stratified by the site variable. Next we examined the combinability of the ACCESS, PRISM, and PROGRESS data in two ways. First, propensity scores were estimated from multinomial logistic models with site as the outcome and education (≤ high school or > higher school), race/ethnicity (white, non-Hispanic or non-white and/or Hispanic), BMI (maternal pre-pregnancy, continuous), gestational age (continuous), mother’s age at birth (continuous), infant sex, and average third trimester PM2.5 (continuous) as the predictors, while allowing for two- and three-way interactions among the predictor variables. Significant terms indicate complex characteristics of the studies that distinguish them from one another. Propensity scores are calculated by taking the inverse of the probabilities obtained from the multinomial logistic models and defined as the conditional probability of an individual belonging to their actual study given covariates. If the probability of being assigned to any of the three studies is similar, it suggests that the sites are comparable in regard to the covariates. The distribution of the probabilities of an individual being assigned to each site was evaluated with boxplots.
Second, log-linear models of multidimensional contingency tables were used to detect and characterize heterogeneous inter-study association patterns (Agresti et al. 2002). Inter-study association patterns refer to interactions between the covariates, exposure and outcome particularly to determine if these were modified by study site. Deviance was used as a measure of fit. A significant deviance indicates that the nested model does not fit as well as the larger model. The variables considered were maternal education (≤ high school or > higher school), site (ACCESS, PRISM, or PROGRESS) age (dichotomized at median), pre-pregnancy BMI (dichotomized at median), and for the purposes of the log linear models only, PM2.5 was included in quartiles, and the outcome birth weight for gestational age z-score was dichotomized at the median. Log-linear models were such that 6-way, 5-way, 4-way, and 3-way site interaction terms could be systematically evaluated. Factors that were not adequately diverse (i.e., PROGRESS included only Hispanics, PROGRESS being predominantly non-smoking) were not included in log-linear models. Log linear models were used in order to determine grouping; exclusion of variables in this step did not preclude their inclusion in the models after grouping was decided. Model construction followed with the model fully parameterized with terms not including site and where k-way site interaction terms were tested for significance in a top-down strategy (i.e., 6-way interaction, then all 5-way interaction terms, etc.) Alpha was set at 10%. All significant site interactions were further explored with odds ratios (OR).
Finally, linear models were used to evaluate the association between third trimester PM2.5 and birth weight for gestational age z-scores, both as continuous variables, according to the pooling indicated by the log linear models. Models were adjusted for maternal education, race, age, and pre-pregnancy BMI.
3. Results
3.1. Descriptive statistics
As seen in Table 1, many of the demographic characteristics varied across the ACCESS, PRISM, and PROGRESS cohorts. Notably, PROGRESS, which was recruited in Mexico, included 100% Hispanic participants whereas 90% of ACCESS and 64% of PRISM participants were non-white and/or Hispanic. In ACCESS and PROGRESS, mothers had similar education levels (61% and 76% ≤ high school, respectively), whereas the majority of women in PRISM had >high school education (73%). PROGRESS had lower mean birth weight z-scores, whereas ACCESS and PRISM birth weight z-scores were more similar. The distribution of infant sex was consistent across studies. PROGRESS had the highest levels of PM2.5 (mean: 23.1 μg/m3) while PRISM had the lowest levels of PM2.5 (mean: 8.23 μg/m3).
Table 1.
Descriptive characteristics for pooled sample and individual cohorts
| Pooled Sample | ACCESS | PRISM | PROGRESS | ||
|---|---|---|---|---|---|
| N | 1966 | 660 | 365 | 941 | |
| 3rd trimester PM2.5 (μg/m3)* | 16.27 (15.93, 16.61) | 10.75 (10.62,10.88) | 8.23 (8.09, 8.38) | 23.13 (22.8, 23.46) | |
| Birth weight for gestational age z-score* | −0.24(−0.29, −0.19) | −0.09 (−0.19,0.00) | −0.11 (−0.21, −0.02) | −0.46 (−0.52, −0.39) | |
| Maternal pre-pregnancy BMI* | 26.6 (26.34, 26.86) | 28.8 (28.3, 29.3) | 25.93 (25.3, 26.55) | 25.23 (24.94, 25.52) | |
| Maternal age at enrollment* | 28.37 (28.12, 28.64) | 27.1 (26.64, 27.56) | 31.5 (30.94, 32.06) | 28.04 (27.69, 28.39) | |
| Sex* | Male | 52% | 52% | 51% | 52% |
| Female | 48% | 48% | 49% | 48% | |
| Race/Ethnicity* | White, Non-Hispanic | 10% | 10% | 36% | 0% |
| Non-White and/or Hispanic | 90% | 90% | 64% | 100%* | |
| Education* | ≥ High School | 62% | 61% | 27% | 76% |
| > High School | 38% | 39% | 73% | 24%* | |
Means (95% CI) shown
P < 0.05, differences tested using ANOVA for continuous variables and Chi-Square for dichotomous variables
3.2. Linear regression and meta-regression models prior to combinability analysis
We first show results from the linear regression and fixed effect meta-regression analysis. Table 2 shows the linear model associations for each individual study and the estimated results of a meta-regression combining the betas and standard errors from the adjusted linear regression models. (i.e., all 3 study sites considered individually). The associations between third trimester PM2.5 and birth weight z-score are negative in PRISM and PROGRESS and in the overall meta-regression but these results did not reach statistical significance in the individual cohorts. A limitation of the meta-regression model is that it does not benefit from differences in the exposure range across studies; that is, the slope estimates are combined without regard to the variable range of the exposure within each study. Specifically, PROGRESS has higher PM2.5 values with non-overlapping IQRs with ACCESS and PRISM (Table 1) yet this expanded exposure range across studies is not taken advantage of in the meta-regression.
Table 2.
Linear regression models within each study and fixed effects meta-regression assessing the relationship between PM2.5 and Fenton z-scores: Prior to combinability analyses
| Linear Models† | Meta Regression‡ | ||||
|---|---|---|---|---|---|
|
| |||||
| N | β* (95% CI) |
P-value | β (95% CI) |
P-value | |
| ACCESS | 660 | 0.036 (−0.017, 0.089) |
0.181 | −0.0015 (−0.013, 0.009) |
0.786 |
| PRISM | 365 | −0.031 (−0.098, 0.036) |
0.362 | ||
| PROGRESS | 941 | −0.002 (−0.014, 0.009) |
0.670 | ||
β represents the change in birth weight z-score per 1 unit increase in third trimester PM2.5 (μg/m3) levels
Linear models were adjusted for maternal education (≤ high school or > higher school), race/ethnicity (white/non-Hispanic or non-white and/or Hispanic), age at birth (continuous), and pre-pregnancy BMI (continuous).
Meta-regression showing results for the combination of the 3 separate study estimates obtained from adjusted linear regression models.
3.3. Combinability analyses
We next examined the combinability of the three studies. Figure 1 shows that the distribution of the probability of being assigned to the three studies varied; thus, differences in covariate and exposure distributions predict study identification. In particular, significant model terms indicated the existence of higher-level interactions. We also explored these interactions excluding PM2.5 and found PRISM was more distinct compared to the similarities in ACCESS and PROGRESS. For additional insight, we continued with further analysis of combinability through log-linear models.
Figure 1.
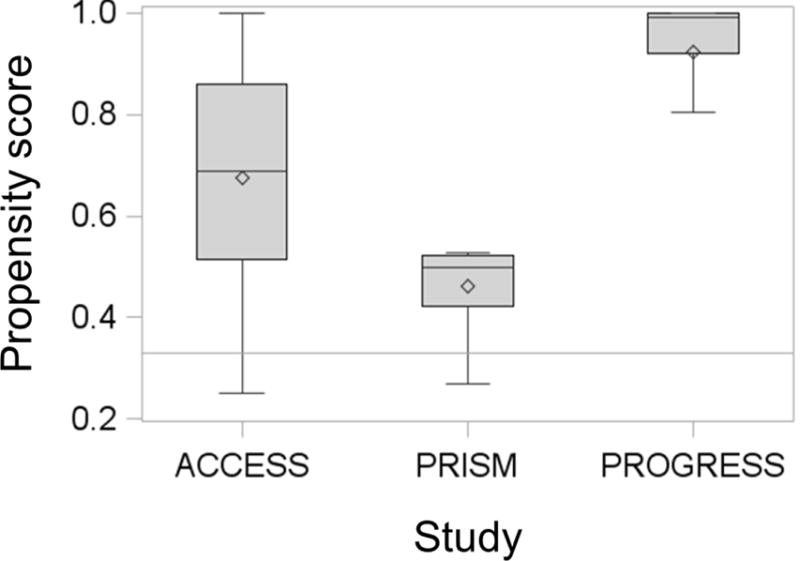
Distributions of propensity scores by cohort study. Box plots depicting propensity scores for ACCESS, PRISM and PROGRESS. Propensity scores are defined as the conditional probability of an individual belonging to their actual study given covariates. Line in the box plot depicts the median, the diamond depicts the mean, the lower and upper bounds of the box depict the first and third quartile respectively, and the whiskers depict the minimum and maximum propensity score values for each cohort.
In the next step, log-linear models were used to systematically evaluate 6-way, 5-way, 4-way, and 3-way site interaction terms (Table 3). Deviance was used as a measure of fit and a significant deviance indicates that the nested model does not fit as well as the larger model. Log-linear models detected a 3-way site interaction, which indicated heterogeneous inter-study association patterns (Table 4). We did not see a significant interaction between PM2.5, birthweight and study which would indicate that the association of interest is similar across studies. We identified that the association between maternal age and BMI differed among the sites (p=0.001). Figure 2 shows a further examination of this association using odds ratios to compare the odds of younger mothers (maternal age <median) having low BMI (<median) when compared to the odds of younger mothers having high BMI (>median). The OR for PRISM was statistically different from ACCESS and PROGRESS which saw associations in the same direction and of similar magnitude. We also found that the association between maternal BMI and birth weight for gestational age z-score differed among sites. Similar to Figure 2, Figure 3, compares the odds of mothers with low BMI having a baby with birth weight z-score below the median when compared to the of mothers with low BMI having a baby with a birth weight z-score above the median. The OR for this association in PRISM differed in directionality from the ORs seen in PROGRESS and ACCESS (Figure 3). In conclusion, the propensity scores implied differences across sites due to high level interactions. Similarly, the log-linear models and ORs suggested that the association patterns among covariates were similar for ACCESS and PROGRESS. Therefore, we combined the ACCESS and the PROGRESS data as a single “surrogate study”.
Table 3.
Log-linear models
| Model | Model Description | Notation | Deviance | DF |
|---|---|---|---|---|
| 1 | Saturated Model | (TEABPW) | 0.00 | 0 |
| 2 | Model excludes 6-way site interaction | (T(EABPW @ 5), EABPW) | 0.12 | 1 |
| 3 | Model excludes 6- and 5-way site interactions | (T(EABPW @ 4), EABPW) | 4.16 | 7 |
| 4 | Model excludes 6-, 5-, and 4-way site interactions | (T(EABPW @ 3), EABPW) | 16.22 | 21 |
| 5 | Model excludes 6-, 5-, 4-, and 3-way site interactions | (T(EABPW @ 2), EABPW) | 45.17 | 41 |
Education (E); mother’s age at birth (A); BMI (B); PM2.5 (P); birth weight for gestation age Fenton z-score (W); site (T).
Table 4.
Comparison of log-linear models
| Model | Test Interactions | P-value | Sig Interactions (P <0.1) |
|---|---|---|---|
| 1 vs 2 | 6-way site int | 0.73 | None |
| 2 vs 3 | 5-way site int | 0.67 | None |
| 3 vs 4 | 4-way site int | 0.60 | None |
| 4 vs 5 | 3-way site int | 0.09 | TBA, TBW |
Mother’s age at birth (A); BMI (B); site (T); Fenton z-score (W)
Figure 2.
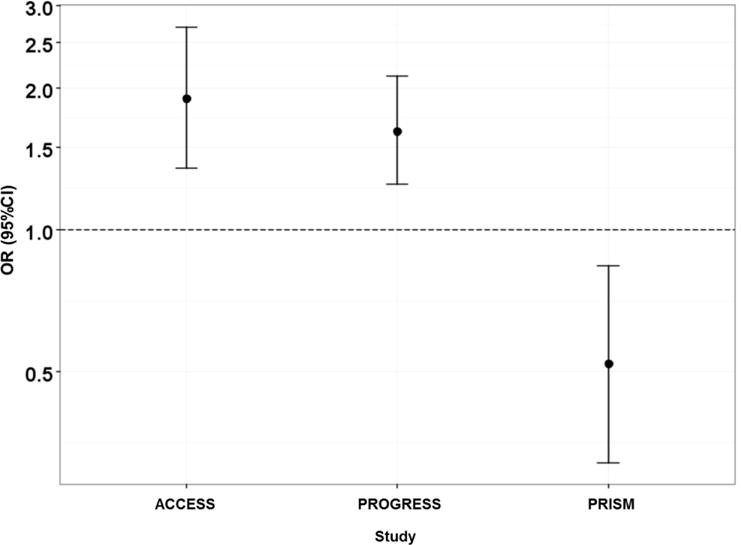
Association between maternal pre-pregnancy BMI and maternal age by site.
Figure 3.
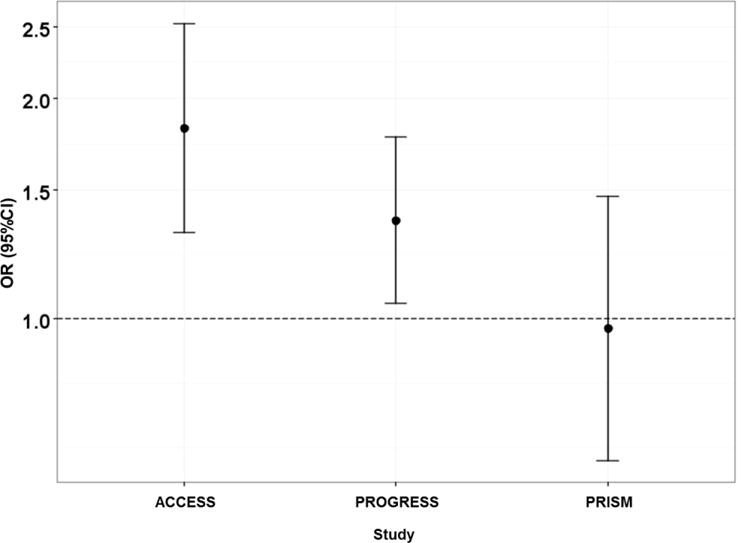
Association between maternal pre-pregnancy BMI and infant birth weight for gestational age Fenton z-score
3.4. Linear regression and meta-regression models informed by combinability analyses
Table 5 shows adjusted linear regression models with a 2-level site variable (ACCESS and PROGRESS as one level and PRISM as another) and a 3-level site variable. The 2-level site model found a significant association (p<0.0001) between third trimester PM2.5 and birth weight for gestational age z-scores; a 5 μg/m3 increase in third trimester PM2.5 levels was associated with a 0.075 decrease in birth weight for gestational age z-score. The linear regression model including a 3-level site variable did not find a significant association (p=0.92) between PM2.5 and birth weight z-score – the primary association of interest.
Table 5.
Linear models with and without surrogate study variable for the association between PM2.5 over pregnancy and birth weight for gestational age z-score.
β represents the change in birth weight z-score per 1 unit increase in PM2.5 (μg/m3) during thethird trimester of pregnancy
Adjusted for maternal education (≤ high school or > higher school), race/ethnicity (white/non-Hispanic or non-white and/or Hispanic), age at birth (continuous), and pre-pregnancy BMI (continuous).
Model 1: Includes study variable (ACCESS, PROGRESS, PRISM)
Model 2: Includes surrogate study variable, Surrogate Site 1 (PROGRESS and ACCESS) and Surrogate Site 2 (PRISM).
The fixed effects meta-regression informed by the combinability analysis, which summarized the results from one model with ACCESS and PROGRESS and a second model with PRISM, also identified a negative association between third trimester PM2.5 levels and birth weight for gestational age z-scores (Table 6) of similar magnitude to the first model.
Table 6.
Fixed effects meta-regression of linear model assessing the relationship between third trimester PM2.5 and birth weight for gestational age z-score: Cohort combinations based on log-linear models
| Linear Models† | Meta Regression‡ | ||||
|---|---|---|---|---|---|
|
| |||||
| N | β* (95% CI) |
P-value | β* (95% CI) |
P-value | |
| Surrogate Study 1# | 1601 | −0.013 (−0.020, −0.005) |
0.001 | −0.0129 (−0.020, −0.006) |
0.0001 |
| Surrogate Study 2¶ | 365 | −0.031 (−0.098, 0.036) |
0.362 | ||
β represents the change in birth weight z-score per 1 unit increase in PM2.5 (μg/m3) during the third trimester of pregnancy.
Surrogate Study 1 refers to ACCESS and PROGRESS.
Surrogate Study 2 refers to PRISM.
Linear models adjusted for maternal education (≤ high school or > higher school), race/ethnicity (white/non-Hispanic or non-white and/or Hispanic), age at birth (continuous), and pre-pregnancy BMI (continuous).
The meta-regression shows the results for the combination of the surrogate study estimates obtained from adjusted linear regression models.
Figure 4 shows an overall negative association between third trimester PM2.5 and the adjusted birth weight for gestational age z-score. The slope for PRISM differed from the slope for ACCESS and PROGRESS, which may suggest a non-linear association between birth weight for gestational age z-score and PM2.5. Results that included report of smoking paralleled those reported here (see Supplemental material).
Figure 4. Site specific linear model within range of exposure: Cohort combinations based on log-linear models.
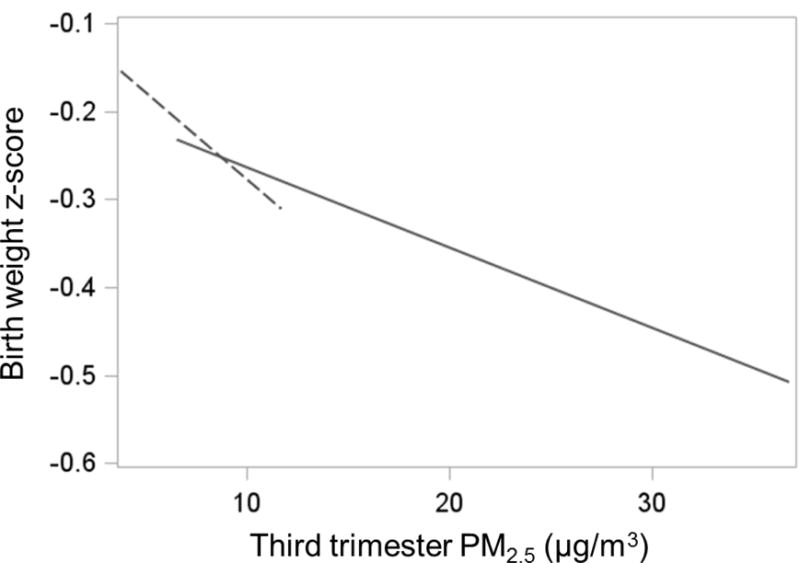
Birth weight z-scores were constructed from the residuals of the pooled analysis linear model that regressed birth weight for gestational age z-scores by maternal education, race/ethnicity, age, and pre-pregnancy BMI and the overall mean birth weight for gestational age z-score was added to the residuals. Dotted line depicts estimate for the PRISM study. Solid line depicts estimate for ACCESS and PROGRESS combined.
4. Discussion
These analyses leveraged data from three North American pregnancy cohorts to exemplify a novel method to assess study heterogeneity and potential combinability of studies for pooled analyses to better inform how to consider site in these analyses. We exemplify these methods in analyses examining associations between ambient PM2.5 exposure and infant birth weight. Notably, these studies took the same approach to modeling air pollution exposures and characterized the outcome using birth weight adjusted for gestational age Fenton z-scores given that both U.S. and international participants were included and z-scores allowed us to include all children and not limit our analysis to only full term children as has been done in previous studies. The combinability approach reported here used propensity scores and log linear models to evaluate higher dimensional associations that serve to indicate heterogeneous inter-study association patterns. The patterns that emerged indicated that these underlying associations were similar between the ACCESS and PROGRESS studies and that these two studies were combinable. Prior to assessing combinability, we did not find a significant association between prenatal PM2.5 and birthweight in analyses considering each cohort separately or the meta-regression which combined the linear regression estimates from the 3 cohorts. Linear models combining studies according to the pooling indicated by the log linear models revealed a significant association between increased levels of third trimester PM2.5 and lower birth weight for gestational age in analyses combining ACCESS and PROGRESS cohort data. The observed associations remained significant after adjustment for potential sociodemographic confounders and other covariates including maternal smoking.
The combinability method presented here allows for the adjustment of known variables across cohorts and minimizes the potential impact of the unknown confounders that “site” might represent. Moreover, the combinability approach takes better advantage of the increased heterogeneity in exposure across studies compared to an analysis simply adjusting for a 3-level site indicator. Notably, significant associations were seen only after combining ACCESS (a cohort in the northeastern U.S.) and PROGRESS (Mexico City sample) likely in part to limited within study exposure contrasts. While the Mexico City sample had higher PM2.5 exposure values with non-overlapping IQRs when compared to the northeastern U.S. samples, we are able to increase exposure contrast by combining it with the ACCESS cohort. This also expanded the range in values for the outcome. This coupled with increased sample size in the pooled analysis likely contributed to enhanced power to detect effects relative to analyses performed prior to assessing combinability.
It is worth pointing out that our results are in line with other global research showing associations between in utero PM2.5 exposure later in pregnancy and birth weight. In Beijing, a 19.8 μg/m3 increase in PM2.5 during the 8th month of gestation was associated with an 18 g decrease in birth weight (Rich et al. 2015). In an analysis of data from the World Health Organization Global Survey on Maternal and Perinatal Health from 2004 through 2008, an increase in seasonally adjusted PM2.5 levels estimated in the month preceding delivery were associated with higher odds of low birth weight in a pooled analysis of 22 countries (Fleischer et al. 2014). In a study of pregnancy outcomes in Canada, PM2.5 exposure late in pregnancy was reported to have stronger associations with SGA and term birth weight than exposure in early and mid- pregnancy (Stieb et al. 2016). We reiterate that we utilized third trimester estimates of air pollution in these analyses to illustrate the approach to assessing combinability of cohort data. While some studies report significant associations between PM2.5 exposure in later pregnancy, others show associations in the first trimester or do not identify specific vulnerable windows (Hao et al. 2016) (Dadvand et al. 2013). Such disparate findings related to the exposure timing in this field are likely related to various methodological issues across studies (Woodruff et al. 2009). Consideration of timing of exposure is another challenge to doing pooled analysis of observational studies in this field and was beyond the scope of our current demonstration.
As the main purpose of our presented analysis was to exemplify a novel combinability approach for pooled analyses, we acknowledge some limitations relative to the specific associations being examined. We did not consider air pollution exposures in microenvironments that might lead to personal exposure levels that differed from our ambient exposure estimated at the home residence. The covariates used to determine the combinability of the studies were not exhaustive and we cannot rule out the possibility that additional variables might yield different results. The analyses were limited to the covariates that were available for all three studies which had adequate diversity as noted previously. Mother’s prenatal smoking status was not considered in the main analysis for a number of reasons. The distribution of PM2.5 levels did not vary by report of maternal smoking in any of the 3 cohorts (Supplemental table S1). Inclusion of maternal smoking did not alter the distribution of propensity scores (Figure S1). Maternal smoking was excluded from the log linear model step used to determine grouping due to inadequate diversity (PROGRESS being practically non-smoking). Moreover, studies indicate that tobacco smoke exposure is a major contributor to indoor air pollution in inner-city homes (Wallace et al. 2003) (McCormack et al. 2009) and that variations in indoor source particles (e.g., from smoking and/or cooking sources) are largely uncorrelated with variations in outdoor source particles, which is the main exposure considered herein (Wilson et al. 2000). Thus, while particles of indoor origin are an important predictor of fetal development in and of themselves, they are unlikely to confound associations between ambient particulate matter and birthweight. In secondary analyses presented in supplemental material, we show that inclusion of maternal smoking modestly strengthened the association between PM2.5 and birth weight z-score after grouping PROGRESS and ACCESS together in both the analyses using the surrogate study variables (Table S3) and the meta-regression (Table S4). Nonetheless, we cannot rule out potential residual confounding due to unmeasured host and environmental factors that may influence birth weight.
Pooled analyses of longitudinal observational studies are increasingly being done to inform policy and public health. Meta-analysis of observational studies present particular challenges given heterogeneity in the distribution of covariates across studies and variable design. We demonstrate that assessing the combinability of heterogeneous populations prior to combining their data may allow for a better understanding of underlying cohort differences and provide increased power to detect associations that would be undetected by more customary methods for combining cohorts.
Supplementary Material
Highlights.
Examined association between prenatal PM2.5 and birth weight data from 3 studies.
Used novel combinability method to integrate data, account for study heterogeneity.
PROGRESS and ACCESS studies were comparable with regard to covariates.
Higher 3rd trimester PM2.5 associated with lower BWGA z-scores.
Acknowledgments
This work was supported by the National Institutes of Health (grant UG3OD023337, T32 HD049311-09 supported MJR); the National Institute of Environmental Health Sciences (grants R01 ES010932, R01 ES013744, R21 ES021318, R01 ES021357, P30 ES023515; R00ES023450 supported ACJ) and the National Heart Lung and Blood Institute (grants U01 HL072494, R01 HL080674, R01HL095606) and U2CES026555. MBE was supported by the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital. This study was also supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico
We thank the ABC (American British Cowdray) Hospital in Mexico for providing research facilities and the ACCESS, PRISM and PROGRESS staff for data collection.
Abbreviations
- CI
confidence interval
- LBW
low birth weight
- PM2.5
Particulate matter less than or equal 2.5 microns in diameter
- PM10
Particulate matter less than or equal 10 microns in diameter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A, Coull BA. The analysis of contingency tables under inequality constraints. J Stat Plan Infer. 2002;107:45–73. [Google Scholar]
- Brunst KJ, Wright RO, DiGioia K, Enlow MB, Fernandez H, Wright RJ, Kannan S. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr. 2014;17:1960–1970. doi: 10.1017/S1368980013003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Braun JM, Byun HM, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics-Uk. 2013;5:271–281. doi: 10.2217/epi.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. The Journal of pediatrics. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, Gehring U, Glinianaia SV, Gouveia N, Ha EH, Leem JH, van den Hooven EH, Jalaludin B, Jesdale BM, Lepeule J, Morello-Frosch R, Morgan GG, Pesatori AC, Pierik FH, Pless-Mulloli T, Rich DQ, Sathyanarayana S, Seo J, Slama R, Strickland M, Tamburic L, Wartenberg D, Nieuwenhuijsen MJ, Woodruff TJ. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environmental health perspectives. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Gold DR, Oken E. Prenatal Exposure to Traffic Pollution: Associations with Reduced Fetal Growth and Rapid Infant Weight Gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer NL, Merialdi M, van Donkelaar A, Vadillo-Ortega F, Martin RV, Betran AP, Souza JP, O’Neill MS. Outdoor Air Pollution, Preterm Birth, and Low Birth Weight: Analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environmental health perspectives. 2014;122:425–430. doi: 10.1289/ehp.1306837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- Gehring U, Wijga AH, Fischer P, de Jongste JC, Kerkhof M, Koppelman GH, Smit HA, Brunekreef B. Traffic-related air pollution, preterm birth and term birth weight in the PIAMA birth cohort study. Environmental research. 2011;111:125–135. doi: 10.1016/j.envres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Hao YP, Strosnider H, Balluz L, Qualters JR. Geographic Variation in the Association between Ambient Fine Particulate Matter (PM2.5) and Term Low Birth Weight in the United States. Environmental health perspectives. 2016;124:250–255. doi: 10.1289/ehp.1408798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatric and perinatal epidemiology. 2008;22:587–596. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W, Nawrot TS. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environmental health perspectives. 2012;120:1346–1352. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Tellez-Rojo MM, Moody E, Wang Y, Lyapustin A, Kloog I. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environmental science & technology. 2015;49:8576–8584. doi: 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, Lyapustin A, Wang YJ, Schwartz J. A new hybrid spatio-temporal model for estimating daily multiyear PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–590. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy pm(2.5) exposure, premature birth and birth weight in Massachusetts. Environ Health-Glob. 2012;11 doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Chiu YHM, Coull BA, Just AC, Maxwell SL, Schwartz J, Gryparis A, Kloog I, Wright RJ, Wright RO. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environmental research. 2015;137:268–277. doi: 10.1016/j.envres.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, Escobedo L, Wu J. Low birth weight and air pollution in California: Which sources and components drive the risk? Environ Int. 2016;92–93:471–477. doi: 10.1016/j.envint.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, Eggleston P, Diette GB, Center for Childhood Asthma in the Urban Environment In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009 Feb;117(2):294–8. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM, Sadd JL, Pastor M. Ambient air pollution exposure and full-term birth weight in California. Environ Health-Glob. 2010;9 doi: 10.1186/1476-069X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwanaji-Enwerem JC, Colicino E, Trevisi L, Kloog I, Just AC, Shen J, Brennan K, Dereix A, Hou L, Vokonas P, Schwartz J, Baccarelli AA. Long-term ambient particle exposures and blood DNA methylation age: findings from the VA normative aging study. Environ Epigenet. 2016;2 doi: 10.1093/eep/dvw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke. 2007;38:264–270. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AMN, Ballester F, Beelen RMJ, Chatzi L, Cirach M, Danileviciute A, Dedele A, van Eijsden M, Estarlich M, Fernandez-Somoano A, Fernandez MF, Forastiere F, Gehring U, Grazuleviciene R, Gruzieva O, Heude B, Hoek G, de Hoogh K, van den Hooven EH, Haberg SE, Jaddoe VWV, Klumper C, Korek M, Kramer U, Lerchundi A, Lepeule J, Nafstad P, Nystad W, Patelarou E, Porta D, Postma D, Raaschou-Nielsen O, Rudnai P, Sunyer J, Stephanou E, Sorensen M, Thiering E, Tuffnell D, Varro MJ, Vrijkotte TGM, Wijga A, Wilhelm M, Wright J, Nieuwenhuijsen MJ, Pershagen G, Brunekreef B, Kogevinas M, Slama R. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Resp Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- Pereira AP, Dias MA, Bastos MH, da Gama SG, Leal Mdo C. Determining gestational age for public health care users in Brazil: comparison of methods and algorithm creation. BMC research notes. 2013;6:60. doi: 10.1186/1756-0500-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocobelli G, Dublin S, Enquobahrie DA, Mueller BA. Birth Weight and Birth Weight for Gestational Age in Relation to Risk of Hospitalization with Primary Hypertension in Children and Young Adults. Maternal and child health journal. 2016;20:1415–1423. doi: 10.1007/s10995-016-1939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Liu KB, Zhang JL, Thurston SW, Stevens TP, Pan Y, Kane C, Weinberger B, Ohman-Strickland P, Woodruff TJ, Duan XL, Assibey-Mensah V, Zhang JF. Differences in Birth Weight Associated with the 2008 Beijing Olympics Air Pollution Reduction: Results from a Natural Experiment. Environmental health perspectives. 2015;123:880–887. doi: 10.1289/ehp.1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Hlth. 2012;5:369–381. [Google Scholar]
- Stieb DM, Chen L, Beckerman BS, Jerrett M, Crouse DL, Omariba DW, Peters PA, van Donkelaar A, Martin RV, Burnett RT, Gilbert NL, Tjepkema M, Liu S, Dugandzic RM. Associations of Pregnancy Outcomes and PM2.5 in a National Canadian Study. Environmental health perspectives. 2016;124:243–249. doi: 10.1289/ehp.1408995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Tu W, Tedders SH. Spatial variations in the associations of term birth weight with ambient air pollution in Georgia, USA. Environ Int. 2016:92–93. 146–156. doi: 10.1016/j.envint.2016.04.005. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, Lindemans J, Russcher H, Steegers EA, Miedema HM, Jaddoe VW. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environmental health perspectives. 2012a;120:746–751. doi: 10.1289/ehp.1104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, Russcher H, Lindemans J, Miedema HM, Steegers EA, Jaddoe VW. Air pollution exposure and markers of placental growth and function: the generation R study. Environmental health perspectives. 2012b;120:1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace LA, Mitchell H, O’Connor GT, Neas L, Lippmann M, Kattan M, Koenig J, Stout JW, Vaughn BJ, Wallace D, Walter M, Adams K, Liu LJ, Inner-City Asthma Study Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003 Jul;111(9):1265–72. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJJ, Perstin J, Preston TJ, Wiley MJ, Wong AW. Oxidative Stress in Developmental Origins of Disease: Teratogenesis, Neurodevelopmental Deficits, and Cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: why and how. J Air Waste Manag Assoc. 2000 Jul;50(7):1167–83. doi: 10.1080/10473289.2000.10464164. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell ML, Choi H, Glinianaia S, Hoggatt KJ, Karr CJ, Lobdell DT, Wilhelm M. Methodological issues in studies of air pollution and reproductive health. Environmental research. 2009;109:311–320. doi: 10.1016/j.envres.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian SV, Wright R. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cienc Saude Coletiva. 2008;13:1729–1742. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Doi H. Outdoor air pollution and term low birth weight in Japan. Environ Int. 2015;74:106–111. doi: 10.1016/j.envint.2014.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


