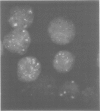
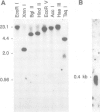
Abstract
A pore-forming peptide is implicated in the potent cytolytic activity of pathogenic Entamoeba histolytica. Using NH2-terminal sequence information of this peptide, the corresponding cDNA was isolated. The cDNA-deduced amino acid sequence revealed a putative signal peptide and a mature peptide of 77 amino acids including six cysteine residues. Computer-aided secondary structure analysis predicted that the peptide would be composed of four adjacent alpha-helices, and CD spectroscopy indicated an all alpha-helical conformation. The tertiary structure appears to be stabilized by three disulfide bonds; the pore-forming activity was not sensitive to heat but was lost in the presence of reducing agents. Sequence homology was found to the saposins and to surfactant-associated protein B, both mammalian polypeptides of similar size and secondary structure but of non-lytic function. In particular, the six cysteine residues were found to be conserved, suggesting a common motif for stabilizing a favourable tertiary structure. Compared with previously characterized toxic peptides also containing three disulfide bonds, the amoeba peptide may represent a distinct class of biologically active peptides.
Full text
PDF
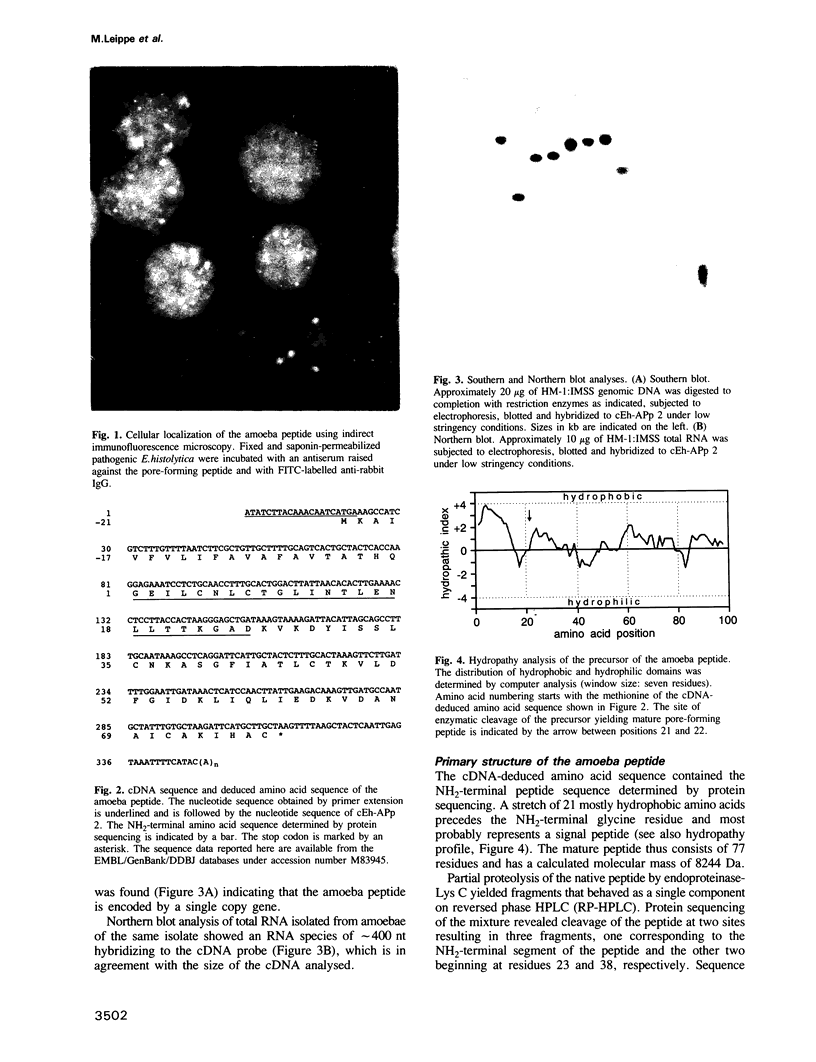
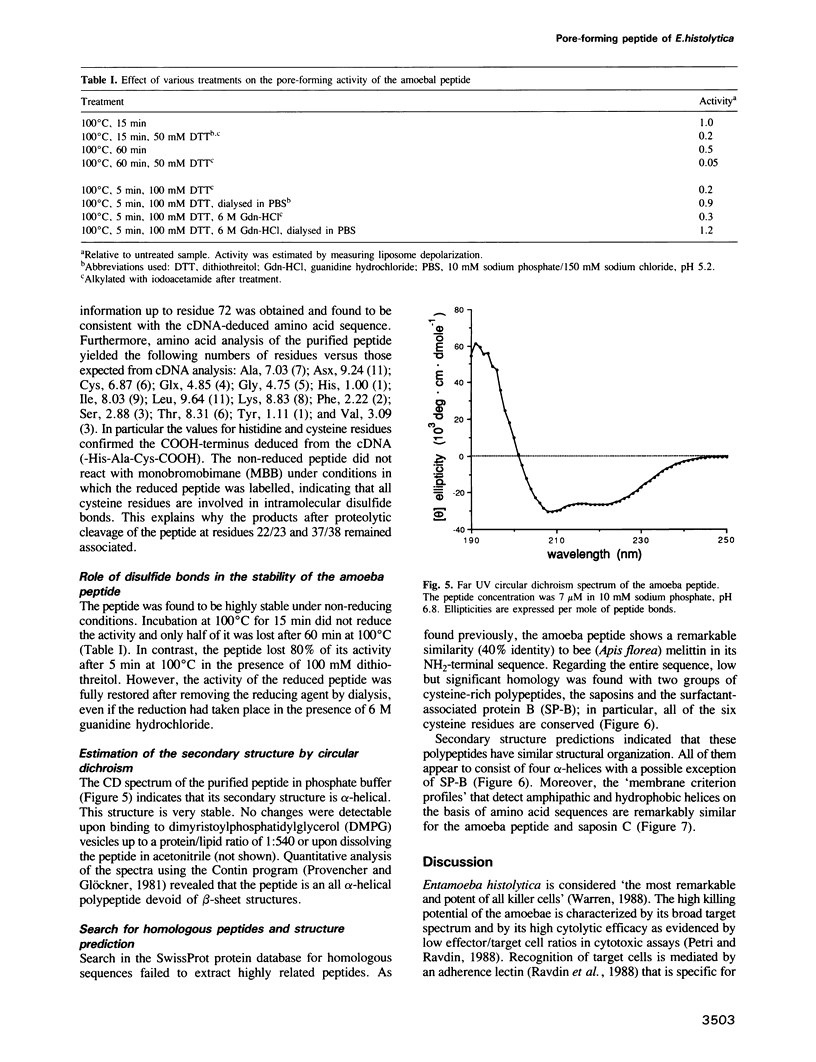


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bontems F., Roumestand C., Gilquin B., Ménez A., Toma F. Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science. 1991 Dec 6;254(5037):1521–1523. doi: 10.1126/science.1720574. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gitler C., Calef E., Rosenberg I. Cytopathogenicity of Entamoeba histolytica. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):73–85. doi: 10.1098/rstb.1984.0110. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Hill C. P., Yee J., Selsted M. E., Eisenberg D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991 Mar 22;251(5000):1481–1485. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- Hollecker M., Larcher D. Conformational forces affecting the folding pathways of dendrotoxins I and K from black mamba venom. Eur J Biochem. 1989 Jan 15;179(1):87–94. doi: 10.1111/j.1432-1033.1989.tb14524.x. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Secondary structures of proteins and peptides in amphiphilic environments. (A review). Proc Natl Acad Sci U S A. 1983 Feb;80(4):1137–1143. doi: 10.1073/pnas.80.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. Int J Pept Protein Res. 1989 Oct;34(4):277–286. doi: 10.1111/j.1399-3011.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Leippe M., Ebel S., Schoenberger O. L., Horstmann R. D., Müller-Eberhard H. J. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7659–7663. doi: 10.1073/pnas.88.17.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Rosenberg I., Bridge M., Gitler C. Diffusion potential cascade. Convenient detection of transferable membrane pores. Biochemistry. 1983 Feb 15;22(4):837–844. doi: 10.1021/bi00273a020. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Rosenberg I. M., Gitler C. An ion-channel forming protein produced by Entamoeba histolytica. EMBO J. 1982;1(7):801–804. doi: 10.1002/j.1460-2075.1982.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Yamamoto Y., Kretz K. A., O'Brien J. S., Kishimoto Y. Saposin A: second cerebrosidase activator protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S., Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991 Mar 1;5(3):301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of the precursor of pulmonary surfactant-associated protein SP-B with prosaposin and sulfated glycoprotein 1. J Biol Chem. 1991 Apr 5;266(10):6035–6037. [PubMed] [Google Scholar]
- Pattus F., Heitz F., Martinez C., Provencher S. W., Lazdunski C. Secondary structure of the pore-forming colicin A and its C-terminal fragment. Experimental fact and structure prediction. Eur J Biochem. 1985 Nov 4;152(3):681–689. doi: 10.1111/j.1432-1033.1985.tb09248.x. [DOI] [PubMed] [Google Scholar]
- Pattus F., Massotte D., Wilmsen H. U., Lakey J., Tsernoglou D., Tucker A., Parker M. W. Colicins: prokaryotic killer-pores. Experientia. 1990 Feb 15;46(2):180–192. [PubMed] [Google Scholar]
- Peitsch M. C., Amiguet P., Guy R., Brunner J., Maizel J. V., Jr, Tschopp J. Localization and molecular modelling of the membrane-inserted domain of the ninth component of human complement and perforin. Mol Immunol. 1990 Jul;27(7):589–602. doi: 10.1016/0161-5890(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B., Finkelstein A. V. Theory of protein secondary structure and algorithm of its prediction. Biopolymers. 1983 Jan;22(1):15–25. doi: 10.1002/bip.360220105. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. A review of the parasite cellular mechanisms involved in the pathogenesis of amebiasis. Rev Infect Dis. 1982 Nov-Dec;4(6):1185–1207. doi: 10.1093/clinids/4.6.1185. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I. Immunobiology of human infection by Entamoeba histolytica. Pathol Immunopathol Res. 1989;8(3-4):179–205. doi: 10.1159/000157148. [DOI] [PubMed] [Google Scholar]
- Rosenberg I., Bach D., Loew L. M., Gitler C. Isolation, characterization and partial purification of a transferable membrane channel (amoebapore) produced by Entamoeba histolytica. Mol Biochem Parasitol. 1989 Mar 15;33(3):237–247. doi: 10.1016/0166-6851(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin V. K., Gupta S., Leung T. K., Taylor V. E., Ohning B. L., Whitsett J. A., Fox J. L. Biophysical and biological activity of a synthetic 8.7-kDa hydrophobic pulmonary surfactant protein SP-B. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2633–2637. doi: 10.1073/pnas.87.7.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Strydom D. J. Homology of functionally diverse proteins. J Mol Evol. 1977 Aug 5;9(4):349–361. doi: 10.1007/BF01796098. [DOI] [PubMed] [Google Scholar]
- Tannich E., Bruchhaus I., Walter R. D., Horstmann R. D. Pathogenic and nonpathogenic Entamoeba histolytica: identification and molecular cloning of an iron-containing superoxide dismutase. Mol Biochem Parasitol. 1991 Nov;49(1):61–71. doi: 10.1016/0166-6851(91)90130-x. [DOI] [PubMed] [Google Scholar]
- Tannich E., Horstmann R. D. Codon usage in pathogenic Entamoeba histolytica. J Mol Evol. 1992 Mar;34(3):272–273. doi: 10.1007/BF00162976. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Molecular mechanisms of cytotoxicity mediated by Entamoeba histolytica: characterization of a pore-forming protein (PFP). J Cell Biochem. 1985;29(4):299–308. doi: 10.1002/jcb.240290404. [DOI] [PubMed] [Google Scholar]
- Young J. D., Young T. M., Lu L. P., Unkeless J. C., Cohn Z. A. Characterization of a membrane pore-forming protein from Entamoeba histolytica. J Exp Med. 1982 Dec 1;156(6):1677–1690. doi: 10.1084/jem.156.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]