Abstract
Human pluripotent stem cells (hPSCs), including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), have great potential as an unlimited donor source for cell-based therapeutics. The risk of teratoma formation from residual undifferentiated cells, however, remains a critical barrier to the clinical application of these cells. Herein we describe external beam radiation therapy (EBRT) as an attractive option for the treatment of this iatrogenic growth. We present the evidence that EBRT is effective in arresting growth of hESC-derived teratomas in vivo at day 28 post-implantation by utilizing a microCT irradiator capable of targeted treatment in small animals. Within several days of irradiation, teratomas derived from injection of undifferentiated hESCs and hiPSCs demonstrated complete growth arrest lasting several months. In addition, EBRT reduced re-seeding potential of teratoma cells during serial transplantation experiments, requiring irradiated teratomas to be seeded at 1×103 higher doses to form new teratomas. We demonstrate that radiation induces teratoma cell apoptosis, senescence, and growth arrest, similar to established radiobiology mechanisms. Taken together, these results provide proof of concept for the use of EBRT in the treatment of existing teratomas and highlight a strategy to increase the safety of stem cell-based therapies.
Keywords: Pluripotent stem cells, Cancer, Irradiation, Tumor cell purging
Graphical Abstract
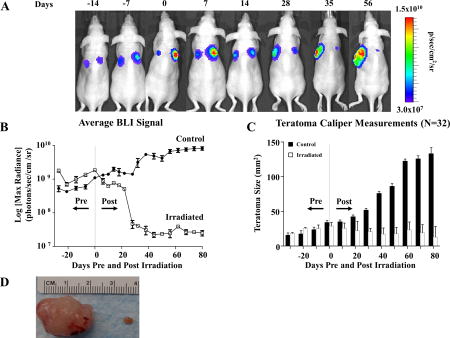
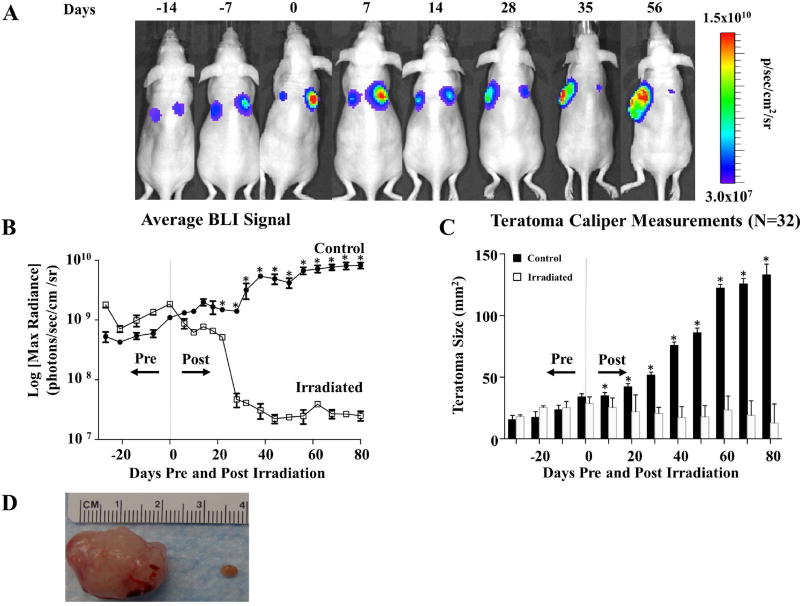
Irradiation arrests hESC-derived teratoma growth in vivo. (A) Representative BLI of teratoma growth and irradiation for immunodeficient mice seeded with 1×106 H9 hESCs constitutively expressing FLuc-GFP on both dorsal flanks. At 28 days post-transplantation (day 0 pre-radiation), the larger of the two teratomas (right side) in this example was irradiated with 6 Gy daily for 3 continuous days (days 28–30 post injection) for a cumulative dosage of 18 Gy while the non-irradiated contralateral teratoma served as control as an un-irradiated control. A subsequent decrease in luciferase signal was observed on the irradiated side, whereas the non-irradiated teratomas demonstrated a progressively increasing luciferase signal. (B) Quantification of teratoma growth over time using BLI of luciferase signal from H9 hESC derivatives demonstrated growth arrest of irradiated tumors vs. control. (C) Changes in in vivo caliper measurements of teratomas over time. Non-irradiated teratomas increased in size over time, whereas irradiated teratomas decreased in size. (D) Explanted gross teratoma specimens from day 130 post seeding. Note the significant reduction in mass in the irradiated teratoma on the right compared to the non-irradiated teratoma on the left. *p <0.05
INTRODUCTION
Recent reports of tumor formation in victims of unregulated “stem cell tourism” have heightened concerns about the safety of stem cell transplantation [1–3]. Because transplantations frequently occur at or near anatomically sensitive sites (e.g., spinal cord, brain, heart, or eye), even small tumors can be clinically devastating and may result in significant dysfunction. Thus far, strategies to control stem cell misbehavior have focused on the identification and removal of unwanted cells pre-transplantation [4, 5]. Ensuring a 100% pure cell product, however, remains challenging. Pre-transplant cell separation methods have been criticized for their low efficiency, high expense, and potential impairment of cell survival and engraftment [6]. Thus, it is critical that patients be monitored post injection for teratoma development using non-invasive imaging. In combination with specific biomarkers (e.g., CEA, AFP, HCG), magnetic resonance imaging, for example, has been able detect teratomas >17 mm3 in rodents with a sensitivity of >87% [7], enabling early treatment. Perhaps the most well studied therapeutic strategy is the incorporation of “suicide” genes into stem cells [8]. This approach, however, has largely relied on viral integration of kill switches and remains a topic of regulatory discussion.
In this study, we evaluate the feasibility of external beam radiation therapy (EBRT) [9], one of the primary modalities employed in oncologic treatment of solid tumors, for the treatment of hPSC-derived teratomas in mice. Using this platform, we demonstrate the ability of EBRT to decrease teratoma load in vivo for both hESCs and hiPSCs. Furthermore, we explore the underlying mechanisms of teratoma eradication by investigating the efficacy of EBRT to induce growth arrest, senescence, and disruption of vasculature, as well as to reduce the re-seeding potential of hPSC-derived teratomas.
RESULTS AND DISCUSSION
Radiation effects on hESC-derived teratomas were initially tested using a H9 hESC line that constitutively expresses the luciferase-GFP (FLuc-GFP) fusion protein [10, 11]. A murine in vivo model was employed in which teratomas were seeded contra-laterally via the subcutaneous injection of 1×106 H9 hESCs on both dorsal flanks of the same immunodeficient mouse. At 28 days post-injection, a microCT irradiator was used to treat the larger of the two teratomas, which was irradiated with 6 Gy of radiation for 3 continuous days for a cumulative dosage of 18 Gy. The non-irradiated contralateral teratoma served as control (Supplemental Figure 1A–C and Supplemental Figure 2). Compared to non-irradiated teratomas that grew by over 1 order of magnitude as measured by bioluminescence imaging (BLI) (p<0.001) (Figure 1A–B), irradiated teratomas had a 1–2 log decrease in luciferase signal (n=32 per teratoma group). BLI results were confirmed by weekly caliper measurements as well as via gross histology of explanted teratomas (p<0.001, Figure 1C–D). Importantly, the growth of irradiated teratomas was inhibited indefinitely following treatment until the mice were sacrificed. Taken together, these findings demonstrated the capacity of radiotherapy treatment to significantly hinder hESC-derived teratoma growth in vivo.
Figure 1.
Irradiation arrests hESC-derived teratoma growth in vivo. (A) Representative BLI of teratoma growth and irradiation for immunodeficient mice seeded with 1×106 H9 hESCs constitutively expressing FLuc-GFP on both dorsal flanks. At 28 days post-transplantation (day 0 pre-radiation), the larger of the two teratomas (right side) in this example was irradiated with 6 Gy daily for 3 continuous days (days 28–30 post injection) for a cumulative dosage of 18 Gy while the non-irradiated contralateral teratoma served as control as an un-irradiated control. A subsequent decrease in luciferase signal was observed on the irradiated side, whereas the non-irradiated teratomas demonstrated a progressively increasing luciferase signal. (B) Quantification of teratoma growth over time using BLI of luciferase signal from H9 hESC derivatives demonstrated growth arrest of irradiated tumors vs. control. (C) Changes in in vivo caliper measurements of teratomas over time. Non-irradiated teratomas increased in size over time, whereas irradiated teratomas decreased in size. (D) Explanted gross teratoma specimens from day 130 post seeding. Note the significant reduction in mass in the irradiated teratoma on the right compared to the non-irradiated teratoma on the left. *p <0.001.
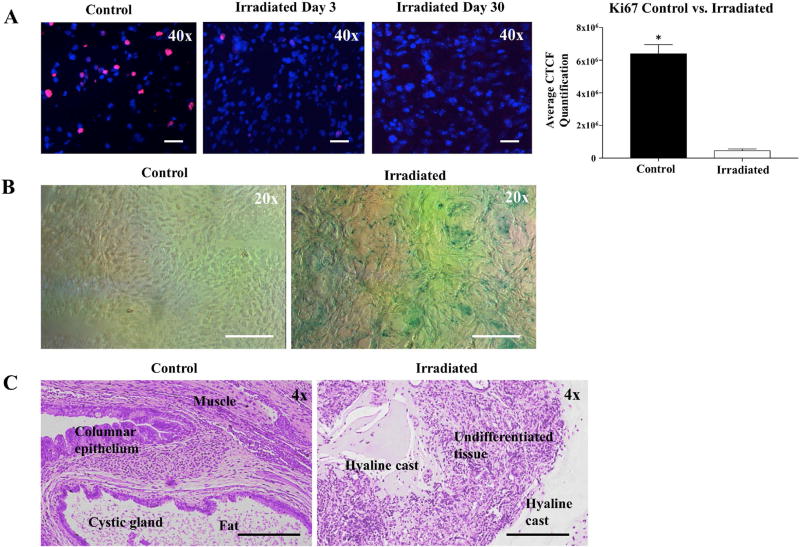
To confirm that treated teratomas were exposed to ionizing radiation, a subset of teratomas (n=3 per group) were explanted immediately after microCT irradiation and stained for γ-H2AX, a marker of DNA double stranded breaks. Teratomas treated with radiation demonstrated positive staining for both γ-H2AX and TUNEL, signifying the presence of DNA damage and initiation of apoptotic pathways, respectively (Supplemental Figure 3A–B). To investigate the mechanisms by which radiotherapy halts teratoma growth, we next assessed cellular proliferation and senescence. Radiation exposure resulted in a sharp decline in Ki67 staining, a marker of dividing cells, at day 0 compared to day 3 with a near-complete elimination of positive staining by day 30 (Figure 2A). In addition, we found that irradiated teratomas demonstrated significantly higher levels of cellular senescence than control counterparts as shown by increased β-galactosidase staining at day 30 (Figure 2B). Finally, to assess the effects of radiation upon structural integrity of hESC-derived teratomas, we compared the histology of non-irradiated and irradiated teratomas at week 14 post-treatment. Although H&E staining of control teratomas demonstrated an expected abundance of differentiated tissues from all three germ layers, irradiated teratomas exhibited aberrant structural morphology with numerous hyaline casts replacing cell depots (Figure 2C). Taken together, these results suggest that EBRT induced cellular apoptosis and cell division arrest, followed by cellular senescence (Supplemental Figure 4) [12, 13], which resulted in hyaline casting and inhibition of differentiated tissue growth, largely paralleling established therapeutic mechanisms in radiobiology [14].
Figure 2.
Irradiation arrests cell growth and induces senescence in teratomas. (A) Cellular proliferation as measured through Ki67 staining. Irradiated cells demonstrated progressively decreasing Ki67+ cells at days 3 and 30 compared to the non-irradiated control. Quantification of Ki67 staining at day 30 also revealed significantly higher Ki67 levels in non-irradiated cells than in irradiated cells. (B) Irradiated cells stained blue-green, demonstrating greater staining for the senescence marker β-galactosidase than non-irradiated control cells. (C) H&E stained sections of explanted tissues from non-irradiated teratomas (control) and irradiated teratomas. Control teratomas demonstrated normal differentiated teratoma histology and contained mature derivatives from all 3 germ layers. Irradiated teratomas contained hyaline casts that have sloughed off from dead cells. Furthermore, irradiated teratomas were found to lack derivatives from all 3 germ layers, indicating that irradiation exposure causes cell death and inhibits differentiation of tumorigenic stem cells. *p <0.01.
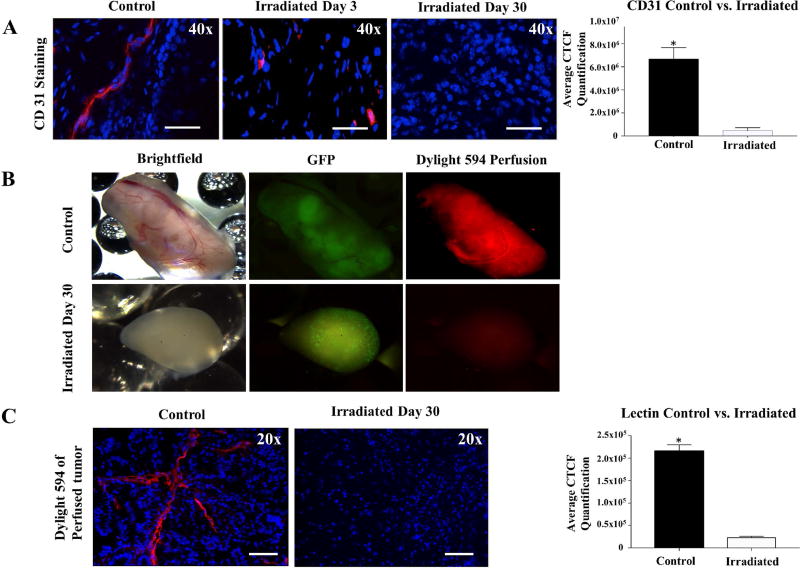
Previous studies have demonstrated that therapeutic effects of irradiation upon cancers are mediated by the destruction of tumor vasculature [15]. To explore the mechanisms contributing to teratoma growth arrest, we assessed the vascular component of treated teratomas before and after irradiation. Immunostaining of irradiated teratomas for CD31, a marker for vascular endothelial cells, revealed a significant decrease in CD31+ structures as early as 3 days post-irradiation (Figure 3A). By day 30 following treatment, irradiated teratomas exhibited low levels of CD31 staining, indicating that radiation resulted in the destruction of teratoma vasculature. Perfusion of animals bearing control versus irradiated teratomas with a DyLight 594 Tomato Red Lectin dye 30 days after treatment confirmed the absence of functional vasculature in irradiated teratomas (Figure 3B). As Tomato Red Lectin is a stable glycoprotein with a biodistribution pattern limited largely to the vasculature [16], analysis of DyLight 594 fluorescence allowed for the assessment of the quantity and integrity of functional vessels within the teratoma. Histological analysis of the perfused teratomas revealed a near absence of vessels carrying Lectin dye in teratomas at 30 days post-radiation, in contrast to control teratomas, which were well-perfused by the Lectin dye (Figure 3C). These results indicate that radiation disrupted teratoma vasculature and constitute a vital mechanism by which teratoma growth may be inhibited.
Figure 3.
Irradiation results in disruption of vascular supply to teratomas in vivo. (A) CD31 staining demonstrated a decrease in vascular endothelial cells at days 3 and 30 following radiation exposure. Quantification of CD31 staining revealed destruction of teratoma vasculature following irradiation. (B) Perfusion of animals bearing control and irradiated teratomas 30 days after radiation treatment with DyLight 594 Lectin dye confirmed presence of functional vasculature in non-irradiated teratomas and absence of functional blood vessels in irradiated teratomas. (C) DyLight 594 staining of a section of the perfused teratoma. Quantification of the Tomato Red Lectin staining at day 30 confirmed significantly higher fluorescence levels in non-irradiated cells than in irradiated cells. *p <0.01.
Data from published studies have shown that undifferentiated hPSCs demonstrate hypersensitivity to radiation-induced cell death [17]. To confirm that undifferentiated hPSCs demonstrate hypersensitivity to radiation-induced cell death, we next irradiated undifferentiated FLuc-GFP H9 hESCs [11]. In vitro irradiation of undifferentiated hESCs with a single dose of 18 Gy resulted in rapid reduction of luciferase signal by BLI as well as destruction of hESC colonies under light microscopy (Supplementary Figure 5A–C). This observation is in agreement with findings from previous studies, which showed hESCs to be highly susceptible to apoptotic stimuli such as genotoxic stress [18].
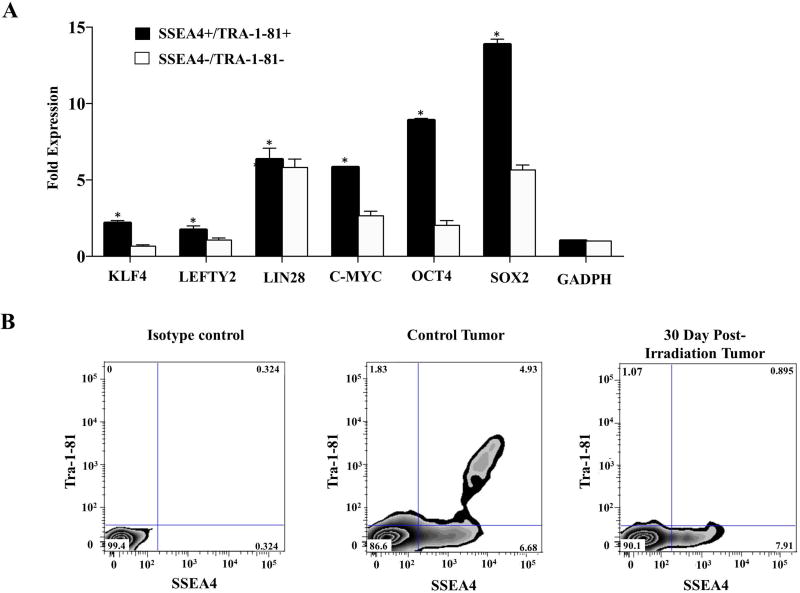
We next aimed to determine whether the selective effects of radiation on undifferentiated pluripotent stem cells contributed to growth arrest following teratoma irradiation. We first dissociated teratomas at day 30 post-injection and used flow cytometry to quantitatively assess for presence of cell populations expressing cell surface markers associated with pluripotency. Dissociated teratomas were observed to contain a small population of cells co-expressing both SSEA-4 and Tra-1-81. Single cell PCR of teratoma digests confirmed that teratoma cells co-expressing SSEA-4 and Tra-1-81 expressed higher levels of pluripotency transcription factors than cells in the SSEA-4/Tra-1-81 double-negative fraction (Figure 4A) [19, 20].
Figure 4.
Irradiation reduces residual teratoma cell populations that express markers of pluripotency. (A) Comparison of pluripotent gene expression in SSEA-4/TRA-1-81 double-positive and double-negative cell populations. (B) Explanted teratomas were dissociated prior to irradiation and at day 30 post-irradiation. Flow cytometry was used to quantitatively assess for presence of cell populations expressing the pluripotency cell surface markers Tra-1-81 and SSEA-4. Irradiation was found to decrease the SSEA-4/TRA-1-81 double-positive fraction of teratoma cells. *p <0.05.
To test the effects of EBRT on SSEA-4/Tra-1-81 double-positive cells, we next dissociated teratomas 30 days post-irradiation and performed analysis of SSEA-4 and TRA-1-81 expression by flow cytometry. Irradiation was found to markedly decrease the number of SSEA-4/TRA-1-81 double-positive teratoma cells (Figure 4B). Interestingly, there was a larger degree of down-regulation of Tra-1-81 compared to SSEA-4, likely due to the documented presence of SSEA-4+ on non-pluripotent cell types such as stromal cells of mesenchymal origin [21, 22].
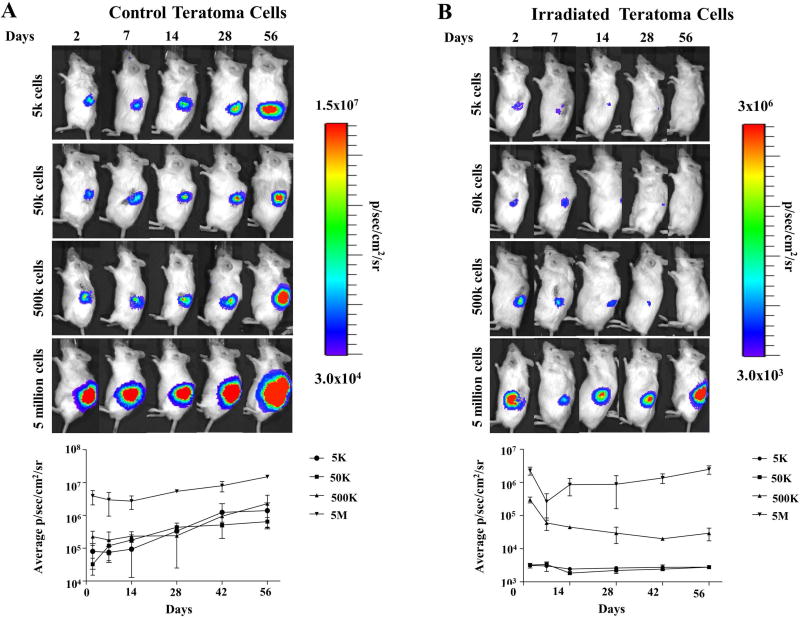
We next tested the capacity of irradiated teratoma cells to serially re-seed teratomas in vivo. Control and irradiated teratomas were digested into single cell suspensions 3 days after irradiation and transplanted into the kidney capsule of SCID mice, a site especially conducive to teratoma formation due to its high vascularity [23]. Whereas non-irradiated teratoma cells were able to readily reform teratomas with as few as 5×103 cells, teratoma cells from irradiated teratomas digests required a minimum of 5×106 cells to re-seed tumors (Figure 5A–B). The higher number of teratoma cells required to re-form teratomas following irradiation suggests there is a direct effect of irradiation upon the tumorigenicity of teratoma cells. However, as irradiated cells were still able to re-form teratomas albeit at 3-fold higher seeding doses, we believe other factors such as disruption of the host vasculature also play a pivotal role in the observed effects of radiation on teratoma growth arrest. To assess the relationship of tumorigenicity and the presence of pluripotent cells, we compared expression of pluripotent markers (e.g., Tra-1-81 and SSEA-4) in un-irradiated and irradiated teratomas with hESCs serving as control. Non-irradiated teratomas were found to harbor a small population of double-positive cells not observed in irradiated teratomas, suggesting that this population of double-positive cells is associated with increased tumorigenicity (Supplemental Figure 6). To ensure that the effects of radiation we observed were not limited to the H9 hESC line, we also performed identical treatment assays in a hiPSC line expressing the FLuc-GFP reporter gene and found similar results (Supplemental Figure 7). The susceptibility of hiPSCs suggests that findings from irradiation may be extrapolated to other hPSC lines as well.
Figure 5.
Irradiation inhibits serial seeding capacity of teratoma cells. (A) Control non-irradiated teratomas were digested into single cell suspensions and transplanted into the kidney capsule of SCID mice to test their ability to serially re-form teratomas. Control teratoma cells not exposed to radiation were able to readily reform teratomas with as few as 5×103 cells. (B) Irradiated teratomas were similarly explanted and digested into single cell suspensions 3 days after irradiation, then serially transplanted into kidney capsules. A minimum of 5×106 cells were required to reform teratomas following irradiation.
Finally, to confirm the safety of EBRT, we stained for Ki67, TUNEL, and beta galactosidase in samples of the liver, intestine, and muscle taken 5 mm from irradiated sites (Supplemental Figure 8). The preservation of cell proliferation and absence of apoptosis and cellular senescence suggested that EBRT is an effective and safe strategy for the treatment of hPSC- induced teratomas.
CONCLUSION
The tumorigenic potential of hESCs and hiPSCs presents a major barrier to the translation of ongoing stem cell research [6, 24]. A thorough assessment of potential solutions for teratoma formation is thus essential for hPSCs to safely achieve clinical translation [25]. The results of our study provide a “proof of concept” that EBRT is a viable strategy to treat teratoma formation by damaging tumorigenic cells and disrupting tumor vasculature. hPSC-associated teratomas harbor small populations of SSEA-4/Tra-1-81 double-positive cells enriched for pluripotent gene expression. These cells can be selectively ablated by irradiation due to their radiosensitive nature with limited damage to surrounding tissue. Further optimization of this promising strategy may improve the safety of hESC- and hiPSC-based cell therapies, facilitating the future use of hPSC-derived cell products for patients.
Supplementary Material
Acknowledgments
We would like to thank the National Institutes of Health R01 HL132875, R01 HL123968, and R01 HL133272 (JCW); California Institute of Regenerative Medicine DR2A-05394 and RT3-077998 (JCW); Fondation Leducq Grant 11CVD02; American Heart Association 10SDG4280129 (PKN) and 13EIA14420025 (JCW); Burroughs Welcome Foundation 1015009 (JCW); Howard Hughes Medical Institute (AL); and Stanford Cardiovascular Institute (AL) for their support.
Footnotes
Author contributions
- Andrew Lee: Conception and design, collection and/or assembly of data, data analysis and interpretation; manuscript writing.
- Chad Tang: Collection and/or assembly of data, data analysis, manuscript writing.
- Wan Xing Hong: Collection and/or assembly of data
- Sujin Park: Collection and/or assembly of data
- Magdalena Bazalova: Collection and/or assembly of data
- Geoff Nelson: Collection and/or assembly of data
- Veronica Sanchez-Freire: Collection and/or assembly of data
- Isaac Bakerman: Collection and/or assembly of data
- Evgenios Neofytou: Collection and/or assembly of data
- Wendy Zhang: Collection and/or assembly of data
- Andrew Connolly: Collection and/or assembly of data, data analysis
- Charles K. Chan: Collection and/or assembly of data, data analysis
- Edward E. Graves: Conception and design, provision of study materials or patients
- Irving L. Weissman: Conception and design, provision of study materials or patients
- Patricia K. Nguyen: Collection and/or assembly of data, data analysis, and interpretation; manuscript writing.
- Joseph C. Wu: Conception and design, financial support, collection and/or assembly of data, data analysis and interpretation; final approval of manuscript writing
Disclaimers
None
References
- 1.Amariglio N, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuriyan AE, et al. Vision loss after intravitreal injection of autologous "stem cells" for AMD. N Engl J Med. 2017;376(11):1047–1053. doi: 10.1056/NEJMoa1609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz AL, et al. Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem-Cell Tourism". N Engl J Med. 2016 doi: 10.1056/NEJMc1600188. [DOI] [PubMed] [Google Scholar]
- 4.Tang C, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29(9):829–34. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MO, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riegler J, et al. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Reports. 2016;6(2):176–87. doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao F, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9(1):107–17. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, et al. Development of a micro-computed tomography-based image-guided conformal radiotherapy system for small animals. Int J Radiat Oncol Biol Phys. 2010;78(1):297–305. doi: 10.1016/j.ijrobp.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JC, et al. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4(4):297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 11.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc. 2009;4(8):1192–201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu PC, et al. Accelerated cellular senescence in solid tumor therapy. Exp Oncol. 2012;34(3):298–305. [PubMed] [Google Scholar]
- 13.Liao EC, et al. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 2014;5:e1255. doi: 10.1038/cddis.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31(4):363–72. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 16.Bergers G, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24(5):268–74. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson KD, et al. Effects of ionizing radiation on self-renewal and pluripotency of human embryonic stem cells. Cancer Res. 2010;70(13):5539–48. doi: 10.1158/0008-5472.CAN-09-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews PW, et al. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3(4):347–61. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 20.Kannagi R, et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–61. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gang EJ, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 22.Brimble SN, et al. The cell surface glycosphingolipids SSEA-3 and EA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25(1):54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- 23.Prokhorova TA, et al. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009;18(1):47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 24.Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 25.Neofytou E, et al. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125(7):2551–7. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.