Abstract
Background and aims
Elemental uptake in serpentine floras in eastern North America is largely unknown. The objective of this study was to determine major and trace element concentrations in soil and leaves of three native pseudo-metallophyte C4 grasses in situ at five sites with three very different soil types, including three serpentine sites, in eastern USA.
Methods
Pseudo-total and extractible concentrations of 15 elements were measured and correlated from the soils and leaves of three species at the five sites.
Results
Element concentrations in soils of pseudo-metallophytes varied up to five orders of magnitude. Soils from metalliferous sites exhibited higher concentrations of their characteristic elements than non-metalliferous. In metallicolous populations, elemental concentrations depended on the element. Concentrations of major elements (Ca, Mg, K) in leaves were lower than typical toxicity thresholds, whereas concentrations of Zn were higher.
Conclusions
In grasses, species can maintain relatively low metal concentrations in their leaves even when soil concentrations are richer. However, in highly Zn-contaminated soil, we found evidence of a threshold concentration above which Zn uptake increases drastically. Finally, absence of main characteristics of serpentine soil at one site indicated the importance of soil survey and restoration to maintain serpentinophytes communities and avoid soil encroachment.
Keywords: C4 grass, accumulation, excluder, Serpentine, Calamine, Pseudo-metallophytes
Introduction
Metal and metalloid trace elements have variable concentrations in parental bedrock that define natural background concentrations in soils (Gao et al. 1998; Alloway 2013). Based on variation in parent rock composition and alteration processes, some soils contain elements at concentrations well above global median values (Kabata-Pendias 2000). For example, serpentine soils derived from ultramafic bedrock is richer in Ni and Cr than other soils (Massoura et al. 2006; Alloway 2013); likewise, concentrations of Cd and Zn also vary with underlying bedrock type (Jyoti et al. 2015). Trace metals may also be elevated from anthropogenic sources such as smelting, military or agricultural activities (Alloway 2013). Indeed, metalliferous sites can be characterized as primary, secondary, or tertiary, based on whether soil metals occur naturally, are resulted from mining activities, or were deposited as pollution from smelters or other sources, respectively. Sites can also be classified by the particular elevated elements, which are mainly calamine (Cd, Pb and Zn), copper-cobalt outcrops (Faucon et al. 2016) and serpentine (Cr, Co, Ni) in temperate climate (Baker and Brooks 1989).
Metals in soils can have consequences for vegetation. At high concentrations, as in serpentine soils, trace elements can induce phytotoxicity and reduce plant fitness (Clemens 2001; Lin and Aarts 2012). Many species found in metalliferous sites, called metallophytes, are able to cope with elevated metal concentrations and form unique, endemic plant communities (Baker et al. 2010; Faucon et al. 2016; Isnard et al. 2016). Some plants called pseudo-metallophytes have the ability to grow on both metalliferous and non-metalliferous soils (Pollard et al. 2002; Pollard et al. 2014).
In the eastern US, many ultramafic outcrops are found along the Appalachian orogenic belt from Québec and Newfoundland south to Georgia and Alabama (Rajakaruna et al. 2009; Alexander 2009). These areas exhibit common soil properties of the “serpentine syndrome”: high Cr, Co and Ni concentrations in soil, Ca:Mg ratio below a value of 1.0, and deficiency of essential macronutrients (Brady et al. 2005; Kazakou et al. 2008). Serpentine sites are also characterized by poor plant productivity, high rates of endemism and distinct vegetation from those of neighboring areas (Whittaker 1954 in Brady et al. 2005). Most serpentine sites, termed serpentine barrens, in eastern North America were under ice during the last glaciation (Last Glacial Maximum, 23,000–15,000 years ago, Prentice et al. 1991; Williams 2003), which means they represent a relatively new ecosystem without much time for plant species divergence, but possibly for ecotypic variation (Burgess et al. 2015b). Compared to similar soils in unglaciated sites across the world, Eastern serpentine sites present only two endemic species (Harris and Rajakaruna 2009; Boufford et al. 2014), namely Adiantum viridimontanum (Pteridaceae) and Minuartia marcescens (Caryophyllaceae), whereas the Western serpentine sites contain more than 246 endemic species among 670 species (Anacker et al. 2011).
The serpentine plant community is mainly characterized by patches of typical grasslands or savannas, as islands embedded within Eastern deciduous forest. Disturbances such as mowing, mining, wildfires, and grazing help maintain grasslands or savannas on Eastern serpentine by prohibiting plant succession to forest (Latham 1993; Arabas 2000; Burgess et al. 2015a). Although Eastern serpentine may contain patches of unusual woodland and wetland communities, these sites are dominated by grasses, particularly several C4 grasses that are also major components of tallgrass prairie of the Great Plains in Midwest Region (Bever et al. 1996; Ji et al. 2013). Despite the importance of this grass species on serpentine outcrops, little has been investigated about their metal uptake properties in part, because no known hyperaccumulator species are found there (Rajakaruna et al. 2009).
Tolerant metallophytes exhibit alternate adaptive traits enabling them to either resist uptake or detoxify trace elements within their tissues (Baker 1981; Baker 1987). They present three different patterns of metal homeostasis: excluders maintain low concentrations in above-ground tissue over a large range of soil concentrations, indicators show similar concentrations as soils, and hyperaccumulators concentrate trace elements in above-ground tissue parts independent of soil levels (Baker 1981; Van der Ent et al. 2013; Pollard et al. 2014). To date, research has focused on hyperaccumulators because they hold promise for detoxifying metal-polluted soils through phytoremediation–mainly phytoextraction and phytomining (Cunningham et al. 1995; Ali et al. 2013; Van Der Ent et al. 2015).
Learning more about the metal uptake properties of other tolerant species, especially grasses, is important because of their utility in stabilizing and revegetating metal contaminated areas (Chaney et al. 1997; Li et al. 2000), which can be new environments for these plants. A good example is the revegetation of the superfund site called Palmerton, a calamine site managed by the Le-high Gap Nature Center. The north-facing slope of the mountain was contaminated with chalcophile elements (cadmium, lead, and zinc) due to emission from two zinc smelters operated from roughly 1898 until 1980. Revegetation of the site began in 2002. Our samples for this study were collected in an area that received aerial application of fertilizer, lime, and a mixture of different cultivars of grass seeds (Latham et al. 2007a), including several C4 grasses. Currently, information about elemental concentrations are scare and come from greenhouse experiments (Glassman and Casper 2012). Not all elements and their interaction were determined.
To date, we know of no comprehensive study that examines uptake for a suite of metals in a native grasses species in metalliferous (serpentine and calamine) and in non-metalliferous sites. Since pseudo-metallophytes can still show higher metal concentrations on metalliferous soils, they can cause metallicolous and non-metallicolous congeneric populations to be considered sub-species (Boyd and Martens 1998) or simply different ecotypes of the species or edaphic type (Pauwels et al. 2005; Gonneau et al. 2014; Burgess et al. 2015b).
We aim to examine variation and correlation of major and trace element concentrations in soils from different sites that include three soil types: serpentine (primary and secondary metalliferous), calamine (tertiary metalliferous), and non-metalliferous site. We expected that concentrations of characteristic elements for two types of metalliferous sites were higher than in the non-metalliferous site and with higher variation of the other elements. We also examine the variation associated with individuals (local variation) and species within site for all elements. Furthermore, we compare metal uptake in the three native grasses, which are considered to be metal excluding plants, across the different soils. To achieve these objectives, we measured trace element concentrations in soils and in aboveground plant tissues in sites. The three species we target, Andropogon gerardii, Schizachyrium scoparium, and Sorghastrum nutans, are dominant C4 grasses in Eastern North America. We sampled these grasses from three serpentine grasslands, one calamine site polluted by nearly 100 years of zinc smelting, and one non-metalliferous site where land management practices maintain a grassland.
Materials and methods
Site studies and sampling
In fall 2014, leaves (less than two grams) and rooting soil (approximatively 1.0 l using a stainless steel shovel) from the same plants were collected from five sites corresponding to three edaphic type: serpentine group (SERP) with three sites, calamine (CAL, one site), and nonmetalliferous (NMET, one site). The serpentine sites are located in Chester County, PA (Rajakaruna et al. 2009): Nottingham (NOT, 39°44″8.53″N 76° 2″9.94″ W) in Nottingham County Park; Sugartown (SGW, 40°00″22.7″N 75°31″56.7″W) within the boundaries of Willisbrook Preserve; and Unionville (UNI, 39°55″ 15.2″N 75°43″30.9″W), mostly within the boundaries of the ChesLen Preserve. The calamine site is located on Blue Mountain, near the town of Palmerton in Carbon County, PA (PAL, 40°47′9.60″N 75°37′8.40″W) The non-metalliferous site is located at Fort Indiantown Gap in Lebanon County, PA (FIG, 40°26′24.2″N 76°34′53.15″W). It is a military training area managed by the Pennsylvania National Guard (Latham et al. 2007b; Ferster et al. 2008). Grassland vegetation is maintained by removing woody vegetation mechanically or by fire.
At each site, we collected eight individuals with at least 30 m fair away for each other of each of three C4 grass species: Andropogon gerardii (A. ger), Sorghastrum nutans (S. nut), and Schizachyrium scoparium (S. sco). Due to loss during the drying process, the soils from Palmerton (PAL) were analyzed for five individuals of each species.
Plant and soil analysis
Leaf samples were cleaned three times tap water and one time with ultrapure water (18 mΩ.cm), dried for 42 h at 50 °C, and crushed into small pieces according to Rees et al. (2015). Crushed leaves (0.5 g) were digested with a mixture of HNO3/H2O2 using a DigiPrep system (SCP science, USA) following a method outlined in Gonneau et al. (2014). For N measurement, approximately 0.25 g of fine ground material (< 200 μm) was weighed into a tin capsule (Elemental Microanalysis, Okehampton, Devon, UK) on a microbalance. Analysis of N concentrations was conducted by combustion using Vario Max C/N analyser (Elementar, Germany).
All of the soils were dried at 65 °C for 48 h and sieved to remove grains with size above 2.0 mm. Soil pH was measured from a solution consisting of 1:5 ratio of soil to ultrapure water. Pseudo-total concentrations (an assessment of the maximum potentially soluble element and not bound in silicates) of major elements (Al, Ca, Fe, K and Mg) and trace elements (Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn) were determined by EPA method 3050, which involves digestion of 1.0 g ground soil (< 200 μm) in aqua regia (mixture of HNO3/H2O2/HCl) in a DigiPrep system.
The soil extractible quantity of each element, the fraction considered available to plants, was measured as follows. Phosphorus was extracted by mixing 1.0 g of soil with a solution of 0.025 N HCl and 0.03 N NH4F (Bray-1) for 5 min. The amount of phosphorus was determined by colorimetric analysis at 880 nm (Hach, DR 6000, USA). To extract other elements, we used two solutions: DTPA (Al, Cd, Co, Cr, Cu, Mn, Ni and Pb), and ammonium acetate (Ca, K, Mg, Na). For the DTPA extraction, 10 g of soil was mixed in 20 mL of 0.005 M DTPA +0.01 M CaCl2 + 0.01 M TEA at pH 7.3 for 2 h (Lindsay and Norvell 1978). For the ammonium acetate extraction, 1 g of soil was mixed in 10 mL of 1 M ammonium acetate (NH4OAc) solution at pH 7.0 for 2 h (Thomas 1982) to determine cation-exchange capacity (CEC) and Ca, K, Mg, and Na. All solutions were filtered through a 0.45 μm membrane (nylon) before measuring the elemental concentration using inductive coupled plasma optical emission spectroscopy (ICP-OES, Spectro Genesis, Spectro Analytical Instruments, Germany). For each analysis, the instrument was calibrated using matrix-matched element standards, and ten blanks were used to determine quantification limits. A control solution (EuH4, SCP science, USA) was also analyzed to verify accurate values.
Data processing
All statistical analyses were performed using R v2.10.14. Before analysis, all data were log-transformed in order to improve normality and variance homogeneity of residuals. All parameters were analyzed by a nested two-ways analysis of variance with site and species nested within site followed by post-hoc analysis. Variance components were estimated for site, species within site, and samples within species within site. Variance components were estimated for each factor using the restricted maximum likelihood (REML) method with the lmer function inside the lme4 package. Constrained Analysis of Proximities (CAP), a constrained ordination technique) was used to examine the relationships between plant species identity, site and soil chemical characteristics using “capscale” command in the “vegan” package in R. Firstly, CAP analysis was performed on the pseudo-total concentrations in rooting soil samples and secondly for extractible concentration of elements and pH. A third CAP was used to examine elemental concentrations in leaves. For each CAP analysis, the permutation-based “anova.cca” method was performed to test the significance of site and species within site as a predictor of plant and soil chemistry, and the “plot.cca” method to visualize the results. Finally, for each edaphic group (SERP, CAL and NMET), multiple correlation tests between all soil and leaf parameters were performed using the Pearson method and FDR control for type I errors. Based on our analyses for extractible quantities of elements, we separated collections at the SGW serpentine site into different edaphic groups. We grouped collections under S. scoparium with those from NOT and UNI but made A. gerardii and S. nutans a group of their own.
Results
Variation of soils properties
All pseudo-total concentrations of major and trace elements showed significant differences between sites and, for some elements, differences between species (Table 1). Pseudo-total and extractible concentrations of elements in rooting soil varied from one to five orders of magnitude (Tables S2 and S3). Percentage of variance associate from sample to site factor varied from 66.7% for Fe to 100% for Zn (Table S1). Sites more often explained a greater percentage of variance than species within site (Table S1).
Table 1.
Nested analyses of variance for pseudo-total and extractible concentrations of a) major and b) trace elements in rooting soil and in leaves accounted for Site and Species (Site)
| a) Source | Df | Al | Ca | Fe | K | Mg | Ca:Mg | N:P | P | |
| F | F | F | F | F | F | F | F | |||
| Pseudo-Total | ||||||||||
| Site | 4 | 10.1 *** | 22.8 *** | 12.1 ** | 29.2 *** | 56.4 *** | 127 *** | nd | nd | |
| Species (Site) | 9 | 1.3 ns | 2.91 * | 2.47 * | 2.49 * | 4.71 ** | 2.89 ns | nd | nd | |
| Residuals | 89 | |||||||||
| Extractible | ||||||||||
| Site | 4 | 55.9 *** | 20.7 *** | 26.7 *** | 0.537 ns | 96.5 *** | 193 *** | nd | 28.4 *** | |
| Species (Site) | 9 | 0.43 ns | 1.19 ns | 4.95 ** | 2.03 * | 3.24 ** | 5.18 ** | nd | 4.83 ** | |
| Residuals | 89 | |||||||||
| Leaves | ||||||||||
| Site | 4 | 4.3 ** | 72.1 *** | 23.2 *** | 10.6 *** | 56.3 *** | 144 *** | 14.2 *** | 18.2 *** | |
| Species (Site) | 9 | 5.52 *** | 10.8 *** | 0.706 ns | 0.761 ns | 5.66 *** | 11.2 *** | 5.1 *** | 8.44 *** | |
| Residuals | 98 | |||||||||
| b) Source | Df | Cd | Co | Cr | Cu | Mn | N | Ni | Pb | Zn |
| F | F | F | F | F | F | F | F | F | ||
| Pseudo-Total | ||||||||||
| Site | 4 | 930 *** | 58.8 *** | 102 *** | 45.2 *** | 16.5 ** | nd | 180 *** | 58.8 *** | 181 *** |
| Species (Site) | 9 | 2.39 * | 2.79 * | 2.48 ** | 3.37 ** | 1.96 ns | nd | 6.79 *** | 2.12 * | 2.36 * |
| Residuals | 89 | |||||||||
| Extractible | ||||||||||
| Site | 4 | 150 *** | 43.1 *** | nd | 3.29 * | 18.4 *** | nd | 91.2 *** | 8.26 *** | 65.2 *** |
| Species (Site) | 9 | 3.66 ** | 2.1 * | nd | 1.11 ns | 1.64. ns | nd | 4.81 *** | 3.86 ** | 2.92 * |
| Residuals | 89 | |||||||||
| Leaves | ||||||||||
| Site | 4 | 43.7 *** | 1.83 ns | 3.03 ns | 2.74 * | 19.9 *** | 1.92 ns | 1.97 ns | 16.3 *** | 116 *** |
| Species (Site) | 9 | 2.31 * | 1.32 ns | 1.75 ns | 6.17 *** | 1.85 ns | 2.47 * | 0.44 ns | 2.28 * | 1.24 ns |
| Residuals | 98 |
nd not determined. Significant level: ns p > 0.05;
p < 0.05;
p < 0.01;
p < 0.001
CAP analysis with all pseudo-total concentrations showed clear distinction of three types of soil based on trace elements concentrations but not a clear separation by species under which the soil was collected (Fig. 1a). Both site and species within site effect were highly significant (p < 0.001). CAP1 and CAP 2 explained 71.7% and 24.5% of variance respectively. Three main part were observed with (i) PAL, the calamine site, showed higher pseudo-total concentrations of Cd, Pb, and Zn than other sites (Table S2 and S3), (ii) FIG, and (iii) three serpentine sites, displaying the highest concentrations in Co, Cr and Ni. Compared to the two other serpentine sites, SGW had lower concentrations of Co and Ni (but not Cr).
Fig. 1.
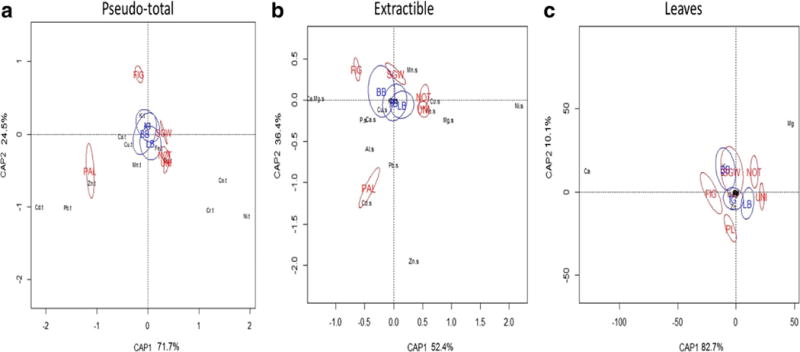
CAP ordination space of elemental concentrations in a pseudo-total, b extractible and c in leaves with position of species in blue and site in red. NOT: Nottingham, SGW: Sugartown, UNI: Unionville, PAL: Palmerton and FIG: Fort Indiantown Gap
Pseudo-total concentrations of other elements (Al, Ca,Cu, Fe, K, Mg and Mn) much variation in the CAP analysis or contribute to the separation of sites by edaphic group (Table S2 and S3)., Finally the Ca:Mg ratio in rooting soil in serpentine was characteristically lower than 1.0 and much lower than in FIG and PAL (Table S2). Extractible soil concentrations of all elements except K differed between sites (Table 1). Soil pH was similar between sites (Table S3). The total percentage of variance explained by site, species within site, and samples within species within site ranged from <10% for Cu and K to >90% for Al, Fe, P, Mn, and Zn (Table S1). The percentage of variance explained by site varied more among elements than the percentage of variance explained by species within site (Table S1). Concentrations of Cd, Cr and Pb were below detection limit for most individuals except in PAL (Table S2 and S3).
For extractible quantities of elements, CAP analysis likewise, showed significant differences according to site and species within site (Fig. 1b). CAP1 which explained 52.4% of the variance was negatively correlated with Ca:Mg ratio and positively correlated with Ni and Mg, thereby separating serpentine sites from FIG and PAL (Fig. 1b). Extractible concentrations of Cd, Pb and Zn were negatively correlated with the CAP2 axis, which explained 36.4% of the variance. These elements are highest in soils from PAL. Finally, soils from FIG and SGW were separated from NOT and UNI on this axis. Among species, A. gerardii and S. scoparium showed the greatest separation while S. nutans overlapped with both (Fig. 1b).
Examining extractible quantities of elements individually, Al, Ca, Cd, Cu, P, Pb and Zn were higher in PAL (Figs.2 and 3, Table S3). Mg concentrations were higher in NOT and UNI than in SGW and FIG (Fig.2). Ni concentrations were higher in NOT and UNI than in SGW and then in FIG and PAL (Fig. 2b). Al, Ca, Cu, and Mn differed also among species within sites (Table S2). At SGW A. gerardii and S. nutans showed higher Ca:Mg ratio (2.40 and 1.76, respectively) than S. scoparium (0.368) (Table S3); Ca:Mg ratio below 1.0 are typical on serpentine soil and are thought to range from 0.238 to 0.596. At FIG, soils under S. nutans and S. scoparium were more acidic (average pH 5.82 and 5.73 respectively) than soils under A. gerardii (average pH 6.86, p < 0.001).
Fig. 2.

Concentrations of Ca and Mg (mg kg−1) in five sites in ENA: pseudo-total, extractible and in leaves. NOT: Nottingham, SGW: Sugartown, UNI: Unionville, PAL: Palmerton and FIG: Fort Indiantown Gap
Fig. 3.

Concentrations of Ni and Zn (mg kg-1) in five sites in ENA: pseudo-total, extractible and in leaves. NOT: Nottingham, SGW: Sugartown, UNI: Unionville, PAL: Palmerton and FIG: Fort Indiantown Gap
Element concentrations in leaves
Concentrations in leaves differed among sites and species for many elements (Table 1). The total percentage of variance explained by site, species within site, and samples within species within site was less than 25% for K, Co, Cr, and Ni and greater than 90% for Al, Cu, Mn, N, N:P ratio and Pb (Table S1). For Al, Cu, Mn, N, N:P ratio and Pb, most of variance was explained by samples. Site explained a higher percentage of variance for Ca, Fe, Mg, Ca:Mg ratio, and Cd. Species within site explained a higher percentage of variance only for P.
Site and species within site were also significant by CAP analysis (p < 0.001; Fig. 1c). CAP1, which explained 82.7% of the variance, was negatively correlated with Ca and positively correlated with Mg (Fig. 1c). This axis separated the FIG site in the Ca direction and UNI/NOT in the Mg direction. CAP2, which explained 10.1% of the variance, was negatively correlated with Zn, separating PAL from the other sites. Species showed clear separation without an overlap, with a greater difference between A. gerardii and S. scoparium (Fig. 1c).
Consistent with serpentine syndrome, plants from the serpentine sites had higher Mg concentrations and lower Ca concentrations and Ca:Mg ratios (Table S4 and Fig. 2c). Only N, Co and Ni in leaf tissue did not vary between sites (Tables 1 and S4, Fig. 3). Cd, Pb, and Zn were higher in the calamine site PAL (Table S4 and Fig. 3). Concentrations of Cd were below 10 mg kg-1 and concentrations of Zn reached 1000 mg kg−1 at PAL (Fig. 3). Concentrations of Cd and Zn were below 5 mg kg−1 and 50 mg kg−1 in the other sites respectively (Fig. 3). Other elements did not present particular patterns by edaphic type (Table S4). N:P ratio were higher in NOT and UNI (N:P = 12) than three others sites with N:P < 9 (Table S4). Major elements showed more differences between species than did trace elements. Within sites, the greatest differences between three species were found at SGW with lower concentrations of Ca and Ca:Mg ratio for S. scoparium, lower concentrations of Mg and Na for S. nutans, and higher concentrations of Pb for A. gerardii. At UNI, S. scoparium showed higher concentrations of Cu than S. nutans, and at PAL A. gerardii showed the lowest concentrations of Mn (Table S4).
Correlation within soil and leaf parameters
Many of the concentrations for different elements were significantly correlated (p < 0.05), both in leaves and in rooting soils for four group from CAP analysis based on pseudo-total and extractible concentrations (Fig. 4 and S1). In order to describe the strongest correlations, only |r| values above 0.5 were taken into account.
Fig. 4.

Correlation between major and trace elements concentrations in leaves at a Nottingham, Unionville and S. scoparium at Sugartown, b soil from A. gerardii and S. nutans at Sugartown, c Palmerton and d Fort Indiantown Gap. The three species at Sugartown were separated into two groups after our soil analyses indicated that S. scoparium clustered with the serpentine group while A. gerardii and S. nutans did not
On this basis, some positive correlations among elements measured in leaves, such as Co, Cr and Pb, were very similar among the four groups (Fig. 4). K and P were positively correlated except in serpentine group. Al and Fe were positively correlated at PAL and FIG. In the serpentine group and FIG, there were positive correlations between Al and Co, Cr and Pb and Cu and Pb. There were positive correlations between K and Mn and between Mg and P for S. nutans and S. scoparium at SGW and for PAL. A positive correlation between Co and Zn, Cr and Zn, Mn and Zn was found only for FIG and for S. nutans and S. scoparium at SGW. Some of the strongest correlations between elements were found only in one group namely between Cu, Pb and Zn at FIG or between major elements such as Ca, K, N and P for individuals of S. nutans and S. scoparium at SGW. At PAL, Cd was postively correlated with Pb and Zn. A negative correlation between elements was less common. For S. nutans and S. scoparium at SGW Cd was negatively correlated with Co, Cr, Pb and Zn (Fig. 4b). At PAL, Ca and Mn were negatively correlated, and Mg and P were both negatively correlated with Cu and Zn (Fig. 4c).
Correlations between elemental concentrations in leaves with either pseudo-total or extractible soil concentrations were mostly weak or non-significant. Among the strong relationship between levels in soil and plant, were positive correlations in the serpentine group for Ni and Zn, a positive correlation for Mg and negative correlations for Cu and Pb at PAL, and a positive correlation for Ca at FIG (Table S5).
Pseudo-total concentrations of elements in soils were mainly positively correlated between them (Fig. S1). Pseudo-total concentrations of siderophile elements (Co, Cr, Ni and Mn) were positively correlated in soils. The exception was for Mn at PAL (Fig. S1). Major elements showed mostly positive correlations between pseudo-total and extractible concentrations. Except for a few cases, extractible elements were less correlated than pseudo-total concentrations. Trace elements, for example, showed lower correlations for FIG and PAL compared to serpentine sites. Cd and Zn were positively correlated for Palmerton for pseudo-total and extractible concentrations (Fig. S1).
Discussion
In this study, we evaluated characteristic element concentrations and their variability in rooting soil and in leaves of the three dominant C4 grasses (Andropogon gerardii, Schizachyrium scoparium, and Sorghastrum nutans). Soils properties showed important variability and not only for elements expected to be high in two metalliferous edaphic group. Results showed the importance of the measurement of many elementals in rooting soil since soil at SGW showed non-serpentine properties even if parental bedrock seems to correspond to this edaphic type. Three C4 species are tolerant to metalliferous soils properties and generally showed low trace element concentrations in leaves despite a wide range of concentrations in non-metalliferous to metalliferous soils. Only Zn, at a contaminated site, was considerably elevated in leaf tissue.
Soil composition under pseudo-metallophyte species
The metal composition of soils under the three C4 grasses were clustered more by the edaphic group and by site than by species identity, suggesting no spatial sorting of species by soil characteristics. The metalliferous sites studied here showed higher concentrations of trace elements than did the non-metalliferous site by one to five orders of magnitude. Pseudo-total concentrations of most trace elements in rooting soils were within the range of values found in 4857 soil sampling sites distributed in the contiguous United States (Smith et al. 2013; Woodruff et al. 2015). Concentrations in two metalliferous types of soils were mostly higher than the third quartiles of those samples, indicating metalliferous soils characterized by high concentrations.
We found three main distinguishing characteristics of ultramafic soils at two of three serpentine sites, NOTand UNI: elevated concentrations of some trace elements (especially nickel, cobalt, chromium), a Ca:Mg ratio < 1, and deficiency in essential plant nutrients like phosphorus, but not K (Brady et al. 2005; Massoura et al. 2006; Kazakou et al. 2010). While P concentrations in three serpentine sites did not differ from the non-metalliferous native grassland FIG (Table S3), P, measured by the Bray-1 method, was at least four to ten times lower than reported values for two non-metalliferous native grasslands in the Great Plains, Firmi and Cedar Creek where the focal grasses occur (Johnson et al. 2010). Similar differences were observed for P, measured using the Melich-3 method, among soils collected under the same species in East Coast serpentine sites and two other grasslands in Iowa (Ji et al. 2012); such differences were not found for NH4-N and NO3-N.
Due to their similar origin and affinity to iron (Goldschmidt 1937), relatively high pseudo-total concentrations of Ni, Co, and Cr, which were higher in serpentine, are found together in soils. Extractible concentrations of Ni in serpentine soils were one order of magnitude higher than in others sites, but there was less of difference between serpentine and others sites for Cr and Co. Cr is not detected by DTPA extraction, as it is mainly included in resistant mineral phases, which do not dissolve significantly during this extraction. Indeed, both extractible quantities and the mobility of these three elements differ. Analyzing the mobility of Ni, Cr, and Co in six serpentine sites in Poland, Kierczak et al. (2016) showed that the highest mobile element was Ni whereas Cr was the least mobile.
The Ca to Mg ratio was low (<0.3) in most serpentine soils except under S. nutans and A. gerardii at SGWand typically lower than 1 (Brady et al. 2005; Rajakaruna et al. 2009). Still the values were higher than reported for other serpentine sites in the eastern US such as in Maryland (0.03–0.15; Alexander 2009) and more similar to some serpentine sites in France or in the eastern Europe (Massoura et al. 2006). Compared to most serpentine outcrops elsewhere, serpentine sites in northeastern regions of North America were previously glaciated, and deposits by made by moving glaciers may have ameliorated some aspects of serpentine soils detrimental to plant growth (Rajakaruna et al. 2009). Alternatively, these sites present inherently different mineralogy and serpentinization process.
At Palmerton, the calamine site, Cd and Zn concentrations in some soil samples were close to high values found just after smelting ceased and before revegetation (Brown et al. 1994; Li et al. 2000) but lower than some concentrations in some localities on this mountain with 40,000 mg kg−1 (Palazzo et al. 2003). In all case, observed values here exceeded the maximum values recorded in another survey of contaminated areas in the US (76.8 and 1700 mg kg−1 respectively, Woodruff et al. 2015). Smelting activities, like those at Palmerton, represent the main source of pollution among industrial sources, with a large increase and enrichment of trace elements in the environment compared to geogenic concentrations (Nagajyoti et al. 2010). Cd and Zn extractible concentrations were closed to concentrations observed in another superfund site, Callahan Mine in Brooksville, ME, USA (Mansfield et al. 2014). However, our measured Cd and Zn pseudo-total concentrations were 10 and 15 times lower, respectively, than concentrations observed in other calamine sites occurs in the south of France (mining waste pile) and in Belgium (smelter activities) (Bert et al. 2002; Escarré et al. 2011).
Trace element concentrations at FIG were below detection limits or within the range of values found for non-metalliferous soils in the US (Smith et al. 2013). Nevertheless, a few samples under S. nutans at FIG showed high Pb pseudo-total concentrations (50–300 mg kg−1). These soils presented a different relationship with Fe compared to the other soils at the same site indicating a local increase of Pb, likely from anthropogenic sources (Fig. 1). This specific area corresponds to a major military shooting range (Ferster et al. 2008). Concentrations is higher than concentrations observed in mining site in Callahan Mine in Brooksville, ME, USA (Mansfield et al. 2014) but lower than some mining site in Southern of France where Pb hyperaccumulator occurs (Escarré et al. 2011). Lead is a major component of bullets, and the abrasion of Pb bullets passing through soil could result in contamination with smaller metallic Pb particles and may have contributed to the total content of soil Pb content (Astrup et al. 1999; Bennett et al. 2007). Due to its relatively low solubility, Pb has a long residence time in soils. Robinson et al. (2008) reported variable Pb concentrations in a Swiss military shooting range with higher maximum concentrations (> 1000 mg kg−1) compared to FIG.
Evaluating elemental uptake in excluder strategies
The elemental profiles in leaves of the three C4 grasses studied here qualitatively mirror variation in the soil. Moreover, differences in leaf concentrations of Ca and Mg concentrations between serpentine and non-serpentine sites structured CAP analysis. The mean Ca concentration was below 2500 mg kg−1 for serpentine populations of S. scoparium and Sorghastrum nutans at NOT and UNI, but the mean concentration of Mg exceeded the deficiency threshold (2000 mg kg−1). Both Ca and Mg concentrations presented here were much lower than concentrations found by Kazakou et al. (2010) in 21 plant species, including dicots, collected in situ at four serpentine sites in Lesbos Island but within the range of concentrations for 14 different vascular plants at a serpentine site in Maine, USA (Pope et al. 2010). Different reports demonstrated that grasses have a lower Ca requirement than dicots because their Type II cell walls are composed of cellulose fibers encased in glucuronoarabinoxylans and contain high levels of hydroxycinnamates with very low levels of pectin and structural proteins (Vogel 2008; O’Dell and Rajakaruna 2011; Marschner 2012). The Ca:Mg ratio < 1.0 found in leaves for our study populations resulted from lower concentrations of Ca. These results are consistent with the tolerance of Ca deficiency hypothesis and/or Mg toxicity proposed by Kruckeberg (1954 in Kazakou et al. 2008). For other major, essential elements, the C4 grasses presented concentrations near those considered deficient for many grasses or other crops (Marschner 2012). Indeed, most plants presented Fe, P and K concentrations below 100, 2000 and 15,000 mg kg−1 respectively. These findings are similar to those of Pope et al. (2010) who sampled 14 species at a serpentine site in Maine and showed that some Poaceae presented lower than 1000 mg kg−1. The N:P ratio, another important stress indicator, is within the normal range [10, 20] (Güsewell 2004) for all plant samples from the serpentine group except for A. gerardii and S. nutans at SGW. However, lowest N:P ratio in three species at PAL and FIG reflect N limitation for last sites.
For trace elements, our results indicate that the C4 grasses are excluder species and can maintain consistently low trace element concentrations in aboveground tissues over a wider range of soil concentrations. Reduced elemental concentrations in leaves, result from different mechanisms, including the sequestration of most trace elements in the roots, restricting uptake and transport to the shoots (Baker 1981) and influences of soil microbe (Doubková et al. 2011). Any such mechanism failed only for Zn at Palmerton because concentrations in leaves are at least ten times higher than in others sites, suggesting a potential break point or critical level of Zn in soil where homeostasis breaks down. Due to a large difference in Zn concentrations, for both pseudo-total and extractible concentrations, between Palmerton and the four other sites, we did not identify the breaking point in our study. Indeed, Zn, and to a lesser extent Cd, concentrations in leaves at Palmerton were higher than the toxicity threshold for most plants (Krämer 2010). Grasses cultivated on soil from PAL in the greenhouse show high concentrations of Zn in their aboveground tissues (Li et al. 2000; Palazzo et al. 2003), and there is evidence that soil microbes influence levels of Zn in C3 grasses from the site (Glassman and Casper 2012) and S. nutans (Casper, unpublished data). Mans-field et al. (2014) found that two species in the Salicaceae showed concentrations higher than 1000 mg kg−1 at the Callahan Mine in Brooksville, ME, USA. Indeed, it is usual to observed high concentrations of Zn in aboveground tissues in the plant families Brassicaceae, Poaceae or Salicaceae (Broadley et al. 2007). Broadley et al. (2001) found significant variation in shoot metal content occurred at the level of order and above, suggesting an ancient evolution of this trait.
In contrast, Ni concentrations were similar for plants of the same species at different sites despite important variation between sites in soil Ni levels. The availability of Ni in soils and its subsequent transfer to plants depends on its mineralogical origin (Kabata-Pendias 2000; Massoura et al. 2006) as well as on soil characteristics such as pH, conductivity, organic matter and clay content (Kabata-Pendias 2004), and even plant species identity. Using Ni spiked soil, Doherty et al. (2008) found that Ni concentrations in leaves of S. nutans were similar across seed and microbial communities from serpentine and non-serpentine origin, and was marginally affected by Ni dose. Other studies have shown differences between plant species in Ni content (Baker 1981; Kazakou et al. 2010; DeHart et al. 2014).
Finally, species accounted for more variation in leaf element concentrations than they did for soil element concentrations, suggesting species-specific differences in homeostasis in response to soil properties. Thus, foliar elemental profiles of Schizachyrium scoparium were characterized by relatively high concentrations of Co, Cr, Cu and Ni whereas Andropogon gerardii and Sorghastrum nutans showed higher concentrations of Ca at PAL and K at FIG. Leaf concentrations represent the product of many factors including soil properties as described above and several potential filtering processes in roots, including metal efflux from the plasma membrane (Curie et al. 2009), metal chelation by phytochelatins and metallothioneins (Schat et al. 2002; Devez et al. 2009), and compartmentalization within the vacuole (Shaw et al. 2005). Even in species known to exclude trace elements, Kazakou et al. (2010) found higher Mg and Cr concentrations and lower Ca to Mg ratios in plants from serpentine soils compared to non-serpentine soils.
Phytostabilization and site management strategies
Much research focuses on using hyperaccumulators to rehabilitate metal-contaminated land through phytoextraction (Hammer and Keller 2003; Broadhurst et al. 2015) and phytomining (Bani et al. 2007; Van Der Ent et al. 2015) and metallophytes are the optimal choice for vegetation in restoration ecology and remediation in mining sites. Phytostabilization represents another way to remediate, especially in soils with high trace element concentrations like those at industrially contaminated sites The three C4 grasses studied here are present across a range of soil types including those with high concentrations of Pb and Cd, which are considered hazardous in soil (Järup 2003). With low metal concentrations in leaves and the absence of any correlations between metal levels in soils and plants, members of the Poaceae are excellent candidates for phytoremediation of the many Pb contaminated areas found across the northern United States (Burt et al. 2003; Woodruff et al. 2015). The could also be used for phytostabilization of serpentine sites, areas contaminated with Ni by industrial activities, or asbestos quarries (Rodríguez-Seijo and Andrade 2015).
Elemental concentrations in soils under S. nutans and A. gerardii at Sugartown (SERP) were closer to non-metalliferous soils than soils under S. scoparium in same site and were spatially separated from S. scoparium (C. Gonneau, personal observation). These soils properties indicate loss of the serpentine syndrome (Kazakou et al. 2008) and a shift to non-serpentines conditions approximatively since 100 years ago (Burgess et al. 2015a). When bordering woodlands encroach on grasslands they create a richer soil layer over the serpentine soils as their leaves drop and decompose (Haegele 2011), alter the mycorrhizal community (Cumming and Kelly 2007), and lead to the disappearance of a particular and unique flora and fauna (Rajakaruna et al. 2009; Latham and McGeehin 2012). The tallgrass prairie is one of the most critically endangered ecosystems in North America mainly due to the invasion of woody plants encroachment (Hoekstra et al. 2005). Mesophication is also a main problem for grassland, with growing dominance by Acer, Nyssa and more mesic Quercus and Fagus species (Burgess et al. 2015a). To sustain this species on many serpentines areas, a Restoration and Management Plan is implemented, which includes prescribed burning and trees and alien species removal as well the soil organic matter (Latham et al. 2007a; Gustafson et al. 2008; Latham 2008). The establishment of state law to protect this extreme environment is also important (O’Dell 2014) since serpentine site represent a globally rare ecosystem and a cluster of rare species give the site national or even global significance (Baker et al. 2010; Mengoni et al. 2010). Understanding plant metal uptake in grasses and their distribution in serpentines sites can inform better management strategies.
Supplementary Material
Acknowledgments
The authors thank the Lehigh Gap Nature Center, Natural Lands Trust, Joseph Hovis from PA Department of Military and Veterans Affairs, and Chester County Facilities & Parks for authorization to collect samples on their land, Roger Latham for his help to identify sites, Bianca Charbonneau, Aurora MacRae-Crerar for their help in sampling process and Kinsey Miller for her help in lab. We also thank David Vann for his help on ICP analysis and Rengyi (Emily) Xu for her statistical guidance and analysis of plant data. We are very grateful to the Daldal Lab at University of Pennsylvania for the utilization of their lab equipment and platform.
Research reported in this publication was supported by National Institute Environmental Health Sciences of the National Institutes of Health under award number P42 ES023720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Responsible Editor: Antony Van der Ent.
Electronic supplementary material: The online version of this article (doi:10.1007/s11104-017-3198-9) contains supplementary material, which is available to authorized users.
Contributor Information
Cédric Gonneau, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Sanjay K. Mohanty, Department of Earth and Environmental Science, University of Pennsylvania, Philadelphia, PA 19104, USA
Lee H. Dietterich, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104, USA
Wei-Ting Hwang, Department of Biostatistics and Epidemiology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Jane K. Willenbring, Department of Earth and Environmental Science, University of Pennsylvania, Philadelphia, PA 19104, USA
Brenda B. Casper, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104, USA
References
- Alexander EB. Serpentine Geoecology of the eastern and southeastern margins of North America. Northeast Nat. 2009;16:223–252. doi: 10.1656/045.016.0518. [DOI] [Google Scholar]
- Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals—concepts and applications. Chemosphere. 2013;91:869–881. doi: 10.1016/j.chemosphere.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Alloway BJ. Sources of Heavy Metals and Metalloids in Soils. In: Alloway BJ, editor. Heavy Metals in Soils. Springer; Netherlands: 2013. pp. 11–50. [Google Scholar]
- Anacker BL, Whittall JB, Goldberg EE, Harrison SP. Origins and consequences of serpentine endemism in the California Flora. Evolution. 2011;65:365–376. doi: 10.1111/j.1558-5646.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- Arabas KB. Spatial and temporal relationships among fire frequency, vegetation, and soil depth in an eastern north American serpentine barren. J Torrey Bot Soc. 2000;127:51–65. doi: 10.2307/3088747. [DOI] [Google Scholar]
- Astrup T, Boddum JK, Christensen TH. Lead distribution and mobility in a soil embankment used as a bullet stop at a shooting range. J Soil Contam. 1999;8:653–665. [Google Scholar]
- Baker AJM. Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr. 1981;3:643–654. doi: 10.1080/01904168109362867. [DOI] [Google Scholar]
- Baker AJM. Metal Tolerance. New Phytol. 1987;106:93–111. doi: 10.1111/j.1469-8137.1987.tb04685.x. [DOI] [Google Scholar]
- Baker AJM, Brooks R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Baker AJ, Ernst WH, van der Ent A, et al. Metallophytes: the unique biological resource, its ecology and conservational status in Europe, Central Africa and Latin America. Ecol Ind Pollut Camb Univ Press Camb. 2010:7–40. [Google Scholar]
- Bani A, Echevarria G, Sulpe S, et al. In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania) Plant Soil. 2007;293:79–89. doi: 10.1007/s11104-007-9245-1. [DOI] [Google Scholar]
- Bennett JR, Kaufman CA, Koch I, et al. Ecological risk assessment of lead contamination at rifle and pistol ranges using techniques to account for site characteristics. Sci Total Environ. 2007;374:91–101. doi: 10.1016/j.scitotenv.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Bert V, Bonnin I, Saumitou-Laprade P, et al. Do Arabidopsis Halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol. 2002;155:47–57. doi: 10.1046/j.1469-8137.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- Bever JD, Morton JB, Antonovics J, Schultz PA. Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. J Ecol. 1996;84:71–82. doi: 10.2307/2261701. [DOI] [Google Scholar]
- Boufford DE, Kartesz JT, Shi S, Zhou R. Packera Serpenticola (Asteraceae; Senecioneae), a new species from North Carolina, U. S. a. Syst Bot. 2014;39:1027–1030. doi: 10.1600/036364414X682274. [DOI] [Google Scholar]
- Boyd R, Martens S. Nickel hyperaccumulation by Thlaspi montanum var. montanum (Brassicaceae): a constitutive trait. Am J Bot. 1998;85:259. [PubMed] [Google Scholar]
- Brady KU, Kruckeberg AR, Bradshaw HD. Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol Syst. 2005;36:243–266. [Google Scholar]
- Broadhurst CL, Chaney RL, Davis AP, et al. Growth and cadmium phytoextraction by Swiss chard, maize, Rice, Noccaea Caerulescens, and Alyssum murale in pH adjusted Biosolids amended soils. Int J Phytoremediation. 2015;17:25–39. doi: 10.1080/15226514.2013.828015. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Willey NJ, Wilkins JC, et al. Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol. 2001;152:9–27. doi: 10.1046/j.0028-646x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, et al. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Brown SL, Chaney RL, Angle JS, Baker AJM. Phytoremediation potential of Thlaspi Caerulescens and bladder Campion for zinc- and cadmium-contaminated soil. J Environ Qual. 1994;23:1151. doi: 10.2134/jeq1994.00472425002300060004x. [DOI] [Google Scholar]
- Burgess J, Szlavecz K, Rajakaruna N, et al. Vegetation dynamics and mesophication in response to conifer encroachment within an ultramafic system. Aust J Bot. 2015a;63:292–307. doi: 10.1071/BT14241. [DOI] [Google Scholar]
- Burgess J, Szlavecz K, Rajakaruna N, Swan C. Ecotypic differentiation of mid-Atlantic Quercus species in response to ultramafic soils. Aust J Bot. 2015b;63:308–323. [Google Scholar]
- Burt R, Wilson MA, Mays MD, Lee CW. Major and trace elements of selected Pedons in the USA. J Environ Qual. 2003;32:2109. doi: 10.2134/jeq2003.2109. [DOI] [PubMed] [Google Scholar]
- Chaney RL, Malik M, Li YM, et al. Phytoremediation of soil metals. Curr Opin Biotechnol. 1997;8:279–284. doi: 10.1016/s0958-1669(97)80004-3. [DOI] [PubMed] [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Cumming JR, Kelly CN. Pinus virginiana Invasion influences soils and arbuscular mycorrhizae of a serpentine grassland. J Torrey Bot Soc. 2007;134:63–73. doi: 10.3159/1095-5674(2007)134[63:PVIISA]2.0.CO;2. [DOI] [Google Scholar]
- Cunningham SD, Berti WR, Huang JW. Phytoremediation of contaminated soils. Trends Biotechnol. 1995;13:393–397. [Google Scholar]
- Curie C, Cassin G, Couch D, et al. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot. 2009;103:1–11. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHart KS, Meindl GA, Bain DJ, Ashman T-L. Elemental composition of serpentine plants depends on habitat affinity and organ type. J Plant Nutr Soil Sci. 2014;177:851–859. doi: 10.1002/jpln.201300485. [DOI] [Google Scholar]
- Devez A, Achterberg E, Gledhill M. Metal ion-binding properties of phytochelatins and related ligands. Met Ions Life Sci. 2009;5:441–481. [Google Scholar]
- Doherty JH, Ji B, Casper BB. Testing nickel tolerance of Sorghastrum nutans and its associated soil microbial community from serpentine and prairie soils. Environ Pollut. 2008;151:593–598. doi: 10.1016/j.envpol.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Doubkova P, Suda J, Sudova R. Arbuscular mycorrhizal symbiosis on serpentine soils: the effect of native fungal communities on different Knautia arvensis ecotypes. Plant Soil. 2011;345:325–338. doi: 10.1007/s11104-011-0785-z. [DOI] [Google Scholar]
- Escarre J, Lefebvre C, Raboyeau S, et al. Heavy metal concentration survey in soils and plants of the les Malines Mining District (southern France): implications for soil restoration. Water Air Soil Pollut. 2011;216:485–504. doi: 10.1007/s11270-010-0547-1. [DOI] [Google Scholar]
- Faucon M-P, Stradic SL, Boisson S, et al. Implication of plant-soil relationships for conservation and restoration of copper-cobalt ecosystems. Plant Soil. 2016;403:153–165. doi: 10.1007/s11104-015-2745-5. [DOI] [Google Scholar]
- Ferster B, Leppo BR, Swartz MT, et al. Lepidoptera of fort Indiantown gap National Guard Training Center, Annville, Pennsylvania. NortheastNat. 2008;15:141–148. doi: 10.1656/1092-6194(2008)15[141:LOFIGN]2.0.CO;2. [DOI] [Google Scholar]
- Gao S, Luo T-C, Zhang B-R, et al. Chemical composition of the continental crust as revealed by studies in East China. Geochim Cosmochim Acta. 1998;62:1959–1975. [Google Scholar]
- Glassman SI, Casper BB. Biotic contexts alter metal sequestration and AMF effects on plant growth in soils polluted with heavy metals. Ecology. 2012;93:1550–1559. doi: 10.1890/10-2135.1. [DOI] [PubMed] [Google Scholar]
- Goldschmidt VM. The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the Chemical Society on March 17th, 1937. J Chem Soc Resumed. 1937:655–673. doi: 10.1039/JR9370000655. [DOI] [Google Scholar]
- Gonneau C, Genevois N, Frérot H, et al. Variation of trace metal accumulation, major nutrient uptake and growth parameters and their correlations in 22 populations of Noccaea Caerulescens. Plant Soil. 2014;384:271–287. [Google Scholar]
- Güsewell S. N : P ratios in terrestrial plants: variation and functional significance. New Phytol. 2004;164:243–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- Gustafson DJ, Halfacre AC, Anderson RC. Practical seed source selection for restoration projects in an urban setting: tallgrass prairie, serpentine barrens, and coastal habitat examples. Urban Habitats. 2008;5:7–18. [Google Scholar]
- Haegele E. Unionville Serpentine Barrens: Analyzing the Relationship Between Soil Profiles and Forest Succession Rate 2011 [Google Scholar]
- Hammer D, Keller C. Phytoextraction of Cd and Zn with Thlaspi Caerulescens in field trials. Soil Use Manag. 2003;19:144–149. doi: 10.1111/j.1475-2743.2003.tb00295.x. [DOI] [Google Scholar]
- Harris T, Rajakaruna N. Adiantum viridimontanum, Aspidotis densa, Minuartia marcescens, and Symphyotrichum rhiannon: additional serpentine endemics from eastern North America. Northeast Nat. 2009;16:111–120. doi: 10.1656/045.016.0509. [DOI] [Google Scholar]
- Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol Lett. 2005;8:23–29. [Google Scholar]
- Isnard S, L’huillier L, Rigault F, Jaffré T. How did the ultramafic soils shape the flora of the new Caledonian hotspot? Plant Soil. 2016;403:53–76. doi: 10.1007/s11104-016-2910-5. [DOI] [Google Scholar]
- Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Ji B, Bentivenga SP, Casper BB. Comparisons of AM fungal spore communities with the same hosts but different soil chemistries over local and geographic scales. Oecologia. 2012;168:187–197. doi: 10.1007/s00442-011-2067-0. [DOI] [PubMed] [Google Scholar]
- Ji B, Gehring CA, Wilson GWT, et al. Patterns of diversity and adaptation in Glomeromycota from three prairie grasslands. Mol Ecol. 2013;22:2573–2587. doi: 10.1111/mec.12268. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Wilson GWT, Bowker MA, et al. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci. 2010;107:2093–2098. doi: 10.1073/pnas.0906710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyoti V, Saini-Eidukat B, Hopkins D, DeSutter T. Naturally elevated metal contents of soils in northeastern North Dakota, USA, with afocus on cadmium. J Soils Sediments. 2015;15:1571–1583. doi: 10.1007/s11368-015-1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata-Pendias A. Trace elements in soils and plants. CRC Press; London: 2000. [Google Scholar]
- Kabata-Pendias A. Soil-plant transfer of trace elements—an environmental issue. Geoderma. 2004;122:143–149. doi: 10.1016/j.geoderma.2004.01.004. [DOI] [Google Scholar]
- Kazakou E, Dimitrakopoulos PG, Baker AJM, et al. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biol Rev. 2008;83:495–508. doi: 10.1111/j.1469-185X.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- Kazakou E, Adamidis GC, Baker AJM, et al. Species adaptation in serpentine soils in Lesbos Island (Greece): metal hyperaccumulation and tolerance. Plant Soil. 2010;332:369–385. doi: 10.1007/s11104-010-0302-9. [DOI] [Google Scholar]
- Kierczak J, Pędziwiatr A, Waroszewski J, Modelska M. Mobility of Ni, Cr and Co in serpentine soils derived on various ultrabasic bedrocks under temperate climate. Geoderma. 2016;268:78–91. doi: 10.1016/j.geoderma.2016.01.025. [DOI] [Google Scholar]
- Kramer U. Metal hyperaccumulation in plants. Annu Rev Plant Biol. 2010;61:517–534. doi: 10.1146/annurev-arplant-042809-112156. [DOI] [PubMed] [Google Scholar]
- Latham RE. The serpentine barrens of temperate eastern North America: critical issues in the Management of Rare Species and Communities. Bartonia. 1993:61–74. [Google Scholar]
- Latham R. Pink hill serpentine barrens restoration and management plan. Continental Conservation; Rose Valley, PA: 2008. www.tylerarboretum.org/wp-content/uploads/.../Latham-PinkHillReport-2008.pdf Accessed 15 July 2016. [Google Scholar]
- Latham R, McGeehin M. Unionville serpentine barrens restoration and management plan. Continental Conservation; Rose Valley, PA: 2012. http://www.continentalconservation.us/Roger%20Latham%20publications_files/Unionville_Barrens_plan_NLT.pdf Accessed 15 July 2016. [Google Scholar]
- Latham RE, Steckel DB, Harper HM, et al. Lehigh Gap Wildlife Refuge ecological assessment. For the Lehigh Gap Nature Center; Slatington, Pennsylvania, by Natural Lands Trust, Media, Pennsylvania: Continental Conservation; Rose Valley, Pennsylvania; and Botanical Inventory, Allentown, Pennsylvania: 2007a. p. 62. [Google Scholar]
- Latham RE, Zercher D, McElhenny P, et al. The role of disturbance in habitat restoration and Management for the Eastern Regal Fritillary (Speyeria idalia idalia) at a military installation in Pennsylvania. Ecol Restor. 2007b;25:103–111. doi: 10.3368/er.25.2.103. [DOI] [Google Scholar]
- Li Y-M, Chaney RL, Siebielec G, Kerschner BA. Response of four Turfgrass cultivars to limestone and Biosolids-compost amendment of a zinc and cadmium contaminated soil at Palmerton, Pennsylvania. J Environ Qual. 2000;29:1440. doi: 10.2134/jeq2000.00472425002900050010x. [DOI] [Google Scholar]
- Lin Y-F, Aarts MGM. The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci. 2012;69:3187–3206. doi: 10.1007/s00018-012-1089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J. 1978;42:421–428. [Google Scholar]
- Mansfield MR, Pope NS, Mittelhauser GH, Rajakaruna N. Diversity and soil-tissue elemental relations of vascular plants of Callahan mine, Brooksville, Maine, U.S.a. Rhodora. 2014;116:283–322. doi: 10.3119/13-23. [DOI] [Google Scholar]
- Marschner P. Marschner’s mineral nutrition of higher plants. Academic Press; San Diego: 2012. [Google Scholar]
- Massoura ST, Echevarria G, Becquer T, et al. Control of nickel availability by nickel bearing minerals in natural and anthropogenic soils. Geoderma. 2006;136:28–37. doi: 10.1016/j.geoderma.2006.01.008. [DOI] [Google Scholar]
- Mengoni A, Schat H, Vangronsveld J. Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant Soil. 2010;331:5–16. [Google Scholar]
- Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8:199–216. doi: 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- O’Dell RE. Conservation and restoration of chemically extreme edaphic endemic flora in the western us. 2014 https://www.researchgate.net/publication/297363167_Conservation_and_restoration_of_chemically_extreme_edaphic_endemic_flora_in_the_western_us. Accessed 24 Jan 2017.
- O’Dell RE, Rajakaruna N. Intraspecific variation, adaptation, and evolution. Serpentine Evol Ecol Model Syst. 2011:97–137. [Google Scholar]
- Palazzo AJ, Cary TJ, Hardy SE, Lee CR. Root growth and metal uptake in four grasses grown on zinc-contaminated soils. J Environ Qual. 2003;32:834–840. doi: 10.2134/jeq2003.8340. [DOI] [PubMed] [Google Scholar]
- Pauwels M, Saumitou-Laprade P, Holl AC, et al. Multiple origin of metallicolous populations of the pseudometallophyte Arabidopsis Halleri (Brassicaceae) in Central Europe: the cpDNA testimony. Mol Ecol. 2005;14:4403–4414. doi: 10.1111/j.1365-294X.2005.02739.x. [DOI] [PubMed] [Google Scholar]
- Pollard AJ, Powell KD, Harper FA, Smith JAC. The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci. 2002;21:539–566. [Google Scholar]
- Pollard AJ, Reeves RD, Baker AJM. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 2014;217–218:8–17. doi: 10.1016/j.plantsci.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Pope N, Harris TB, Rajakaruna N. Vascular plants of adjacent serpentine and granite outcrops on the deer isles, Maine, U.S.a. Rhodora. 2010;112:105–141. doi: 10.3119/09-02.1. [DOI] [Google Scholar]
- Prentice IC, Bartlein PJ, Webb T. Vegetation and climate change in eastern North America since the last glacial maximum. Ecology. 1991;72:2038–2056. doi: 10.2307/1941558. [DOI] [Google Scholar]
- Rajakaruna N, Harris TB, Alexander EB. Serpentine geoecology of eastern north america: a review. Rhodora. 2009;111:21–108. [Google Scholar]
- Rees F, Germain C, Sterckeman T, Morel J-L. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea Caerulescens) in contaminated soils amended with biochar. Plant Soil. 2015;395:57–73. doi: 10.1007/s11104-015-2384-x. [DOI] [Google Scholar]
- Robinson BH, Bischofberger S, Stoll A, et al. Plant uptake of trace elements on a Swiss military shooting range: uptake pathways and land management implications. Environ Pollut. 2008;153:668–676. doi: 10.1016/j.envpol.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Seijo A, Andrade ML. Characterization of soil physico-chemical parameters and limitations for revegetation in serpentine quarry soils (NW Spain) J Soils Sediments. 2015:1–10. doi: 10.1007/s11368-015-1284-2. [DOI] [Google Scholar]
- Schat H, Llugany M, Vooijs R, et al. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot. 2002;53:2381–2392. doi: 10.1093/jxb/erf107. [DOI] [PubMed] [Google Scholar]
- Shaw BP, Prasad MNV, Jha VK, Sahu BB. 16 Detoxification/Defense Mechanisms in Metal-Exposed Plants. Trace Elem Environ Biogeochem Biotechnol Bioremediation. 2005;291:271–289. [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, et al. Geochemical and mineralogical data for soils of the conterminous United States. US Geol Surv Data Ser. 2013;801:19. [Google Scholar]
- Thomas GW. Exchangeable cations. Methods Soil Anal Part 2 Chem Microbiol Prop. 1982:159–165. [Google Scholar]
- Van der Ent A, Baker AJ, Reeves RD, et al. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil. 2013:1–16. [Google Scholar]
- Van Der Ent A, Baker AJ, Reeves RD, et al. Agromining: farming for metals in the future? Environ Sci Technol. 2015;49:4773–4780. doi: 10.1021/es506031u. [DOI] [PubMed] [Google Scholar]
- Vogel J. Unique aspects of the grass cell wall. Curr Opin Plant Biol. 2008;11:301–307. doi: 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Whittaker RH. The ecology of serpentine soils: a symposium. I Introduction Ecology. 1954;35:258–259. [Google Scholar]
- Williams JW. Variations in tree cover in North America since the last glacial maximum. Glob Planet Change. 2003;35:1–23. doi: 10.1016/S0921-8181(02)00088-7. [DOI] [Google Scholar]
- Woodruff L, Cannon WF, Smith DB, Solano F. The distribution of selected elements and minerals in soil of the conterminous United States. J Geochem Explor. 2015;154:49–60. doi: 10.1016/j.gexplo.2015.01.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


