Abstract
Background
The purpose of this study was to assess survival for patients with malignant pleural mesothelioma (MPM), epithelial subtype, utilizing extended pleurectomy-decortication combined with intraoperative photodynamic therapy (PDT) and adjuvant pemetrexed-based chemotherapy.
Methods
From 2005 to 2013, 90 patients underwent lung-sparing surgery and PDT for MPM. All patients had a preoperative diagnosis of epithelial subtype, of which 17 proved to have mixed histology. The remaining 73 patients with pure epithelial subtype are analyzed. All patients received lung-sparing surgery and PDT. 92% also received chemotherapy. The median follow up was 5.3 years for living patients.
Results
Macroscopic complete resection was achieved in all 73 patients. 30 and 90 day mortalities were 3% and 4%, respectively. For all 73 patients (89% AJCC Stage III/IV, 69% N2 disease and median tumor volume 550 ml) the median overall and disease free survivals were 3 years and 1.2 years, respectively. For the 19 patients without lymph node metastases (74% Stage III/IV, median tumor volume 325 ml) the median overall and disease free survivals were 7.3 years and 2.3 years, respectively.
Conclusions
This is a mature data set for MPM that demonstrates the ability to safely execute a complex treatment plan that included a surgical technique that consistently permitted achieving a macroscopic complete resection while preserving the lung. The role for lung-sparing surgery is unclear but this series demonstrates that it is an option, even in advanced cases. The overall survival of 7.3 years for the node negative subset of patients, still of advanced stage, is encouraging. Of particular interest is the overall survival being approximately triple the disease free survival, perhaps PDT-related. The impact of PDT is unclear, but hopefully will be established by an ongoing randomized trial.
Keywords: malignant pleural mesothelioma, extended pleurectomy-decortication, photodynamic therapy
Malignant pleural mesothelioma (MPM) is a virulent, incurable cancer. Chemotherapy is the standard of care, with survival usually cited in the 12–18 month range {1,2,3}. As a curative microscopic (R0) resection is essentially impossible for MPM the goal of surgery is to achieve a macroscopic complete resection (MCR), performed as part of a multimodal treatment plan. Surgery remains controversial for MPM because it is an operation of considerable magnitude that is, technically, palliative and also because reported surgical results are often similar to nonsurgical results.
Still, there do appear to be patients who benefit significantly more from a surgery-based approach than would be expected from chemotherapy alone, emphasizing the importance of patient selection. The art of patient selection for MPM surgery extends beyond the current staging system and includes prognosticators like: gender, platelet count, pain, tumor volume and, in particular, the subtype of the cancer {4,5,6,7,8,9,10}.
Our surgery-based approach has always included intraoperative photodynamic therapy (PDT), but initially included all subtypes of disease and both surgical approaches. We then switched exclusively to EPD after comparing our outcomes of extrapleural pneumonectomy (EPP) versus EPD {11}. Examining a larger cohort of EPD patients we found that histologic subtype was the main prognosticator, but the results still appeared promising for patients with large volume disease, advanced stage and bulky adenopathy {12}. This study reports the results of EPD, intraoperative PDT and chemotherapy on 73 patients limited to epithelial histology but including the unfavorable prognosticators of advanced stage, nodal disease and bulk.
Patients and Methods
This study is an analysis of all patients treated on two prospective clinical trials that were approved by the University of Pennsylvania Institutional Review Board. From 2005 to 2013, 90 patients underwent lung-sparing surgery and PDT for MPM. All patients had a preoperative diagnosis of epithelial subtype, of which 73 ultimately proved to have pure epithelial MPM on final pathology and are the subject of this analysis. Our Multidisciplinary Mesothelioma Program was started in 2007 and, from its inception, 31% of the patients who presented to the Program and had, or were found to have, a diagnosis of epithelioid subtype, underwent surgery. The median age was 65 years (range, 38–81 years), and there were 55 men (75%) and 18 women (25%). All consecutive patients undergoing EPD across two prospective trials with two separate photosensitizers, porfimer sodium (52 patients) and 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) (21 patients) were analyzed. It was the institutional bias to treat de novo patients with adjuvant pemetrexed-based doublet chemotherapy, but 17 patients presented after chemotherapy and were offered surgery (Table 1). Six patients received a full course of chemotherapy prior to surgery and, therefore, were not deemed appropriate for adjuvant chemotherapy. An additional 11 patients were started on chemotherapy and received 1–2 cycles of chemotherapy before going on to have their definitive resection. All of these patients received additional standard adjuvant chemotherapy. Six out of the 73 patients did not receive adjuvant chemotherapy that was planned due to post-operative mortality or rapid recurrence of disease and the initiation of palliative care”
Table 1.
Cohort description
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Survival | Progression Free Survival | |||||||||
|
|
||||||||||
| N | 3yr rate |
+/− SE | Median (months) |
Log rank p- value |
3yr rate |
+/− SE | Median (months) |
Log rank p- value |
||
| All Patients | 73 | 48.8% | 5.9% | 35.64 | · | 16.1% | 4.4% | 12.75 | · | |
| Gender | ||||||||||
| Male | 55 | 41.7% | 6.7% | 27.84 | 0.0142 | 12.7% | 4.5% | 11.47 | 0.1194 | |
| Female | 18 | 72.2% | 10.6% | 56.64 | 24.3% | 11.0% | 20.55 | |||
| Age | ||||||||||
| <65 | 36 | 52.1% | 8.4% | 36.48 | 0.4957 | 16.2% | 6.2% | 13.14 | 0.8642 | |
| > 65 | 37 | 45.5% | 8.3% | 31.68 | 16.2% | 6.1% | 12.75 | |||
| N stage | ||||||||||
| N0 | 19 | 72.9% | 10.4% | 87.48 | 0.0034 | 47.4% | 11.5% | 26.84 | 0.0023 | |
| N1 | 4 | 50.0% | 25.0% | 38.82 | 0.0% | · | 10.02 | |||
| N2 | 46 | 38.8% | 7.2% | 22.92 | 5.4% | 3.6% | 9.51 | |||
| N3 | 4 | 50.0% | 25.0% | 27.42 | 0.0% | · | 11.56 | |||
| N0 | 19 | 72.9% | 10.4% | 87.48 | 0.0012 | 47.4% | 11.5% | 26.88 | 0.0014 | |
| N1 | 4 | 50.0% | 25.0% | 38.82 | 0.0% | · | 10.02 | |||
| N2/N3 | 50 | 39.4% | 7.0% | 22.92 | 5.0% | 3.3% | 10.56 | |||
| N0 | 19 | 72.9% | 10.4% | 87.48 | 0.0005 | 47.4% | 11.5% | 26.84 | 0.0003 | |
| N+ | 54 | 40.3% | 6.7% | 22.92 | 4.6% | 3.0% | 9.51 | |||
| AJCC Stage | ||||||||||
| 1 | 2 | 100.0% | · | · | 0.0483 | 100.0% | · | · | 0.0533 | |
| 2 | 6 | 100.0% | · | · | (all) | 33.3% | 19.3% | 20.17 | (all) | |
| 3 | 37 | 42.2% | 8.3% | 31.68 | 0.4708 | 12.2% | 5.7% | 11.76 | 0.4023 | |
| 4 | 28 | 42.9% | 9.4% | 22.92 | (3 v. 4) | 10.7% | 5.9% | 9.40 | (3 v. 4) | |
| Photosensitizer | ||||||||||
| Photofrin | 52 | 47.4% | 7.0% | 35.38 | 0.8275 | 14.7% | 5.0% | 11.61 | 0.9621 | |
| HPPH | 21 | 47.6% | 10.9% | 32.59 | 19.1% | 8.6% | 14.78 | |||
| Chemotherapy | ||||||||||
| None | 6 | 33.3% | 19.3% | 5.90 | 0.1682 | 16.7% | 15.2% | 5.27 | 0.5778 | |
| Pre-Op Only | 6 | 66.7% | 19.3% | 45.17 | 16.7% | 15.2% | 8.90 | |||
| Post-Op Only | 50 | 49.3% | 7.2% | 35.58 | 17.8% | 5.5% | 15.34 | |||
| Pre & Post Op | 11 | 36.4% | 14.5% | 22.70 | 9.1% | 8.7% | 8.84 | |||
| Pre-Op Only or Post-Op Only | 56 | 51.2% | 6.7% | 40.12 | 0.0335 | 17.7% | 5.1% | 14.92 | 0.2436 | |
| Pre- and Post-Op Chemo | 11 | 36.4% | 14.5% | 22.70 | 9.1% | 8.7% | 8.84 | |||
Preoperative work-up included risk stratification, CT chest, brain imaging and PET scan. Most patients also underwent an outpatient invasive staging procedure including bronchoscopy and staging laparoscopy, while selected patients also underwent contralateral thoracoscopy, triggered by abnormal radiographic findings. Enrollment was broad, including patients with bulky nodal disease, frank chest wall invasion and two patients with cancer detected on the invasive staging workup (one contralateral pleural/one abdominal) who were enrolled after chemotherapy and repeated workup revealed no detectable disease outside of the affected hemithorax.
Criteria for enrollment included: epithelial subtype, deemed medically “fit” for surgery, disease confined to one hemithorax and convincing demonstration that surgery is not standard of care for MPM, as part of informed consent.
Surgical Procedure
The surgical procedure is described elsewhere in detail {13}. All procedures were performed by the same surgeon (JSF). Briefly, the default operative plan was that the parietal pleural surfaces were mobilized, leaving the specimen tethered to the lung, and then removed en bloc with the entire visceral pleura. Every effort was made to preserve diaphragmatic musculature and the skeletonized phrenic nerve. Full thickness diaphragm was resected and primarily, or absorbable patch, reconstructed. Typically the fibrous pericardium was resected, leaving the inner serous layer. Initially full thickness pericardial invasion was reconstructed with permanent prosthetic, but later in the series reconstruction was not performed. The default was to resect the entire visceral pleura but in rare cases where this was not possible, typically minimal involvement, detectable disease was cauterized. When the cancer was highly invasive into the lung, typically massive bulk disease, the electrocautery used to liberate the specimen with a gross clear parenchymal margin. The fissure was always dissected down to the extrapleural plane, typically resulting in skeletonization of the pulmonary artery. A lymphadenectomy was performed, which in the more recent part of the series included harvesting the posterior intercostal lymph nodes (considered N2 for staging). PDT was then performed, delivering light to a predetermined dose as measured by strategically placed isotropic light detectors. The technical aspects and further discussion regarding PDT have been previously described {11,12,18}. In the later part of the series an absorbable lung sealant (Progel™ Pleural Air Leak Sealant, Bard – Davol, Inc.) was applied if substantial parenchymal leaks were noted. In the latter part of the series, volume of tumor specimens was determined by displacement in saline.
Follow up and statistics
Standard follow-up was an office visit and chest CT every three months. All patients were followed and none were lost to follow-up. Patients were treated for recurrence on an individualized basis, as determined by discussion at a multidisciplinary conference or per their own oncologists. Some patients received multiple different types of treatments. Modalities and agents included: vinorelbine, gemcitabine, pemetrexed, cisplatin, carboplatin, ad-interferon gene therapy, SS1P immunotherapy, standard photon radiation and proton radiation. Overall survival (OS) was defined as the time from surgery to death from any cause or last patient contact. Progression-free survival (PFS) was defined as the time from surgery to first documented recurrence, death from any cause, or last patient contact. Survival and PFS were estimated by the method of Kaplan and Meier. The logrank test was used to test equality over strata of selected clinical indications. Statistical significance was set at 0.05. No adjustments for confounders were included in this analysis. All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient demographics and survival statistics are summarized in Table 1. Of the 90 patients who had a preoperative diagnosis of epithelial subtype, 17 (18%) proved to have mixed histology, with MCR achieved in 14/17 (82%). The following analysis relates to the 73 pure epithelial patients, all in whom MCR was achieved. Nodal status was: N0 19/74 (26%), N1 4/74 (5%) and N2 50 (69%). Median tumor volume was measured in a subset of 34 patients at 550 ml (range 250–2200 ml) by measuring saline displacement in a graduated beaker. The subset of the N0 patients who had tumor volume measured ranged from150–900ml, median 325ml. Median tumor volume for N positive was 675ml (250–2200ml). Stage breakdown was Stage I 2/73 (3%), Stage II 6/73 (8%), Stage III 37/73 (50%) and Stage IV, 28/73 (39%). Further breakdown of the N0 group by stage was: T1N0 2/19 (10%), T2N0 3/19 (15%), T3N0 11/19 (57%) and T4N0 3/19 15%). The extent of resections and reconstructions is summarized in Table 2. The average length of stay was 18.3 days.
Table 2.
Extent of Resection
Extent of Resection
|
Number of Patients N=74 |
% |
|---|---|---|
| CWR + DR + PR + PNR | 2 | 2% |
| CWR + DR + PR | 8 | 10% |
| DR + PR | 34 | 46% |
| PR + DR + PNR | 5 | 6% |
| DR + PNR | 1 | 1% |
| RD + CWR | 1 | 1% |
| Gortex Patch - Pericardium | 7 | 9% |
| PR only | 9 | 12% |
| DR only | 9 | 12% |
| CWR only | 1 | 1% |
| Diaphragm replacement with vicryl mesh | 4 | 5% |
Complications
Complications are summarized in Table 3. The 30-day mortality was 2/73 (3%) with an additional death yielding a 90-day mortality of 3/73 (4%). The early causes of death were stroke and myocardial infarction with cardiac tamponade responsible for the day 37 death. Other complications included: atrial fibrillation 21/73 (28%), respiratory failure requiring tracheostomy 14/73 (19%), chyle leak 4/73 (5%), deep venous thrombosis 17/73 (23%), persistent air leak 17/73 (23%) and discharge with Heimlich valve 2/73 (2%). The pneumonia rate of 28% is high. Extra precautions to prevent aspiration were taken and attention to pulmonary toilet, including liberal use of bronchoscopy, was routine. The reason for this high pneumonia rate, therefore, is unclear, but is potentially related to the use of PDT.
Table 3.
Complications
| Complications | (N=74) Number of Patients |
% |
|---|---|---|
| Atrial fibrillation | 21 | 28% |
| Respiratory failure requiring tracheostomy | 14 | 19% |
| Deep venous thrombosis | 17 | 23% |
| Chyle leak | 4 | 5% |
| Diaphragm rupture | 3 | 4% |
| Pericardial patch dehiscence | 1 | 1% |
| Persistent air leak | 17 | 23% |
| Discharged with Heimlich Valve | 2 | 2% |
| Pneumonia | 21 | 28% |
| Exploration for bleeding | 1 | 1% |
| Empyema | 1 | 1% |
| Pericardial effusion requiring drainage | 3 | 4% |
SURVIVAL
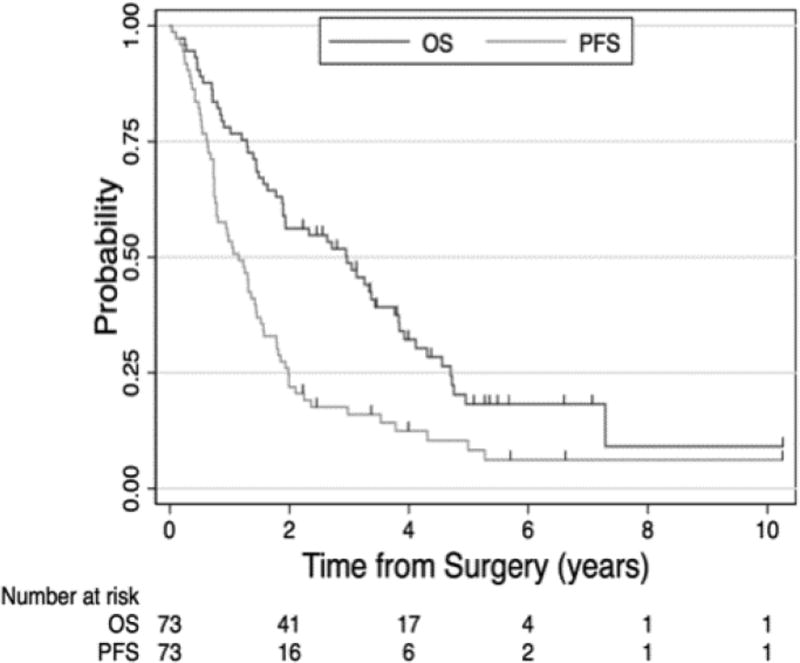
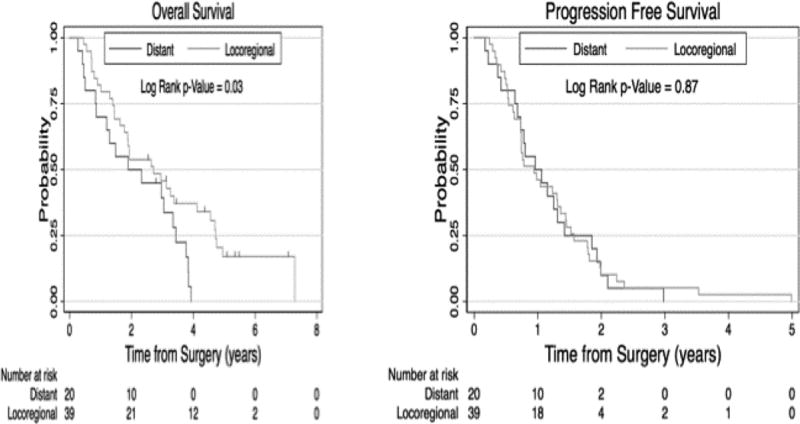
At a median follow up of 5.3 years for living patients, the OS and DFS for all 73 patients were 36 and 14 months, respectively (Figure 1). The 18 patients with mixed histology died within one year after surgery. When broken down by nodal status, the median OS and DFS for N1/2 (93% N2) were 23 and 10 months, respectively, and for N0 disease was 87 and 27 months, respectively (Figure 2). Factors associated with worse survival included male gender OS 26 versus 57 months (p= 0. 0142) and nodal status with N1/2 versus N0, OS 23 versus 87 months (p= 0. 0005). Given the advanced stage patient population included in our clinical trials, there were not enough early stage patients to compare outcomes according to stage grouping. Factors that were not significant with survival included: age <65 years versus >65 years OS 40 versus 30 months (p= 0.41), stage III versus stage IV OS 30 versus 23 months (p= 0.30), and photosensitizer porfimer sodium versus HPPH OS 35 versus 33 months (p=0.72) or preoperative only chemotherapy versus postoperative only chemotherapy (6/74, 8% chemotherapy) versus (50/74, 68%) (Table 1). There was a small statistically significant difference in overall survival but not progression free survival noted if the recurrence was purely local or if there were distant metastases (Figure 3). Local and distant progression were seen in 47 of the patients (64.4%) (Table 4 and 5).
Figure 1. Survival Analysis of entire 73 patient cohort.
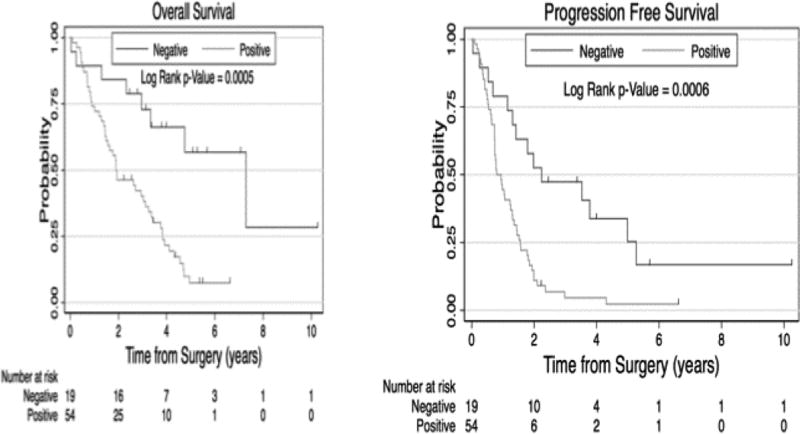
Figure 2. Effect of Lymph Node Metastases on Survival and Progression Free Survival.
Analysis the effect of lymph node metastases was performed, with patients grouped as either node negative (N0) or node positive (N1 or N2). Overall survival for patients with or without lymph node metastases was 7.3 years ± 0.16 years vs 1.9 years ± 0.5 years, respectively. Progression free survival for patients with or without lymph node metastases was 2.2 years ± 1.1 years vs 0.8 years ± 0.1 years, respectively.
Figure 3. Effect of First Treatment Failure Site on Survival and Progression Free Survival.
Analysis of patterns of initial treatment failure was performed, with patients grouped as either experiencing an initial recurrence involving 1) locoregional site(s) vs 2) distant ± locoregional site(s). Overall survival for patients experiencing initial treatment failure at only locoregional vs distant ± locoregional site(s) was 2.7 years ± 0.9 years vs 1.9 years ± 0.9 years, respectively. Progression free survival for patients experiencing only locoregional vs distant ± locoregional recurrence(s) was 1.0 years ± 0.3 years vs 1.0 years ± 0.2 years, respectively.
Table 4.
Statistics for the 60/73 patients with local or distant recurrent disease
| Local Only Progression |
Any Distant Progression |
P Value | |||||
|---|---|---|---|---|---|---|---|
| N | # | % | # | % | |||
| All Patients | 60 | 7 | 11.7% | 53 | 88.3% | · | |
| Gender | Female | 14 | 0 | 0.0% | 14 | 23.3% | 0.6678i |
| Male | 45 | 6 | 10.0% | 39 | 65.0% | ||
| Age | < 65 | 29 | 4 | 6.7% | 26 | 43.3% | 1.000i |
| ≥ 65 | 30 | 3 | 5.0% | 27 | 45.0% | ||
| T Stage | T2 | 12 | 2 | 3.3% | 10 | 16.7% | 0.2192* |
| T3 | 24 | 4 | 6.7% | 21 | 35.0% | ||
| T4 | 23 | 1 | 1.7% | 22 | 36.7% | ||
| N Stage | N0 | 10 | 1 | 1.7% | 9 | 15.0% | 1.000i |
| N+ | 49 | 6 | 10.0% | 44 | 73.3% | ||
| AJCC Stage | Stage 2 | 5 | 0 | 0.0% | 5 | 9.4% | 0.4335* |
| Stage 3 | 30 | 6 | 83.3% | 25 | 47.2% | ||
| Stage 4 | 24 | 1 | 16.7% | 23 | 43.4% | ||
Table 5.
Patterns of Progression
| Progression Status | Frequency | Percent |
|---|---|---|
| No Progression | 13 | 17.81 |
| Local Progression Only | 7 | 9.59 |
| Distant Progression Only | 6 | 8.22 |
| Local and Distant Progression | 47 | 64.38 |
COMMENT
Study limitations
This study suffers the limitations of being retrospective and significant variability in chemotherapy administration. Although all patients received PDT, two different photosensitizers were used. Quantifiable quality of life measures were not obtained.
Study strengths
Follow-up: At a median follow up of 5.3 years, this is a particularly mature data set for MPM. A single surgeon series likely results in more consistency of the largest variable for EPD, surgical technique.
Surgery
With a median tumor volume of 550ml, 68% N2 disease and 89% stage III-IV patients, this does represent a cohort of advanced patients, including patients requiring chest wall resections, bulky nodal disease and tumor volumes greater than 2 liters. A significant portion of this series is comprised of patients who are sometimes denied surgery, or at least not considered candidates for lung-sparing surgery. Whether or not EPD is the optimal approach is debatable, but this study demonstrates that is likely an option for essentially all surgical patients in whom MCR can be achieved. Using the described surgical technique there has not yet been a patient encountered where central bronchovascular invasion mandated EPP to achieve MCR. There were patients with mixed histology in whom MCR could not be achieved, but this was secondary to esophageal and/or aortic invasion and conversion to pneumonectomy would not have helped.
Teamwork
PDT is a particularly complex intraoperative adjuvant therapy that clearly increases the challenges of postoperative care and for which major complications have been reported. Whether or not PDT played a role in these results is unknown, but will hopefully be answered by an ongoing randomized trial (NCT02153229). This study does demonstrate, however, that even this very complicated treatment plan can be safely executed by a focused and dedicated team.
Unique data
The collection of intercostal lymph nodes, previously not described in this context, appears to provide information of prognostic significance. For instance, in 4 patients only these nodes were positive. As tumor volume appears prognostic, the physical measurement of tumor volume provides interesting information, which at a minimum, can be used to validate radiographic volume estimation techniques. The use of a lung sealant appears to have mitigated persistent air leaks, one of the most vexing complications of EPD. While there were still persistent air leaks, 17/73 (23%), the duration of chest tube days generally decreased and discharge with Heimlich valves was eliminated once the sealant was adopted. Over the course of the study management of fully resected pericardium evolved from prosthetic reconstruction to leaving it open in 12% of the patients, without any known early or late complications. This simplified surgery and eliminated potential for patch contamination/infection in the setting of air leaks. The pneumonia rate of 28% is high. Extra precautions to prevent aspiration were taken and attention to pulmonary toilet, including liberal use of bronchoscopy, was routine. The reason for this high pneumonia rate is unclear, but is potentially related to the use of PDT. These data are superficially incorporated into this report, but are the focus of separate upcoming analyses.
Discussion of results
Despite a likely contribution by PDT to the complexity of care and possibly complications, morbidity and mortality are within the range of what is reported for similar patients. With respect to survival, several interesting results were generated. The OS of 3 years achieved for all patients in this advanced stage cohort is consistent with other favorable results in the literature {14,15,16}. The DFS of 14 months being less than half the OS is somewhat unusual. The presence of distant recurrence versus local recurrence portended a 10-month decrease in OS. A greater impact of lymph node status, perhaps a reflection of the more mature follow-up, was noted in this analysis than in our previous reports. The presence of any positive lymph nodes dropped the OS to approximately two years, but if no lymph nodes were involved the median OS was 7.3 years. This last group is particularly interesting as 75% were male, 68% were stage III/IV, median measured tumor volume was 325ml and the DFS was less than a third of the OS.
Value of this study
The surgical approach that was employed was reproducible and reliably permitted lung preservation, to the point where it would potentially allow discrimination between different adjuvant approaches. The study identified a cohort of patients, N0 epithelial, who had a particularly promising outcome. Nineteen patients, however, is too few to draw any definitive conclusions, but this finding is intriguing, especially in light of the OS being more than triple the DFS. More commonly, including some recent promising results, the OS and DFS are nearly the same {15,16}. This OS/DFS disparity could be related to having two lungs instead of one or potentially be a function of an immunologic effect, in this case possibly from PDT. In either case it is a phenomenon worth exploring and exploiting if possible, attempting to nudge MPM more toward a chronic disease until we have a cure.
Challenges in the field
Recent systemic therapy trials have reported a median survival of 18 months and the nonsurgical arm of the MARS trial reported a median survival of 19.5 months for the surgical candidates treated medically{2,17}. As we focus on what is important to patients, OS and quality of life, perhaps this 18 month range, not “one year” is the OS benchmark that should be measured against before declaring a surgery-based approach as beneficial.
Adjuvant therapies are the innovation and potential source of a breakthrough for MPM, but it is difficult or impossible to attribute outcomes to adjuvants at this time. Even with a consistent surgical platform like EPP, nonstaging prognosticators can confound comparisons between series. Lung-sparing surgery is currently so variable that rigorous comparison between series is likely impossible.
Where to go
In order to achieve the goal of knowing which patients should have surgery, which adjuvants are beneficial and which procedure should be performed, the following steps are necessary:
Techniques and quantification of completeness of resection for EPD needs to be refined, defined and standardized. This will allow a lung-sparing approach to be used, like EPP, as a stable platform to evaluate adjuvant therapies.
Future series should incorporate whatever new staging system is developed, but also carefully track and report non-staging prognosticators. This will allow for better comparisons between studies until, hopefully, molecular analyses provide definitive guidance.
Quality of life measurements should be routinely obtained and reported. Until such time that these operations are curative, this will allow for greater discrimination between surgical trials with similar survival results, better informed consenting of patients and stronger evidence to define the role of surgery.
Shift the goal of treatment to OS with maintenance of quality of life, not local control. Until there is a cure, treatments such as immunotherapies that slow progression without significant morbidity, may be more desirable than more morbid treatments geared toward local control.
Embrace innovative adjuvants and capitalize upon the most promising approaches to initiate multicenter trials, paying strict attention to surgical consistency and data gathering.
In conclusion
This study has significant limitations, but validates a surgical approach that consistently permitted lung preservation and yielded some promising results, particularly with respect to outcomes for the subgroup of advanced stage patients without nodal metastases. The impact of nodal metastases and histologic subtype are highlighted by this study, demonstrating the importance of appropriate patient selection for MPM surgery. An unusually prolonged OS:DFS ratio was observed, an intriguing result worth exploration and exploitation if an actionable mechanism is identified. The potential value of intraoperative PDT is again raised, a question that should be answered by an ongoing randomized trial. This study demonstrates a complicated multimodal treatment plan can be safely executed with teamwork. Analysis and critical review of this study reveals areas where generalized improvements can be made in surgery-based trials for MPM.
Acknowledgments
This work was supported, in part, by National Institutes of Health, National Cancer Institute grant number P01 CA-087971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
References
- 1.Damhuis RA, Khakwani A, DeSchutter H, Rich AI, Burgers JA, vanMeerbeeck JP. Treatment patterns and survival analysis in 9014 patients with malignant pleural mesothelioma from Belgium, the Netherlands and England. Lung Cancer. 2015;(89):212–217. doi: 10.1016/j.lungcan.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomized, controlled, open-label, phase 3 trial. Lancet. 2015 Dec 21;:1–10. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol. 2011;6:1304–1312. doi: 10.1097/JTO.0b013e3182208e3f. [DOI] [PubMed] [Google Scholar]
- 5.Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2008;136:605. doi: 10.1016/j.jtcvs.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 6.Richards W, Godleski J, Yeap B, Corson J, Chirieac L, et al. Proposed Adjustments to Pathologic Staging of Epithelial Malignant Pleural Mesothelioma Based on Analysis of 354 Cases. Cancer. 2010;116:1510–17. doi: 10.1002/cncr.24886. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhoff RR, Yang CF, Speicher PJ, Hartwig MG, D-Amico TA, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology and End Results database. J Surg Res. 2015 Jun 1;196(1):23–32. doi: 10.1016/j.jss.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugarbaker D, Richards W, Bueno R. Extrapleural Pneumonectomy in the Treatment of Epithelioid Malignant Pleural Mesothelioma: Novel Prognostic Implications of Combined N1 and N2 Nodal Involvement Based on Experience of 529 Patients. Ann Surg. 2014 Oct;260(4):577–582. doi: 10.1097/SLA.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–31. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL. American Joint Committee on Cancer & American Cancer Society AJCC Cancer Staging Manuel. New York: Springer; 2002. [Google Scholar]
- 11.Friedberg JS, Mick R, Culligan M, Stevenson J, Fernandes A, Smith D, Glatstein E, Hahn S, Cengel K. Photodynamic Therapy (PDT) and the Evolution of a Lung Sparing Surgical Treatment for Mesothelioma. Annals of Thoracic Surgery. 2011 Jun;91(6):1738–45. doi: 10.1016/j.athoracsur.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg JS, Culligan M, Mick R, Stevenson J, Hahn S, Sterman D, Glatstein E, Cengel K. Radical Pleurectomy and Intraoperative Photodynamic Therapy for Malignant Pleural Mesothelioma. Annals of Thoracic Surgery. 2012 May;93:1658–1667. doi: 10.1016/j.athoracsur.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg JS. State of the art in the technical performance of lung sparing operations for pleural mesothelioma. Seminars in Thoracic and Cardiothoracic Surgery. 2013 Summer;25(2):125–143. doi: 10.1053/j.semtcvs.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Sugarbaker DJ, Gill R, Yeap B, Wolf A, DaSilva M, et al. Hyperthermic intraoperative pleural Cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg. 2013 Apr;145:955–63. doi: 10.1016/j.jtcvs.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Lang-Lazdunski L, Bille A, Papa S, Marshall S, Lal R, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and ssytemtic chemotherapy in patients with malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg. 2015 Feb;149:558–66. doi: 10.1016/j.jtcvs.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 16.dePerrot M, Feld R, Leighl N, Hope A, Waddell T, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2016 Feb;151(2):468–475. doi: 10.1016/j.jtcvs.2015.09.129. [DOI] [PubMed] [Google Scholar]
- 17.Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomized feasibility study. Lancet Oncol. 2011 Aug;12(8):763–72. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedberg JS. Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Seminars in Thoracic & Cardiovascular Surgery. 2009 Summer;21(2):177–187. doi: 10.1053/j.semtcvs.2009.07.001. [DOI] [PubMed] [Google Scholar]