Historical Perspective on Prostate Specific Antigen (PSA) Screening
Prostate Cancer (PCa) Epidemiology
PCa is the most commonly diagnosed malignancy in males, but has one of the lowest percentage mortality compared to other cancers. An estimated 180,890 new cases are expected to be diagnosed in 2016 and 26,120 men are expected to die from the disease. A significant dichotomy exists between lifetime risk of being diagnosed versus dying from the disease. 17% of US males are diagnosed with PCa within their lifetime, while only 3% die from the disease.1 The prevalence is highest in African American men, who also have a higher risk of mortality from PCa. Caucasian males have the next highest risk, while incidence is lower among those of Asian descent.
Historical Perspective on PSA Screening
Historically, there have been two ways to screen for PCa: the digital rectal examination (DRE) and PSA blood test. In 1986, the Food and Drug Administration (FDA) approved PSA for use in monitoring PCa recurrence. In 1994, PSA was approved for screening, and was historically used in conjunction with DRE.
Screening with PSA has several limitations. Many men who do not have PCa will screen positive and require a biopsy to rule out cancer, while a few with aggressive disease have low PSA. Because many PCa grow so slowly that they never threaten a patient’s life, there is a danger of over-treatment if these cancers are detected. This is a particularly important issue since treatment for PCa is often associated with significant side effects. Our emphasis going forward should be finding the more aggressive cancers, while avoiding biopsy in those at low risk or those with indolent disease.
Current Screening Recommendations and the Evidence
PCa Screening
Screening is defined as the process of identifying apparently healthy people who may be at increased risk of a disease or condition. Current strategies for managing PCa are mainly aimed at early detection. The potential risks incurred by screening, diagnosis, and the resulting over-treatment of PCa have been well documented within the literature2. These include erectile dysfunction, incontinence, and complications from biopsies, surgery, radiation, or androgen deprivation therapy. The majority of harms associated with over-treatment occur in men in whom PCa would not have been detected in their lifetime had it not been for screening.3 Rates of active surveillance are rising rapidly across the globe.4, 5 Conversely, discontinuing screening altogether has been projected to increase the rates of metastatic disease6 and will preclude the opportunity for many men to receive life saving intervention. Given the substantial advantages and disadvantages associated with historical screening and management paradigms, many providers have been left without a clear roadmap.
Screening Guidelines
Screening recommendations from various organizations differ widely. The U.S. Preventive Services Task Force and Canadian Task Force on Preventive Health Care have recommended against any screening for men of all ages, while most other organizations recommend some variation of shared decision-making. The American Urological Association (AUA) does not recommend routine screening for men ages 50–55, but recommends shared decision-making for men ages 55–69. AUA further mentions that 2-year intervals can be considered to reduce harm.7 NCCN recommends a risk-based screening algorithm, including the patient’s age (consider screening at age 45; in the later 40s 1.0ng/ml is recommended by NCCN as a cutoff for screening within 2 years). Notably, DRE is no longer first-line in the 2015 NCCN guidelines.
Separate European and American randomized screening trials demonstrated divergent results. The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial did not show convincing evidence that PSA screening reduces PCa mortality. However, a large majority of men in the control group received PSA testing before and/or during the study so it was not comparison of screening vs. no screening but rather organized versus opportunistic screening. Furthermore, recent data from the PLCO trial indicate that contamination substantially limited the ability of the PLCO to identify a clinically significant screening benefit.8 The European Randomized Study of Screening for Prostate Cancer found that screening reduces metastasis and PCa death, but also leads to over-diagnosis. In both trials, biopsy was recommended based on a fixed PSA threshold. An alternate approach to a fixed screening protocol is a risk-adapted approach. For example, Thompson et al suggested that optimal PSA screening frequency for men with PSA 0.1 to 1.0ng/ml might be up to every 10 years. This approach has the potential to dramatically reduce the cost of screening, decrease over-detection of inconsequential tumors, and maintain detection of tumors for which treatment has been proven to reduce PCa mortality.9
Screening in the Primary Care Setting
Today, after nearly three decades since PSA was first used as a tool for early detection of PCa, substantial uncertainty surrounds its use. Primary care physicians (PCPs), including internists, order approximately 90% of all PSA screening tests.10 Shared decision-making is ideal but may be difficult to implement in the primary care setting due to several factors, including: the limited time and availability that PCPs have for in-depth discussions about the pros and cons of PSA testing (given the numerous other issues typically covered in a visit); the wide range of information and data that could be discussed during each visit; and the complex tradeoff between immediate harms and long-term benefits.11 Furthermore, shared decision-making rarely occurs with many tests performed by PCPs – often times, it only occurs after an abnormality is detected.
In some cases, spending considerable amounts of time discussing PSA may be seen as an inherent bias towards screening and could result in reduced time spent on other preventive services, thereby resulting in an opportunity cost. Studies have found that only ~half of PCPs are compliant with recommendations to discuss screening with eligible patients, with a large proportion adopting a default “screen all” or “screen none” approach.12 The value of a “detailed discussion” about PSA depends critically on the PCPs’ knowledge. Less than one in five PCPs report confidence in their knowledge about PSA, with a low correlation between confidence and actual knowledge.13, 14 A recent article in JAMA by Eggener et al acknowledged that a novel approach to PCa screening is needed because of the workflow limitations in “discussing this complex decision” in the PCP office.11, 15 Assuming that some element of screening is embraced, there is a need to develop a simple/easy algorithm regarding the role of PSA in PCa screening and to assess when further diagnostic tests are needed.
A New Perspective on PSA Screening in the Primary Care Setting
Rather than the fixed one-size-fits all approach used for screening in the past, there may be ways to use PSA more intelligently for more personalized decisions. We need to avoid PSA tests in men who would have little to no gain by focusing on age and health. Several authors have targeted the relative risks of a baseline PSA and subsequent risk of an abnormal level of greater than 4 in 5 years.16, 17 In a 2011 article, Crawford et al found that a PSA of <1.5ng/ml constitutes a very low risk category for developing PCa (particularly high risk disease) within 4–5 years18. In a follow up piece, they suggested embracing the 1.5ng/ml level and only having further discussions or workup with those above that threshold. An elevated PSA (>1.5ng/ml) becomes case finding (with a focus on identifying men who have a higher risk of having clinically significant disease). In such a case where there is increased suspicion of clinically significant disease, informed decision-making should be employed, as several options are available as next steps. These options include: following up with the patient in 6 months or 1 year, referral to a urologist, or using new techniques, such as MRI or biomarkers to determine whether the patient is at risk of harboring clinically significant disease. For those below the level, recommendations were made to screen again in 5 years. Potential benefits of this approach include reducing the cost of screening, decreasing over detection of inconsequential tumors, and maintaining detection of tumors for which treatment has been proven to reduce PCa mortality.9
Table 1 outlines PSA measurements on approximately 500,000 men from a reference lab outlining PSA levels by age. Approximately 70% of men ages 45 to 70 years of age have a PSA of less than 1.5ng/ml. Some suggest that a change in screening that leads to the biggest health gain is to stop screening older men. This applies to the majority of men over 70 and those over 60 with low PSA (e.g. <1.5ng/ml).19, 20 Indeed, Lilja et al found that a single PSA measured in white men between 44–50 years was highly correlated with any cancer, palpable disease and advanced cancer. At a PSA threshold of 1.5ng/ml theses values were approximately 20%, <15%, and 5% respectively 20–25 years after blood drawn when they were 44–50 years of age. Similarly, Vickers et al showed that for white men with a PSA in the highest 10th grouping (i.e., 1.6ng/ml or greater) at age 45–49 contributed to nearly half of all PCa deaths over the next 25–30 years. They further postulated that low-risk men based on baseline PSA might only need three PSA measurements in their lifetime (i.e., 40s, 50 and age 60). Little is known, however, whether these long-term data apply to men of other racial and ethnic groups.21,22 Crawford et al showed that at least over a 4-year period both Caucasian and African American men were at low risk for any cancer diagnosis (0.51% and 0.54% respectively) when their baseline serum PSA level was <1.5ng/ml.18 Of note, among both African American and Caucasian men a substantial majority of PSA values were <1.5ng/ml (79% and 80% respectively).18
Table 1. PSA measurements of less than 1.5 ng/ml.
(provided with permission of Charles T. Todd Jr., BioReference Laboratories, Inc.)
| Age Group | # of men with PSA < 1.5 ng/ml | % of men by age group with PSA < 1.5 ng/ml | Total # Results |
|---|---|---|---|
| 41–45 | 36,121 | 89.6% | 40,306 |
| 46–50 | 54,616 | 84.1% | 64,920 |
| 51–55 | 69,853 | 76.8% | 90,949 |
| 56–60 | 59,195 | 67.2% | 88,034 |
| 61–65 | 45,386 | 58.1% | 78,072 |
| 66–70 | 34,931 | 51.6% | 67,675 |
| 71–75 | 23,742 | 48.3% | 49,185 |
| 76–80 | 16,255 | 47.1% | 34,506 |
| 81–85 | 9,572 | 46.4% | 20,616 |
| 86–90 | 4,562 | 44.9% | 10,154 |
| 91–96 | 1,266 | 40.1% | 3,154 |
| Grand Total | 355,499 | 64.9% | 547,571 |
Next Generation of Clinical Decision Making Tools
Under the proposed paradigm (Figure 1), PSA can be performed as part of a regular blood panel and only men with a PSA ≥ 1.5ng/ml require shared decision-making about further testing and diagnostic evaluation. This will greatly limit the number of men requiring such a discussion and can be performed either by the PCP or by referral to a urologist. Men with elevated PSA should be evaluated for benign causes. Repeat testing of PSA and secondary molecular tests such as PCA3, SelectMDx, 4KScore, and PHI can be used to refine the specificity of screening to detect high-risk disease (Table 2). However, sampling error is an inherent and well-documented issue with false-negative rates of prostate biopsy procedures reported as high as 25%–35%.23, 24 This results in repeat biopsies, which are associated with additional risks of infection and hospitalization, and with significant costs.
Figure 1. Approach to Shared Care in PCa Diagnosis.
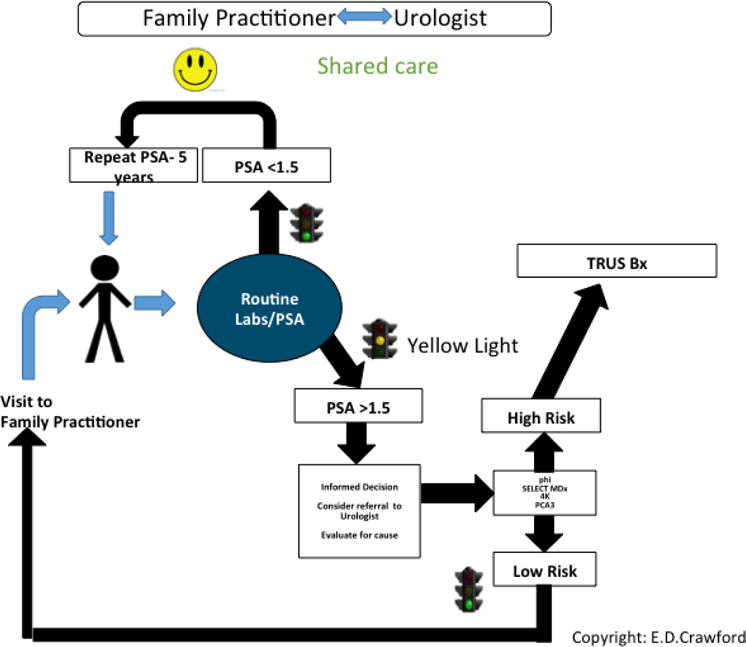
The patient presents to the family practitioner for a routine visit. PSA is obtained with other routine laboratory tests. If PSA is less than 1.5 ng/ml, the patient enters the follow-up pool and has a repeat PSA in 5 years. If PSA is greater than 1.5 ng/ml informed decision is obtained and consideration of referral to urologist. Urologists will evaluate for possible causes including BPH, prostatitis, and PCa. If there is concern for PCa, then a genomic test such as PHI, SelectMDx, 4KScore, or PCA3 is performed to identify men who are at risk of a significant cancer. If the disease is considered low risk, return to the family practitioner for a repeat in one year. If the disease is considered high-risk, consider a TRUS biopsy.
Table 2. Molecular Tests To Identify Gleason Sum equal to or greater than 7.
Secondary molecular tests that may be used to refine the specificity of screening to detect high-risk PCa.
| Assay | PHI | 4KScore | PCA3 | SelectMDx |
|---|---|---|---|---|
| Company | Beckman Coulter |
Opko | Hologic | MDxHealth |
| Specimen | Blood | Serum | *Urine | *Urine |
| Methodology | Immunoassay, 3 protein biomarkers: tPSA and fPSA, proPSA |
Immunoassay, 4 kallikreins biomarkers,: PSA, fPSA, intact PSA, HK-2 |
qPCR, mRNA test, 1 biomarker: PCA3 |
qPCR, 2 mRNA biomarkers: DLX1, HOXC6 |
| Regulatory | FDA/CE | LDT/CLIA/CE | FD/CE | LDT/CLIA/CE |
| List Price ($) | $499 | $1,900 | $500 | $500 |
| Assay Performance | AUC 0.73 | AUC 0.82 | AUC 0.68 | AUC 0.89 |
After DRE
Conclusion
Although many organizations now recommend shared decision-making when it comes to PSA testing, this can present many logistical challenges in daily clinical practice and is not always realistic. Furthermore, we acknowledge that several groups recommend that informed decision-making take place before the PSA test is ordered. However, our aim is to draw a parallel to what happens in the real world for PCPs – i.e., informed decision-making typically comes after the test results (be it blood sugar, blood pressure, cholesterol, or in this case PSA) are known. PCPs are confused about the messages we try to deliver regarding PSA: PSA velocity, age specific PSA, percent free PSA, PSA density, PSA cutoffs of 1ng/ml, 1.5ng/ml, 2.5ng/ml, and 4ng/ml. We believe that a simple message using a PSA cutoff of 1.5ng/ml is reflective of what PCPs often experience with conditions such as mild hypertension and pre-diabetes. In this paper, we have presented an alternative approach in which screening is performed for men with at least a 10-year life expectancy. If the PSA is less than 1.5ng/ml (approximately 70% of men who have a screening PSA), consider a 5-year re-screening interval. If the PSA is ≥ 1.5ng/ml, or the PCP identifies an abnormality on DRE, refer to a specialist or consider a biomarker to assess risk more precisely. This algorithm is similar to that utilized for an elevated blood sugar, where an abnormal result triggers another test such as an A1C hemoglobin. In our algorithm, we recommend that a biopsy should not be performed unless the risk of an aggressive tumor is significant, and following a thorough discussion of benefits and risks with the patient. These discussions should emphasize that the purpose of screening is the early identification of potentially lethal disease, and that in most cases low-risk tumors, if identified, do not require immediate treatment. A potential benefit of this approach is that it could greatly reduce the number of men requiring shared decision-making and further testing to those at greater risk of significant PCa.
Acknowledgments
The authors acknowledge Karen Ventii, PhD for editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 5.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120:3519–3526. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA) Oncology (Williston Park) 2000;14:267–272. 277–268, 280. passim. [PubMed] [Google Scholar]
- 8.Gulati R, Tsodikov A, Wever EM, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23:827–835. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfond J, Choate K, Ankerst DP, Hernandez J, Leach RJ, Thompson IM., Jr Intermediate-Term Risk of Prostate Cancer is Directly Related to Baseline Prostate Specific Antigen: Implications for Reducing the Burden of Prostate Specific Antigen Screening. J Urol. 2015;194:46–51. doi: 10.1016/j.juro.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Aslani A, Minnillo BJ, Johnson B, Cherullo EE, Ponsky LE, Abouassaly R. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191:1737–1742. doi: 10.1016/j.juro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Vickers AJ, Edwards K, Cooperberg MR, Mushlin AI. A simple schema for informed decision making about prostate cancer screening. Ann Intern Med. 2014;161:441–442. doi: 10.7326/M14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vickers AJ, Edwards K, Cooperberg MR, et al. Supplement. Recommendations on Shared Decision Making For Prostate Cancer Screening: Review of the Literature. Ann Intern Med. 2014;161:3. doi: 10.7326/M14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasian GE, Cooperberg MR, Cowan JE, et al. Prostate specific antigen screening for prostate cancer: knowledge of, attitudes towards, and utilization among primary care physicians. Urol Oncol. 2012;30:155–160. doi: 10.1016/j.urolonc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Tasian GE, Cooperberg MR, Potter MB, et al. PSA screening: determinants of primary-care physician practice patterns. Prostate Cancer Prostatic Dis. 2012;15:189–194. doi: 10.1038/pcan.2011.59. [DOI] [PubMed] [Google Scholar]
- 15.Eggener SE, Cifu AS, Nabhan C. Prostate Cancer Screening. JAMA. 2015;314:825–826. doi: 10.1001/jama.2015.8033. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S, Carter HB, Catalona WJ, Moul JW, Schroder FH. Baseline prostate-specific antigen testing at a young age. Eur Urol. 2012;61:1–7. doi: 10.1016/j.eururo.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 17.Crawford ED, Pinsky PF, Chia D, et al. Prostate specific antigen changes as related to the initial prostate specific antigen: data from the prostate, lung, colorectal and ovarian cancer screening trial. J Urol. 2006;175:1286–1290. doi: 10.1016/S0022-5347(05)00706-8. discussion 1290. [DOI] [PubMed] [Google Scholar]
- 18.Crawford ED, Moul JW, Rove KO, Pettaway CA, Lamerato LE, Hughes A. Prostate-specific antigen 1.5–4.0 ng/mL: a diagnostic challenge and danger zone. BJU Int. 2011;108:1743–1749. doi: 10.1111/j.1464-410X.2011.10224.x. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson S, Assel M, Sjoberg D, et al. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: population based cohort study. BMJ. 2014;348:g2296. doi: 10.1136/bmj.g2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen PJ, Connolly D, Tammela TL, et al. Balancing the harms and benefits of early detection of prostate cancer. Cancer. 2010;116:4857–4865. doi: 10.1002/cncr.25474. [DOI] [PubMed] [Google Scholar]
- 21.Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117:1210–1219. doi: 10.1002/cncr.25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Calle C, Patil D, Wei JT, et al. Multicenter Evaluation of the Prostate Health Index to Detect Aggressive Prostate Cancer in Biopsy Naive Men. J Urol. 2015;194:65–72. doi: 10.1016/j.juro.2015.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart GD, Van Neste L, Delvenne P, et al. Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: results of the MATLOC study. J Urol. 2013;189:1110–1116. doi: 10.1016/j.juro.2012.08.219. [DOI] [PubMed] [Google Scholar]


