Abstract
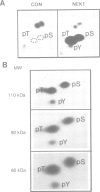
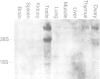
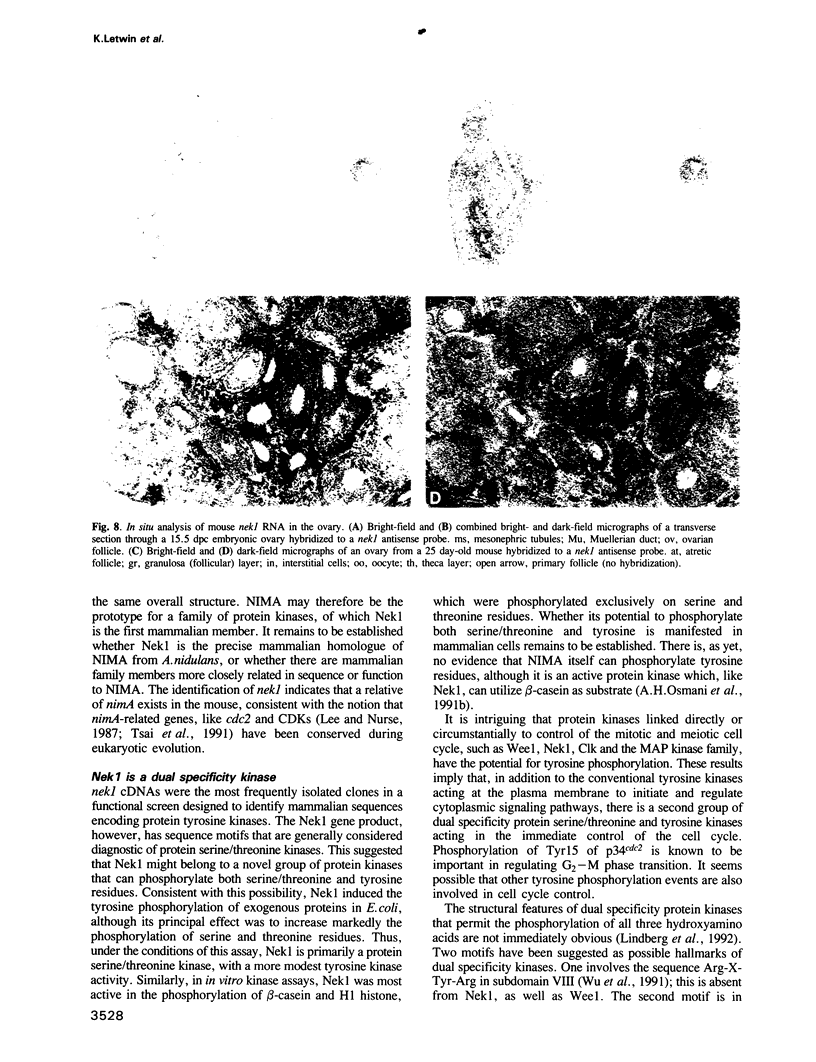
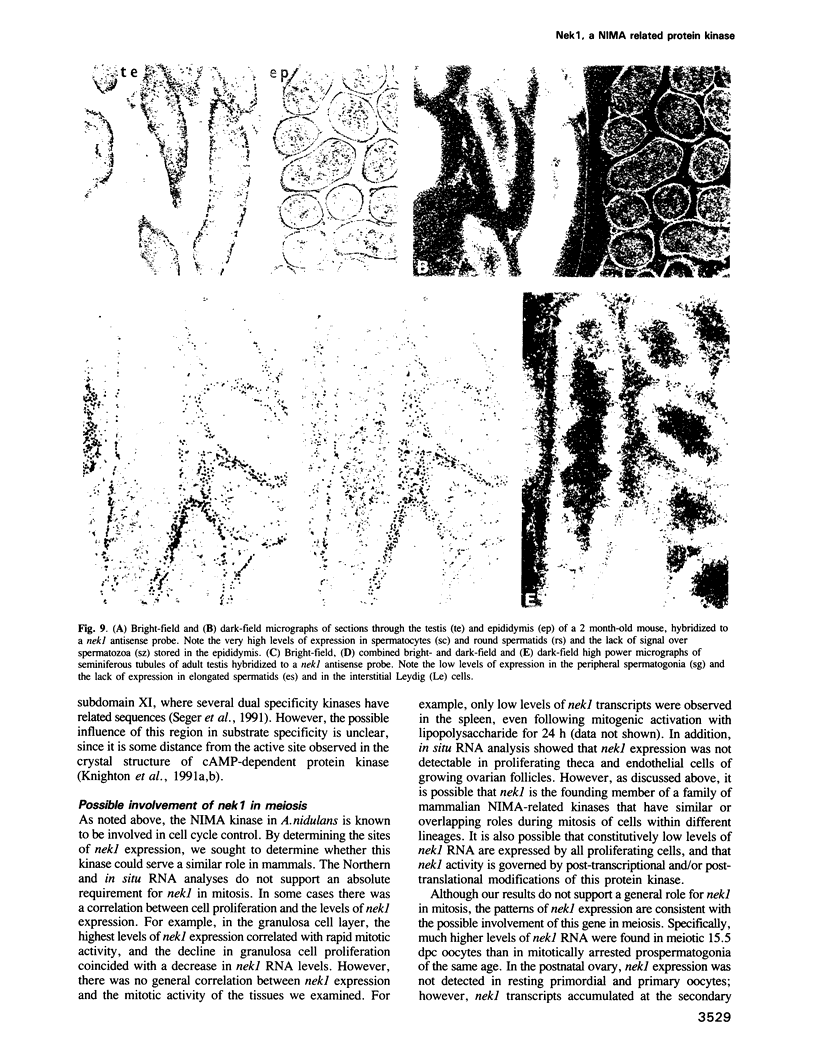
Screening of mouse cDNA expression libraries with antibodies to phosphotyrosine resulted in repeated isolation of cDNAs that encode a novel mammalian protein kinase of 774 amino acids, termed Nek1. Nek1 contains an N-terminal protein kinase domain which is most similar (42% identity) to the catalytic domain of NIMA, a protein kinase which controls initiation of mitosis in Aspergillus nidulans. In addition, both Nek1 and NIMA have a long, basic C-terminal extension, and are therefore similar in overall structure. Despite its identification with anti-phosphotyrosine antibodies, Nek1 contains sequence motifs characteristic of protein serine/threonine kinases. The Nek1 kinase domain, when expressed in bacteria, phosphorylated exogenous substrates primarily on serine/threonine, but also on tyrosine, indicating that Nek1 is a dual specificity kinase with the capacity to phosphorylate all three hydroxyamino acids. Like NIMA, Nek1 preferentially phosphorylated beta-casein in vitro. In situ RNA analysis of nek1 expression in mouse gonads revealed a high level of expression in both male and female germ cells, with a distribution consistent with a role in meiosis. These results suggest that Nek1 is a mammalian relative of the fungal NIMA cell cycle regulator.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amon A., Surana U., Muroff I., Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992 Jan 23;355(6358):368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- Babitzke P., Kushner S. R. The Ams (altered mRNA stability) protein and ribonuclease E are encoded by the same structural gene of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):1–5. doi: 10.1073/pnas.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. Dev Biol (N Y 1985) 1985;1:453–524. doi: 10.1007/978-1-4615-6814-8_11. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Letwin K., Tannock L., Bernstein A., Pawson T. A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 1991 Feb;10(2):317–325. doi: 10.1002/j.1460-2075.1991.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortay J. C., Nègre D., Cozzone A. J. Analyzing protein phosphorylation in prokaryotes. Methods Enzymol. 1991;200:214–227. doi: 10.1016/0076-6879(91)00141-i. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A. A., Erikson R. L. Mouse Erk-1 gene product is a serine/threonine protein kinase that has the potential to phosphorylate tyrosine. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8845–8849. doi: 10.1073/pnas.88.19.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey D., Schieven G. L., Lim M. Y., Marquardt H., Gilmore T., Thorner J., Martin G. S. Novel yeast protein kinase (YPK1 gene product) is a 40-kilodalton phosphotyrosyl protein associated with protein-tyrosine kinase activity. Mol Cell Biol. 1990 Dec;10(12):6244–6256. doi: 10.1128/mcb.10.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dolci S., Williams D. E., Ernst M. K., Resnick J. L., Brannan C. I., Lock L. F., Lyman S. D., Boswell H. S., Donovan P. J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991 Aug 29;352(6338):809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991 Feb 28;349(6312):808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Godin I., Deed R., Cooke J., Zsebo K., Dexter M., Wylie C. C. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991 Aug 29;352(6338):807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Howell B. W., Afar D. E., Lew J., Douville E. M., Icely P. L., Gray D. A., Bell J. C. STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol Cell Biol. 1991 Jan;11(1):568–572. doi: 10.1128/mcb.11.1.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. G., Rosamond J. Isolation of a novel protein kinase-encoding gene from yeast by oligodeoxyribonucleotide probing. Gene. 1990 May 31;90(1):87–92. doi: 10.1016/0378-1119(90)90442-t. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Keshet E., Itin A., Fischman K., Nir U. The testis-specific transcript (ferT) of the tyrosine kinase FER is expressed during spermatogenesis in a stage-specific manner. Mol Cell Biol. 1990 Sep;10(9):5021–5025. doi: 10.1128/mcb.10.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Rosenberg M. P., Mercer J. A., Propst F., Vande Woude G. F., Jenkins N. A., Copeland N. G. Developmental regulation of ovarian-specific Mos expression. Oncogene. 1988 Mar;2(3):235–240. [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Letwin K., Yee S. P., Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988 Dec;3(6):621–627. [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Manova K., Nocka K., Besmer P., Bachvarova R. F. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990 Dec;110(4):1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D., Hermans A., von Lindern M., van Agthoven T., de Klein A., Mackenbach P., Grootegoed A., Talarico D., Della Valle G., Grosveld G. Molecular characterization of the testis specific c-abl mRNA in mouse. EMBO J. 1987 Dec 20;6(13):4041–4048. doi: 10.1002/j.1460-2075.1987.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., McLaren A. X-chromosome activity in foetal germ cells of the mouse. J Embryol Exp Morphol. 1981 Jun;63:75–84. [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Sadowski I., Pawson T. Mutational analysis of a phosphotransfer motif essential for v-fps tyrosine kinase activity. Oncogene. 1988 Dec;3(6):665–672. [PubMed] [Google Scholar]
- Morris N. R. Mitotic mutants of Aspergillus nidulans. Genet Res. 1975 Dec;26(3):237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Motro B., van der Kooy D., Rossant J., Reith A., Bernstein A. Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and Sl loci. Development. 1991 Dec;113(4):1207–1221. doi: 10.1242/dev.113.4.1207. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Grills G. S., Wolgemuth D. J. Evidence for the involvement of the proto-oncogene c-mos in mammalian meiotic maturation and possibly very early embryogenesis. EMBO J. 1988 Mar;7(3):683–689. doi: 10.1002/j.1460-2075.1988.tb02863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- OAKBERG E. F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956 Nov;99(3):391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. A mutation in Aspergillus nidulans that blocks the transition from interphase to prophase. J Cell Biol. 1983 Apr;96(4):1155–1158. doi: 10.1083/jcb.96.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A., Avivi A., Zimmer Y., Givol D., Yarden Y., Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990 Aug;109(4):911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Osmani A. H., McGuire S. L., Osmani S. A. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell. 1991 Oct 18;67(2):283–291. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- Osmani A. H., O'Donnell K., Pu R. T., Osmani S. A. Activation of the nimA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the bimE checkpoint. EMBO J. 1991 Sep;10(9):2669–2679. doi: 10.1002/j.1460-2075.1991.tb07810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Engle D. B., Doonan J. H., Morris N. R. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988 Jan 29;52(2):241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Pu R. T., Morris N. R. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988 Apr 22;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Lee M. S., Ogg S., Falk J. L., Swenson K. I., Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 1991 May;10(5):1255–1263. doi: 10.1002/j.1460-2075.1991.tb08067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Oskarsson M. K., Russell L. B., Nguyen-Huu M. C., Nadeau J., Jenkins N. A., Copeland N. G., Vande Woude G. F. Genetic analysis and developmental regulation of testis-specific RNA expression of Mos, Abl, actin and Hox-1.4. Oncogene. 1988 Mar;2(3):227–233. [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Murray A. W. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992 Jan 23;355(6358):365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Zheng P., Beidler D. R., Zerillo C. Spk1, a new kinase from Saccharomyces cerevisiae, phosphorylates proteins on serine, threonine, and tyrosine. Mol Cell Biol. 1991 Feb;11(2):987–1001. doi: 10.1128/mcb.11.2.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. L., Spudich J. A. Developmentally regulated protein-tyrosine kinase genes in Dictyostelium discoideum. Mol Cell Biol. 1990 Jul;10(7):3578–3583. doi: 10.1128/mcb.10.7.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]