Abstract
Background
Treatment response biomarkers are urgently needed for castration-resistant prostate cancer (CRPC). Baseline and post-treatment circulating tumor cell (CTC) counts of ≥5 cells/7.5 ml are associated with poor CRPC outcome.
Objective
To determine the value of a ≥30% CTC decline as a treatment response indicator.
Design, setting, and participants
We identified patients with a baseline CTC count ≥5 cells/7.5 ml and evaluable post-treatment CTC counts in two prospective trials.
Intervention
Patients were treated in the COU-AA-301 (abiraterone after chemotherapy) and IMMC-38 (chemotherapy) trials.
Outcome measures and statistical analysis
The association between a ≥30% CTC decline after treatment and survival was evaluated using univariable and multivariable Cox regression models at three landmark time points (4, 8, and 12 wk). Model performance was evaluated by calculating the area under the receiver operating characteristic curve (AUC) and c-indices.
Results
Overall 486 patients (122 in IMMC-38 and 364 in COU-AA-301) had a CTC count ≥5 cells/7.5 ml at baseline, with 440, 380, and 351 patients evaluable at 4, 8, and 12 wk, respectively. A 30% CTC decline was associated with increased survival at 4 wk (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.36–0.56; p < 0.001), 8 wk (HR 0.41, 95% CI 0.33–0.53; p < 0.001), and 12 wk (HR 0.39, 95% CI 0.3–0.5; p < 0.001) in univariable and multivariable analyses. Stable CTC count (<30% fall or <30% increase) was not associated with a survival benefit when compared with increased CTC count. The association between a 30% CTC decline after treatment and survival was independent of baseline CTC count. CTC declines significantly improved the AUC at all time-points. Finally, in the COU-AA-301 trial, patients with CTC ≥5 cells/7.5 ml and a 30% CTC decline had similar overall survival in both arms.
Conclusions
A 30% CTC decline after treatment from an initial count ≥5 cells/7.5 ml is independently associated with CRPC overall survival following abiraterone and chemotherapy, improving the performance of a multivariable model as early as 4 wk after treatment. This potential surrogate must now be prospectively evaluated.
Patient summary
Circulating tumor cells (CTCs) are cancer cells that can be detected in the blood of prostate cancer patients. We analyzed changes in CTCs after treatment with abiraterone and chemotherapy in two large clinical trials, and found that patients who have a decline in CTC count have a better survival outcome.
Keywords: Castration-resistant prostate cancer, Treatment outcome Response, Circulating tumor cells, Abiraterone, Chemotherapy
1. Introduction
Prostate cancer is the second most common malignancy in men, and the fifth leading cause of death from cancer worldwide [1]. Although initially responsive to androgen deprivation, lethal castration-resistant prostate cancer (CRPC) ultimately develops. In recent years, unprecedented advances in drug development for CRPC have been observed with the approval of abiraterone, enzalutamide, cabazitaxel, and radium [2–7].
One of the greatest challenges in the current management of CRPC is adequate assessment of response to treatment. A significant proportion of patients present with disease exclusively in bone, which is not amenable to evaluation by the commonly used Response Evaluation Criteria in Solid Tumors (RECIST). Consensus Prostate Cancer Working Group 2 (PCWG2) criteria [8] rely on bone scintigraphy and changes in prostate-specific antigen (PSA) levels to evaluate response to treatment in these patients. Progression according to bone scintigraphy is not evaluable before 16 wk because of the possibility of spurious flare reactions [9], so a confirmatory scan is required after a first scan indicating progression. Likewise, evaluation of prostate-specific antigen (PSA) values for progression is not recommended before 12 wk of treatment. Most studies evaluating PSA declines as a surrogate of survival have yielded negative results [10–12] and treatment discontinuation based solely on rising PSA values is not recommended [8]. Recent studies have reported a stronger association between radiological progression-free survival (rPFS) and overall survival (OS); however, a definition of progression according to rPFS cannot currently be acquired before at least 12–16 wk of treatment, and is difficult to evaluate in men with widespread bone involvement [13]. Improved biomarkers to identify patients not benefitting from anticancer treatment are urgently needed.
Enumeration of the circulating tumor cell (CTC) count has emerged as a powerful biomarker for evaluating prognosis and treatment response in CRPC. The utility of the CellSearch assay (Janssen Diagnostics, Raritan, NJ, USA) in classifying counts into unfavorable (≥5 cells/7.5 ml) and favorable (≤4 cells/7.5 ml) prognostic groups has been proven in prospective trials including IMMC-38, COU-AA-301, AFFIRM, and SWOG-S0421 [14–19]. Association between post-treatment CTC changes and CRPC survival has been reported in terms of CTC conversion (change from unfavorable at baseline to favorable or vice versa) [14], fold-change in CTC [17], and a 30% CTC decline from baseline [16], and it has been shown that CTC count has superior performance to other circulating biomarkers including PSA. CTCs have also been evaluated as a surrogate endpoint in several prospective trials. In the COU-AA-301 trial, a composite biomarker panel comprising CTC and lactate dehydrogenase (LDH) at 12 wk after treatment satisfied the Prentice criteria for surrogacy at the individual patient level [20]. It is envisaged that validation of these results in further prospective clinical trials could contribute to testing trial-level surrogacy so that CTC counts could become a clinical trial endpoint to accelerate drug approval for advanced CRPC.
We carried out a post hoc analysis of data for patients in the prospective IMMC-38 (chemotherapy) and COU-AA-301 (abiraterone) trials with baseline CTC ≥5 cells/7.5 ml, evaluating the value of a 30% CTC decline from baseline at 4, 8, and 12 wk as a biomarker of response to treatment.
2. Patients and methods
2.1. Study population and procedures
We performed a post hoc analysis of the COU-AA-301 and IMMC-38 trials. COU-AA-301 was a phase 3 trial in which postchemotherapy patients with metastatic CRPC were randomly assigned to abiraterone and prednisone or placebo and prednisone. IMMC-38 was a prospective, open-label study in patients with metastatic CRPC undergoing treatment with chemotherapy. Details of the methodology and the final results for both trials have been published elsewhere [2,14,21]. Both studies were approved by local institutional boards. All patients provided written informed consent before participation. CTC counts were measured at baseline and on day 1 of cycle 2 (weeks 4–5), day 1 of cycle 3 (weeks 8–9), and day 1 of cycle 4 (weeks 12–13) in the COU-AA-301 trial. In the IMMC-38 trial, CTC counts were measured in weeks 2–5 (median 4 wk), weeks 6–8 (median 7 wk), and weeks 9–12 (median 11.9 wk). All CTC counts were measured using the CellSearch assay [22]. Hemoglobin (Hb), alkaline phosphatase (ALP), albumin (ALB), and LDH concentrations were measured at baseline and at each study visit. Eastern Cooperative Oncology Group performance status (ECOG-PS) was recorded at baseline. PSA levels were measured every 4 wk in IMMC-38 and every 12 wk in COU-AA-301.
2.2. Statistical analysis
Kaplan-Meier analysis was used to estimate survival. Univariable and multivariable Cox proportional hazards models were used to test the association between the response biomarker and survival. Logistic regression models were used to calculate odds ratios (ORs). Posttreatment CTC response was defined as a 30% decline from baseline at 4, 8, and 12 wk from treatment initiation. A landmark analysis was used to explore the association between CTC response and survival, and specific 4-, 8- and 12-week populations were defined (Supplementary Fig. 1). Bonferroni correction was applied to account for multiple testing at three different time points; p values were considered statistically significant if p < 0.0167. Baseline LDH, ALP, PSA, and CTC data were log-transformed because of positively skewed distributions. The overall performance of the survival models was evaluated by calculating receiver operating characteristic (ROC) curves for 6- and 11-mo survival endpoints (approx. the median and third survival quartile of the data set) and the c-index for each model using the method proposed by Uno et al [23]. The area under the ROC curve (AUC) was compared by calculating the U statistic (nonparametric) [24]. Bootstrapping techniques were used to calculate the 95% confidence interval (CI) of the difference between c-indices. Analyses were performed using SPSS v21 (SPSS Inc., Chicago, IL, USA) and the R statistics package v3.2.1 (R Foundation, Vienna, Austria).
3. Results
Overall, 486 patients with baseline CTC ≥5 cells/7.5 ml participating in the IMMC-38 (n = 122) and COU-AA-301 (n = 364) trials were included in the analysis. The patient inclusion criteria are presented in a CONSORT diagram in Supplementary Figure 1). An analysis of patients with baseline CTC <5 cells/7.5 ml, who had significantly better outcome compared to patients with CTC ≥5 cells/7.5 ml (Supplementary Fig. 2), will be published separately. The median follow-up was 11.2 mo (10.2 mo in IMMC-38; 11.3 mo in COU-AA-301). At the time of analysis, 360 (74.1%) patients had died, with median OS of 11.6 mo (95% CI 10.3–12.8). The median OS for patients with baseline CTC ≥5 cells/7.5 ml was comparable between IMMC-38 (11.5 mo, 95% CI 9.8–13.2) and COU-AA-301 (11.7 mo, 95% CI 10.3–13.1). The median baseline CTC was 19.5 cells/7.5 ml (24 in IMMC-38 and 18 in COU-AA-301). Other baseline characteristics are summarized in Table 1 and Supplementary Table 1.
Table 1.
Baseline characteristics for the whole trial population
| All patients | COU-AA-301 | IMMC-38 | |
|---|---|---|---|
| Patients (n) | 486 | 364 | 122 |
| CTC count (cells/7.5 ml) | 19.5 (9–43.8) | 18 (9–38.5) | 24 (10–97) |
| PSA (ng/ml) | 214.4 (69–579) | 197.3 (64.8–570) | 244 (90–604) |
| ALP (U/l) | 216 (121–385.5) | 205.5 (116–401.5) | 231 (129.8–363.8) |
| LDH (U/l) | 263 (199.3–389.5) | 267 (199.5–384.8) | 250 (199.3–404.8) |
| Hemoglobin (g/dl) | 11.4 (10.3–12.5) | 11.2 (10.2–12.4) | 11.8 (10.8–12.9) |
| Albumin (g/dl) | 3.9 (3.6–4.2) | 4 (3.7–4.2) | 3.7 (3.4–4) |
| ECOG PS, n (%)a | |||
| 0–1 | 419 (87.3) | 315 (86.5) | 104 (89.7) |
| 2 | 61 (12.7) | 49 (13.5) | 12 (10.3) |
CTC = circulating tumor cell; PSA = prostate-specific antigen; ALP = alkaline phosphatase; LDH = lactate dehydrogenase; ECOG PS = Eastern Cooperative Oncology Group performance status.
Six missing baseline ECOG PS values in the IMMC-38 data set.
To define the most appropriate response cutoff, we initially compared the performance of 30% and 50% CTC declines. A 30% cutoff was chosen because of its higher sensitivity in comparison to a 50% CTC decline (Supplementary Tables 2 and 3).
3.1. A 30% CTC response is associated with survival benefit
Overall, 283 (64.3%), 248 (65.3%), and 226 (64.4%) patients experienced a 30% decline in CTC count at 4, 8, and 12 wk, respectively (Table 2). A 30% CTC decline was associated with better survival at 4 wk (14.4 vs 7.9 mo; HR 0.45, 95% CI 0.36–0.56; p < 0.001), 8 wk (15.4 vs 7.9 mo; HR 0.41, 95% CI 0.33–0.53; p < 0.001), and 12 wk (16.1 vs 9.7 mo; HR 0.39, 95% CI 0.3–0.5; p < 0.001). The association was consistent in both the COU-AA-301 and IMMC-38 data sets (Table 2). A 30% CTC decline was associated with survival in multivariable analysis. In addition to a 30% CTC decline, baseline CTC count, and baseline LDH were associated with survival across all three landmark populations (Supplementary Table 4).
Table 2.
Association between survival and CTC responsea
| n (%) | Median OS, mo (95% CI) | HR (95% CI)b | p valueb | |
|---|---|---|---|---|
| Week 4 | ||||
| All patients | 440 | 11.4 (10.5–12.4) | ||
| Response | 283 (64.3) | 14.4 (12.8–15.9) | 0.45 (0.36–0.56) | <0.001 |
| Non-response | 157 (35.7) | 7.9 (6.9–8.9) | ||
| IMMC-38 | 113 | 11.2 (9.7–12.6) | ||
| Response | 75 (66.4) | 12.3 (8.2–16.3) | 0.46 (0.29–0.74) | 0.001 |
| Non-response | 38 (33.6) | 6.8 (4.4–9.2) | ||
| COU-AA-301 | 327 | 11.7 (10.3–13.1) | ||
| Response | 208 (63.6) | 14.4 (13.2–15.5) | 0.44 (0.34–0.57) | <0.001 |
| Non-response | 119 (36.4) | 7.9 (6.9–9) | ||
| Week 8 | ||||
| All patients | 380 | 12.5 (11.1–13.9) | ||
| Response | 248 (65.3) | 15.4 (13.9–16.8) | 0.41 (0.33–0.53) | <0.001 |
| Non-response | 132 (34.7) | 7.9 (15.4–12.5) | ||
| IMMC-38 | 84 | 12.3 (9.4–15.1) | ||
| Response | 56 (66.7) | 17.2 (9.7–24.6) | 0.42 (0.24–0.74) | 0.003 |
| Non-response | 28 (33.3) | 10.2 (5.5–14.9) | ||
| COU-AA-301 | 296 | 12.6 (11.1–14.2) | ||
| Response | 192 (64.9) | 15.4 (14.1–16.7) | 0.4 (0.31–0.53) | <0.001 |
| Non-response | 104 (35.1) | 7.7 (6.7–8.5) | ||
| Week 12 | ||||
| All patients | 351 | 13.8 (12.3–15.3) | ||
| Response | 226 (64.4) | 16.1 (14.6–17.7) | 0.39 (0.3–0.5) | <0.001 |
| Non-response | 125 (35.6) | 9.7 (8.3–11.1) | ||
| IMMC-38 | 79 | 13.6 (10.6–16.6) | ||
| Response | 55 (69.6) | 18.2 (11.7–24.7) | 0.35 (0.19–0.63) | <0.001 |
| Non-response | 24 (30.4) | 13.6 (10.6–16.6) | ||
| COU-AA-301 | 272 | 13.9 (12.2–15.6) | ||
| Response | 171 (62.9) | 15.9 (14.5–17.4) | 0.41 (0.3–0.54) | <0.001 |
| Non-response | 101 (37.1) | 9.7 (7.7–11.7) |
CTC = circulating tumor cell; OS = overall survival; HR = hazard ratio; CI = confidence interval.
Response was defined as a 30% decline in CTC count relative to baseline at each of the landmark time points.
Univariable Cox regression.
Addition of a 30% CTC decline to multivariable survival models significantly enhanced the AUC and c-indices. Addition of baseline CTC count to a multivariable model comprising baseline PSA, LDH, ALB, Hb, ALP, and ECOG PS increased the c-index marginally (0.681 at 4 wk, 0.658 at 8 wk, and 0.669 at 12 wk). Addition of a 30% CTC decline to the model caused a more pronounced increase in the c-index to 0.72 at 4 wk and 0.71 at 8 and 12 wk. Likewise the ROC curves (6- and 11-mo mortality endpoints) showed a significant increase in AUC when a 30% CTC decline was added to the models (Fig. 1).
Fig. 1.
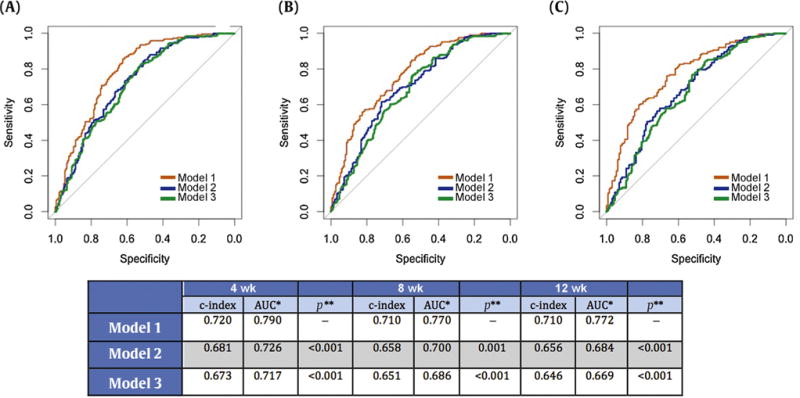
Receiver operating characteristic (ROC) curves for three models at (A) 4 wk, (B) 8 wk, and (C) 12 wk. Model 1 comprised CTC response, baseline CTC (log-transformed), baseline LDH (log-transformed), and baseline ECOG status at 4 wk; and CTC response, baseline CTC (log-transformed), and baseline LDH (log-transformed) at 8 and 12 wk. Model 2 comprised baseline CTC (log-transformed), baseline LDH (log-transformed), and baseline ECOG status at 4 wk; and baseline CTC (log-transformed) and baseline LDH (log-transformed) at 8 and 12 wk. Model 3 comprised baseline LDH (log-transformed) and baseline ECOG status at 4 wk; and baseline LDH (log-transformed) at 8 and 12 wk. CTC = circulating tumor cell; LDH = lactate dehydrogenase; ECOG = Eastern Cooperative Oncology Group; AUC = area under the ROC curve.
*Status variable: survival at 11 mo (yes vs no).
**Comparison of two correlated ROC curves (De Long’s rest) with model 1 as the reference model.
Some 113/486 patients (23.1%) achieved a confirmed 50% PSA response. PSA response was significantly associated with a 30% CTC decline at 4 wk (OR 14.8; p < 0.001), 8 wk (OR 18; p < 0.001), and 12 (OR 13.6; p < 0.001) in both the COU-AA-301 and IMMC-38 populations (Supplementary Table 5).
3.2. CTC response and treatment arm in the COU-AA-301 trial
Of the 364 COU-AA-301 trial participants in the analysis, 245 (67.3%) received abiraterone + prednisone and 119 (32.7%) received placebo + prednisone; the abiraterone cohort had better OS (13.8 vs 9.5 mo; HR 0.75, 95% CI 0.58–0.96; p = 0.02). This benefit was maintained across all three landmark survival populations (Fig. 2), confirming that abiraterone provided a significant survival benefit in patients with baseline CTC ≥5 cells/7.5 ml. Overall, 162 (73.3%) patients receiving abiraterone + prednisone and 46 (43.4%) patients receiving prednisone + placebo had a 30% CTC decline, confirming the intrinsic antitumor activity of prednisone. Treatment arm was not significantly associated with survival when a 30% CTC decline was included in the model. Furthermore, interaction tests between treatment arm and a 30% CTC decline were not significant (p = 0.758), suggesting an equivalent survival benefit for abiraterone and prednisone or prednisone alone in post-chemotherapy patients who achieved a 30% CTC decline (Table 3).
Fig. 2.
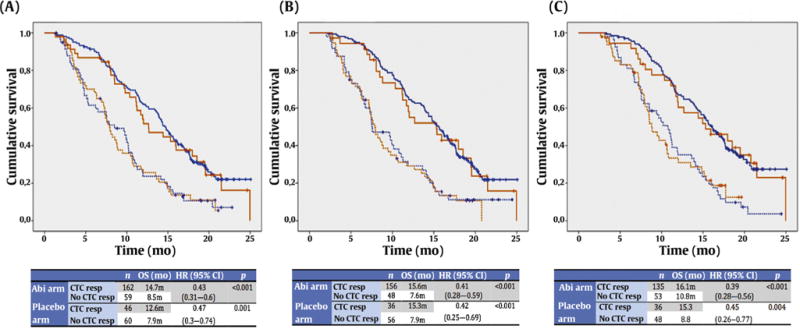
Survival in COU-AA-301 according to treatment arm and CTC response at (A) 4 wk, (B) 8 wk, and (C) 12 wk. Blue lines denote data for patients who received abiraterone + prednisone and red lines patients who received placebo + prednisone. Continuous lines indicate patients with a CTC response and dotted lines patients with no CTC response. CTC = circulating tumor cell; OS = overall survival; CI = confidence interval; Abi = abiraterone; resp = response.
Table 3.
Effect of treatment arm on multivariable models with and without CTC response in the COU-301 trial
| Model without CTC responsea
|
Model with CTC responseb
|
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Week 4 | 0.65 (0.49–0.84) | 0.001 | 0.87 (0.65–1.17) | 0.352 |
|
| ||||
| Week 8 | 0.65 (0.49–0.86) | 0.003 | 0.9 (0.66–1.24) | 0.529 |
|
| ||||
| Week 12 | 0.73 (0.53–0.98) | 0.041 | 0.86 (0.63–1.18) | 0.360 |
CTC = circulating tumor cell; HR = hazard ratio for treatment arm (abiraterone vs placebo); CI = confidence interval.
Model includes: treatment arm; baseline CTC count (log-transformed); lactate dehydrogenase (log-transformed); albumin; alkaline phosphatase (log-transformed); hemoglobin; prostate-specific antigen (log-transformed); and Eastern Cooperative Oncology Group performance status.
Model includes: 30% CTC response at 4, 8, or 12 wk; treatment arm; baseline CTC count (log-transformed); lactate dehydrogenase (log-transformed); albumin; alkaline phosphatase (log-transformed); hemoglobin; prostate-specific antigen (log-transformed); and Eastern Cooperative Oncology Group performance status.
3.3. Stable CTC count and CTC conversion
We investigated the utility of a stable CTC count, defined as a change from baseline that did not exceed a 30% decline or a 30% increase, at each of the prespecified time points. Overall, 57 (13%), 43 (11.3%), and 42 (12%) patients experienced a stable CTC count at 4, 8 and 12 wk, respectively. A 30% CTC decline showed a significant OS benefit when compared to a stable CTC count at all time points, but no difference was observed when comparing stable and progressive (>30% increase) CTC counts (Fig. 3).
Fig. 3.
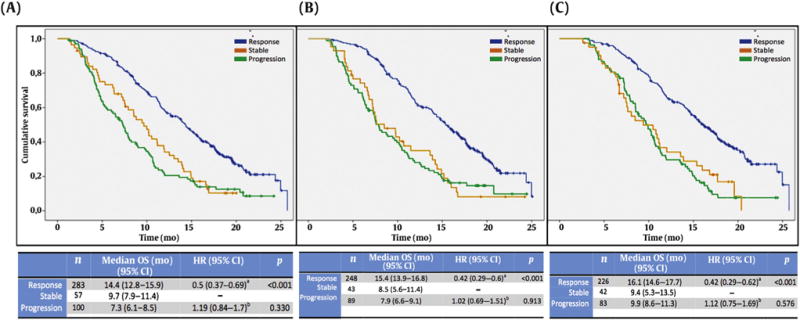
Overall survival (OS) according to circulating tumor cell (CTC) response at (A) 4 wk, (B) 8 wk, and (C) 12 wk. The hazard ratio (HR) and 95% confidence interval (CI) were determines using Cox regression with CTC response as the categorical variable and stable disease as the reference covariable.
aStable versus response.
bStable versus progression.
Overall, 165 (37.5%), 193 (44.3%), and 154 (43.9%) patients achieved conversion to a favorable CTC count of <5 cells/7.5 ml at 4, 8, and 12 wk, respectively. Patients achieving such CTC conversion also had a significant OS benefit at all time points studied (Supplementary Table 6). We compared AUC values for CTC conversion and 30% CTC response (6-mo OS) among all patients and among patients with baseline CTC ≥10 and ≥30 cells/7.5 ml (Supplementary Table 7). Although the AUC was consistently higher for a 30% CTC decline than for CTC conversion, no significant differences were found except for patients with high baseline CTC (≥10 cells/7.5 ml) at 4 wk (AUC 0.701 vs 0.624; p = 0.008).
4. Discussion
The prognostic value of baseline CTC has been evaluated in a number of studies in which patients received chemotherapy [14,17,18] and androgen receptor (AR) signaling inhibitors [19,20]. The value of a post-treatment change, defined as the percentage change from baseline in the manner for other established treatment response biomarkers such as PSA decline or a change in diameter of target lesions (RECIST), has been suggested by our group in a report on a large single-centre series [16] but has not been explored in a clinical trial data set to date. This is the first report to exclusively study patients whose CTC response could be evaluated (ie, with baseline CTC ≥5 cells/5.7 ml), amounting to approximately 50% of patients with advanced CRPC (47.2% in COU-AA-301 and 57.9% in IMMC-38). An analysis of patients with baseline CTC <5 cells/7.5 ml will be published separately.
This pooled post hoc analysis for two prospective clinical trials shows that a 30% CTC decline as early as 4 wk after treatment initiation can effectively distinguish between patients benefiting from improved OS and patients not benefiting from treatment who may require a switch to an alternative therapeutic regimen.
We previously reported separate data showing that a 30% CTC decline was associated with improved OS in a smaller cohort [16]. Using larger prospective series, we now report that a post-treatment 30% CTC decline is associated with longer OS in patients treated with abiraterone + prednisone, corticosteroids alone, and chemotherapy. We considered the choice of a 30% cutoff for a number of reasons. When compared with a 50% CTC decline, although global AUC and c-index values did not differ significantly, a 30% CTC decline was a more sensitive biomarker; a test for early identification of nonresponders should value sensitivity over specificity to minimize the risk of false negatives and unnecessary discontinuation of potentially effective treatments. Likewise, establishing a percentage decline criterion for response is more sensitive than a conversion from ≥5 to <5 cells/7.5 ml. Critically, it is difficult to consider a patient whose CTC count falls from 100 to 5 cells/ 7.5 ml after three cycles as a “nonresponder” while considering a patient whose CTC count falls from 5 to 4 cells/7.5 ml as a “responder”. The CTC threshold of ≥5 cells/7.5 ml, initially chosen to differentiate patients with and without cancer (false-positive cells identified incorrectly as CTCs by detection platforms), has limitations when estimating disease response. We also found that patients in whom CTCs do not decrease following treatment have similar OS to those whose CTCs rise following treatment, suggesting that a treatment switch may need to be considered in both groups.
Importantly, we found that the effect of a post-treatment CTC decline was equivalent in patients treated with chemotherapy and AR signaling inhibitors. HR values for responders participating in the IMMC-38 (chemotherapy) and COU-AA-301 (abiraterone after chemotherapy) trials were very similar, which supports the validity of CTC count as a response biomarker in both treatment groups. The similar median OS and baseline characteristics of both populations support the suitability of pooled analysis.
Addition of a 30% CTC post-treatment decline to multivariable models can provide independent and additional information on outcome to that provided by baseline CTC. Addition of a 30% CTC decline to the multivariable models significantly increased AUC values at all time points studied.
When analyzing the COU-AA-301 data set separately, CTC response was able to identify patients with longer survival in both the abiraterone and prednisone arms of the study. Although the frequency of a 30% CTC decline was significantly lower in the prednisone than in the abiraterone arm of COU-AA-301, patients experiencing a 30% CTC decline on prednisone had median OS comparable to that for participants experiencing a CTC response in the abiraterone arm, and higher than that for nonresponders who received abiraterone, suggesting that corticosteroids had antitumor activity in these patients.
Our study has a number of limitations. Although this is the largest analysis of patients with baseline CTC ≥5 cells/7.5 ml, limitations arising from its unplanned post hoc nature must be acknowledged. Furthermore, only 858/1195 (71.8%) patients enrolled in the COU-AA-301 trial could be evaluated for CTCs. Although CTCs were investigated until progression in the IMMC-38 study, these were only determined at 4, 8, and 12 wk in the COU-AA-301 study. Moreover, the value of a stable CTC count was not investigated in the COU-AA-301 and IMMC-38 data sets independently owing to a lack of sufficient events. Finally, although both median OS and baseline characteristics were similar in the data sets for both trials, approximately three times as many patients were treated with abiraterone (COU-AA-301) than with chemotherapy (IMMC-38).
5. Conclusions
In conclusion, we believe that changes in CTCs as early as 4 wk after treatment can identify patients not benefiting from treatment. Clinical trials are now under way to explore the benefit of a treatment switch in nonresponding patients. Further prospective phase 3 trials are needed to confirm the surrogate value of CTC and the CTC-LDH panel already reported for the COU-AA-301 trial [20]. We envisage that the clinical qualification of CTC count as a intermediate endpoint biomarker of OS in advanced prostate cancer may be close to a positive conclusion.
Supplementary Material
Acknowledgments
We acknowledge support from Prostate Cancer UK and Movember for the London Movember Prostate Cancer Centre of Excellence at The Institute of Cancer Research and Royal Marsden, and from an Experimental Cancer Medical Centre grant from Cancer Research UK and the Department of Health (C51/A7401). The authors acknowledge NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden and The Institute of Cancer Research. David Lorente conducted this work within the Medicine Doctorate framework of Universidad de Valencia. David Olmos ia also supported by the Fundación Científica de la Asociación Española Contra el Cáncer and a 2014 Stewart Rahr-Prostate Cancer Foundation Young Investigator Award.
Funding/Support and role of the sponsor: The authors were supported by a Movember/Prostate Cancer UK Centre of Excellence Program grant and an FP7 EU grant. The sponsors played no role in the study.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.05.023.
Footnotes
Author contributions: David Lorente had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lorente, Olmos, Mateo, McCormack, de Bono. Acquisition of data: Lorente, Olmos, Mateo, Bianchini, Seed, Flohr, Crespo, Figueiredo, Miranda, Baeten, Molina, Kheoh, McCormack, Terstappen, Scher, de Bono.
Analysis and interpretation of data: Lorente, Olmos, Mateo, de Bono. Drafting of the manuscript: Lorente, Olmos, Mateo, de Bono.
Critical revision of the manuscript for important intellectual content: Lorente, Olmos, Mateo, Bianchini, Seed, Flohr, Crespo, Figueiredo, Miranda, Baeten, Molina, Kheoh, McCormack, Terstappen, Scher, de Bono. Statistical analysis: Lorente, Olmos, de Bono.
Obtaining funding: de Bono, Terstappen.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: David Lorente certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: David Lorente, Joaquin Mateo, Diletta Bianchini, George Seed, Penny Flohr, Mateus Crespo, Ines Figueiredo, Susana Miranda, and Johann S. de Bono are employees of the Institute of Cancer Research, which has a commercial interest in abiraterone. Kurt Baeten, Arturo Molina, Thian Kheoh, and Robert McCormack are employees of Janssen Pharmaceuticals, which commercializes abiraterone acetate (Zytiga) and the CellSearch CTC assay.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012v1.0. Cancer incidence and mortality worldwide. Lyon, France: International Agency for Research on Cancer; 2013. (IARC CancerBase No. 11). [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Shah S, Efstathiou E, et al. Phase 11 study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–61. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halabi S, Armstrong AJ, Sartor O, et al. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J Clin Oncol. 2013;31:3944–50. doi: 10.1200/JCO.2013.50.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Garrett-Mayer E, Ou Yang Y-C, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 12.Petrylak DP, Ankerst DP, Jiang CS, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99–16. J Natl Cancer Inst. 2006;98:516–21. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 13.Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol. 2015;33:1356–63. doi: 10.1200/JCO.2014.55.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 15.Danila DC, Heller G, Gignac G, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–8. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 16.Olmos D, Arkenau H, Ang JE, et al. Ciruclating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:1136–42. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisher M, Danila DC, Fizazi K, et al. Circulating tumor cell (CTC) enumeration in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide post-chemotherapy (phase 3 AFFIRM study) J Clin Oncol. 2015;33(15 Suppl):5035. [Google Scholar]
- 20.Scher HI, Heller G, Molina, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33:1348–55. doi: 10.1200/JCO.2014.55.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 22.Kraan J, Sleijfer S, Strijbos MH, et al. External quality assurance of circulating tumor cell enumeration using the CellSearch system: a feasibility study. Cytometry B Clin Cytom. 2011;80:112–8. doi: 10.1002/cyto.b.20573. [DOI] [PubMed] [Google Scholar]
- 23.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–17. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


