Abstract
Purpose
To assess hypo-fractionated particle beam therapy (PBT)'s efficacy relative to that of photon stereotactic body radiotherapy (SBRT) for early stage (ES) non-small cell lung cancer (NSCLC).
Methods
Eligible studies were identified through extensive searches of the PubMed, Medline, Google-scholar, and Cochrane library databases from 2000 to 2016. Original English publications of ES NSCLC were included. A meta-analysis was performed to compare the survival outcome, toxicity profile, and patterns of failure following each treatment.
Results
72 SBRT studies and 9 hypo-fractionated PBT studies (mostly single-arm) were included. PBT was associated with improved overall survival (OS; p = 0.005) and progression-free survival (PFS; p = 0.01) in the univariate meta-analysis. The OS benefit did not reach its statistical significance after inclusion of operability into the final multivariate meta-analysis (p = 0.11); while the 3-year local control (LC) still favored PBT (p = 0.03).
Conclusion
Although hypo-fractionated PBT may lead to additional clinical benefit when compared with photon SBRT, no statistically significant survival benefit from PBT over SBRT was observed in the treatment of ES NSCLC in this hypothesis-generating meta-analysis after adjusting for potential confounding variables.
Keywords: Lung cancer, Early stage, PBT, SBRT, Stereotactic, Particle beam
Lung cancer is the most common cancer and the leading cause of cancer-related deaths worldwide [1]. Non-small cell lung cancer (NSCLC), which represents the majority of lung cancer diagnosed, often presents in late stages. Low-dose (LD) CT screening of patients who are at a high risk to develop lung cancer has been associated with a decrease in lung cancer related mortality and a high specificity in selected studies [2–5]. Its clinical adaptation in the high-risk population has caused an increase in the number of patients diagnosed with stage I NSCLC in recent years [2–8]. Early-stage (ES) NSCLC has traditionally been treated with surgery in operable patients. As an alternative, excellent clinical outcome following high dose irradiation delivered with stereotactic body radiotherapy (SBRT) has been observed in inoperable patients with ES, lymph node negative, NSCLC [9]. This treatment technique is also known as stereotactic ablative radiotherapy (SABR). An ablative dose is delivered to the tumor with SBRT over one to two weeks. This is administered under daily image guidance to ensure accurate tumor localization and maximal sparing of the surrounding normal tissue. SBRT has been quickly adopted into clinical practice worldwide. Its efficacy was found to be potentially comparable to surgery in selected patients [10–12]. As more patients are diagnosed with ES NSCLC through LD-CT screening, high dose irradiation with precision, such as SBRT, may become increasingly considered for this disease. However, SBRT is not without limitations. For example, its application is still limited by tumor location with severe toxicity more frequently encountered in patients with centrally located lesions [13]. This problem may be mitigated by increasing dose fractionation because of photons' physical characteristics. However, tumor proximity to critical thoracic structures still prohibits the utility of SBRT in many patients due to the risk of severe toxicity associated high doses to these structures if a therapeutic dose were to be delivered. Also, photon SBRT poses great challenges in the delivery of subsequent high dose re-irradiation for loco-regional recurrence in many patients as a result of the high dose already delivered to critical thoracic organs during the first course of treatment. As an emerging technology, particle beam (proton and heavy ions, such as carbon ions) therapy (PBT) possesses unique physical properties that allow the irradiation of tumors at any depth within the body with a very sharp dose gradient at the distal edge of the tumor target [14]. This may greatly decrease the dose to the healthy tissues surrounding the tumor in the setting of high dose irradiation in comparison with photon SBRT. Heavy ions, such as carbon ions, also have a biological advantage over photons due to the higher probability of tumor DNA damage associated with their high linear energy transfer (LET). In recent years, more facilities have been delivering PBT for the treatment of ES NSCLC. In this comprehensive critical review and hypothesis-generating meta-analysis, we aim to analyze and compare the efficacy of hypo-fractionated PBT, which is delivered with highly-advanced technology, with that of photon SBRT, a relatively more mature technique that has been in clinical use for over a decade, in the treatment of ES NSCLC.
Methods
Search strategy and selection criteria
A systematic search was conducted in PubMed, Medline, Google-scholar, and the Cochrane library for studies published between January 2000, and June 2016. The subject heading “non-small cell lung cancer/ carcinoma” was combined with the following terms: “early stage”, “stage I”, “T1”, “T2”, “stereotactic body radiation therapy”, “stereotactic body radiotherapy”, “stereo-tactic ablative radiotherapy”, “SBRT”, “SABR”, “hypo-fractionated radiotherapy”, “particle beam therapy”, “proton therapy”, “carbon ion therapy”, “carbon ion radiotherapy”, and “carbon ion radiation therapy”. Relevant articles, abstracts, and review articles were selected and reviewed. The references from these sources were searched for additional studies. Proceedings of the annual meetings of the American Society of Radiation Oncology, American Society of Clinical Oncology, and the European Society of Radiotherapy & Oncology from 2000 onward were manually searched for relevant abstracts, then a search for a fully published manuscript was done. The Physician Data Query (PDQ) clinical trials database was searched for relevant ongoing trials. The last search was conducted on July 1, 2016.
Only studies published in English in peer-reviewed journals were included. Eligible studies include prospective or retrospective studies of SBRT or SABR, and hypo-fractionated PBT, such as proton therapy and carbon ion therapy, as definitive treatment for ES NSCLC (T1, T2, or T3, N0, M0 per the 7th edition of cancer staging by the American Joint Committee on Cancer (AJCC)). Only the latest study with the most comprehensive report of clinical outcome and treatment toxicity was selected when multiple studies on the same patient population from the same institution were found. Multiple reports from the same institution were included if the patient populations were from different time periods, treated differently, or the reports on the same patient population complement each other in data reporting. The search was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [15], and a structured literature search schema was followed (Suppl. Fig. 1).
Data extraction
Studies were extracted independently by two investigators. All relevant characteristics, such as first author, publication year, country, study design, age, sample size, tumor stage, follow up period, clinical outcome (including patterns of failure and survival data), and treatment related toxicity, were collected. For each study, the radiation dose fractionation regimen used was recorded. For survival endpoints, 1, 2, 3, 4, and 5 year data were collected when available. Local control was defined as freedom from any local progression. Survival data were extracted from Kaplan-Meier survival curves when survival rates were not explicitly stated. The biologically effective dose (BED10) for tumor was calculated based on the linear-quadratic equation: BED10 = D [1 + d/(α/β)], where D and d represents the total and fractional radiation dose. α/β for tumor equals to 10 Gy−1.
Statistical analysis
The clinical outcomes of interest include local control, overall survival, progression-free survival at 1, 2, 3, 4 and 5 years and treatment related toxicity. Descriptive statistics and exploratory data analysis were used to summarize the data, including summary tables, box-plots, proportions, mean, median, and range. Summary estimates of event proportion, and relative risk (RR) were estimated from the available data at each time point, using the weighted regression model, and the random-effects model/ mixed-effects model (Supplementary material) [16,17]. Continuous data such as tumor size and median follow-up were evaluated using weighted mean with 95% confidence intervals (CI). Dichotomous data such as adverse effects were summarized using proportion and relative risk with 95% CI. Forest plots were used to evaluate relative risk on local control and 3-year survival between two conditions such as T1 vs. T2 groups, where 95% confidence intervals for RR in each study were represented by horizontal lines and the point estimate by a square. The height of each square is inversely proportional to the standard error of the estimate. The summary RR is represented by a diamond with horizontal limits at the 95% confidence interval and width inversely proportional to its standard error. A multivariate meta-regression model was used to assess treatment effect, including covariates, such as patient/tumor characteristics, and treatment modality, to account for population heterogeneity. Data analysis was performed using the meta-analysis package “meta” [18,19] and statistical software R (version 3.31, R Foundation, Vienna, Austria).
Results
Study selection and characteristics
The study selection process is shown in Suppl. Fig. 1. Among 501 relevant publications on SBRT and 113 relevant publications on PBT, 205 full text articles for SBRT and 19 full text articles for hypo-fractionated PBT (proton and carbon ion therapy) were assessed for eligibility. Of these, 72 studies on photon SBRT and 9 studies on PBT for ES NSCLC were selected [10,12,20-99]. Relevant information on the long term clinical outcome was also extracted from an abstract for 1 phase 2 SBRT trial [20,21]. Two SBRT studies reporting the results of the same phase 2 trial complemented each other, and were counted as 1 study. One phase 3 study comparing SBRT and surgery (only the SBRT data was used) [12], 10 phase 2 [20-31], 1 phase 1 [32], and 56 retrospective [33-51,53-89] photon SBRT studies were included in the survival outcome comparison analysis. Among them, treatment-related toxicities were reported in all the prospective studies [12,20-32], and 40 retrospective studies [34,37,39-40,42,44-47,49,52-61,65,67-73,76-78, 80-82,84-89]. These studies were included in the treatment-related toxicity comparison analysis. For 1 retrospective study without toxicity reporting, a similar retrospective study reporting on patients from the same institution treated in the same fashion and in similar time periods was used in the toxicity analysis [51,52]. Patterns of failure were analyzed based on the information provided in 57 studies, which included 1 phase 3 (only the SBRT data was used), 10 phase 2, 0 phase 1, and 46 retrospective studies [12,20-31,34-46,48-51,54-57,59-65,68-71,73-75,77-79,82,84-90]. Among them, 1 retrospective study was not included in the survival outcome or toxicity profile analysis [90]. It was included in the patterns of failure analysis because of a lack of information in a study from the same institution that was used for survival outcome analysis. At last, 8 studies providing adequate clinical information were included in the clinical outcome comparison for T1 vs. T2 NSCLC analysis [10,24,30,45,58,59,87,90]. Among them, 1 retrospective study was not included in previous analyses as a study of larger sample size was included instead [10,73]. PBT studies included 4 prospective studies (1 phase 2, and 3 phase I/II), and 5 retrospective studies [91-99]. All were included in the survival outcome and toxicity profile comparison analyses [91-99]. For patterns of failure analysis, 8 studies were included [92-99]. Five studies were included in the clinical outcome comparison for T1 vs. T2 NSCLC analysis [91-93,97,99]. The study characteristics are shown in Table 1. Overall, no significant difference in gender, age, functional performance status, the percentage of patients with a pathological diagnosis, utilization of PET/CT staging, treatment time period, % operable patients (%Op), the percentage of patients receiving at least 100 Gy10 at the tumor target periphery, and the tumor location was observed. On the contrary, PBT studies appear to be associated with longer follow up time, larger tumors, and higher T stage. The details of the individual studies, including dose prescription information, are shown in Suppl. Tables 1 & 2, while no patients treated with PBT was selected based on respiratory motion amplitude.
Table 1.
Study characteristics.
| PBT | SBRT | p value | |
|---|---|---|---|
| Studies (total n) | 9 | 72* | – |
| Patients (total n) | 614 | 7291 | – |
| Gender (% male) | 0.23 | ||
| Median (range) | 69% (20%, 87%) | 58% (20%, 95%) | |
| Median age (SE) | 75.75 (2.51) | 74.09 (0.86) | 0.53 |
| ECOG PS 0-1/ KPS 80–100 | |||
| Median (range) | 89% (60%, 98%) | 78% (24%, 100%) | 0.23 |
| Pathological diagnosis | |||
| Median (range) | 100% (0,100%) | 100% (0,100%) | 0.97 |
| PET/CT staging (%) | 71% | 81% | 0.44 |
| Treatment Period (year) | |||
| Median (SE) | 2006(1.08) | 2007(0.36) | 0.37 |
| Operability% | 38% | 16% | 0.07 |
| Median follow up (months) | |||
| Mean (SE) | 39.99 (3.82) | 27.79 (1.29) | 0.003 |
| Median tumor size (cm) | |||
| Mean (SE) | 2.92 (0.19) | 2.41 (0.07) | 0.02 |
| Tumor stage (% T1) | |||
| Median (range) | 57% (0, 90%) | 71% (0, 100%) | 0.05 |
| % BEDperiphery ≥ 100 Gy10 | 50% | 63.6% | 0.62 |
| Tumor location (% P only) | 30% | 28.6% | 0.93 |
2 studies reporting on the same phase II trial and 1 study and 1 abstract reporting on the same phase II trial in a complementary manner were counted as 1 single study each. The p value was obtained with the meta-regression model weighted by sample size described in Supplementary materials, using statistical software R with the statistical package “Meta” [18,19].
Survival outcome
Based on the mixed-effects model [17], the overall survival (OS) and progression-free survival (PFS) following PBT were significantly higher than that following SBRT for ES NSCLC (Fig. 1A & B). The 1, 3 and 5 year OS for PBT vs. SBRT were 91.7% (95% CI 82% - 100%) vs. 86.9% (95% CI 70% - 100%), 69.5% (95% CI 39% - 100%) vs. 58.8% (95% CI 30% - 88%), and 60% (95% CI 23% –97%) vs. 41.3% (95% CI 2% – 81%), p < 0.05. The survival advantage associated with PBT remained significant when studies including patients with peripheral tumors only were analyzed, p < 0.05. In the fitted meta-regression model, PBT remains to be associated with better OS than SBRT (p = 0.03 for 2-year OS & 0.01 for 3-year OS). The 1, 3 and 5 year PFS for PBT vs. SBRT were 85.3% (95% CI 76% – 95%) vs. 80.2% (95% CI 66% – 94%), 63.5% (95% CI 37% – 90%) vs. 50.7% (95% CI 31% - 70%), and 57.2% (95% CI 19% -95%) vs. 37.7% (95% CI 10% - 66%), p < 0.05. Overall, no statistically significant difference in local control (LC) was observed between PBT and SBRT (Fig. 2). The 1, 3 and 5 year LC for PBT vs. SBRT were 96.3% (95% CI 90-100%) vs. 95% (95% CI 84-100%), 87.4% (95% CI 73-100%) vs. 86.1% (95% CI 63-100%), and 87.2% (95% CI 73-100%) vs. 80.8% (95% CI 46-100%).
Fig. 1.
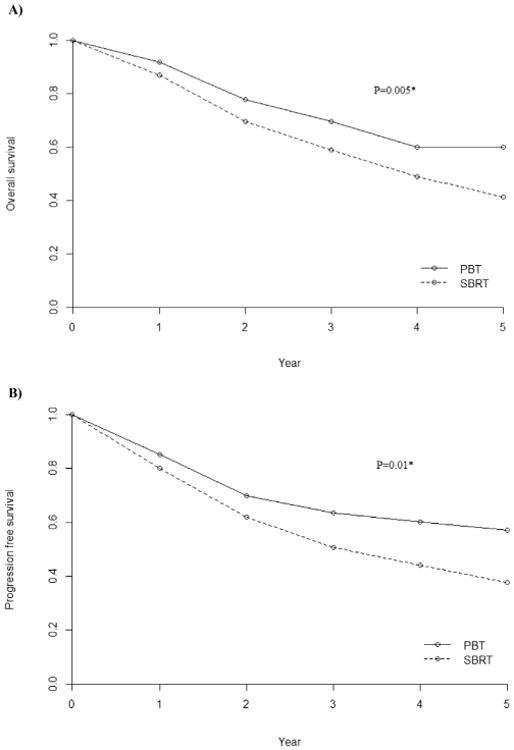
A) Overall survival (OS), and B) Progression-free survival (PFS) following particle beam therapy (PBT) and photon stereotactic body radiation therapy (SBRT) for node-negative, early stage non-small cell lung cancer. A mixed-effects model [17] with an interaction term of treatment and time was used to assess the differences in OS (p = 0.005) and PFS (p = 0.01) between the two treatments.
Fig. 2.
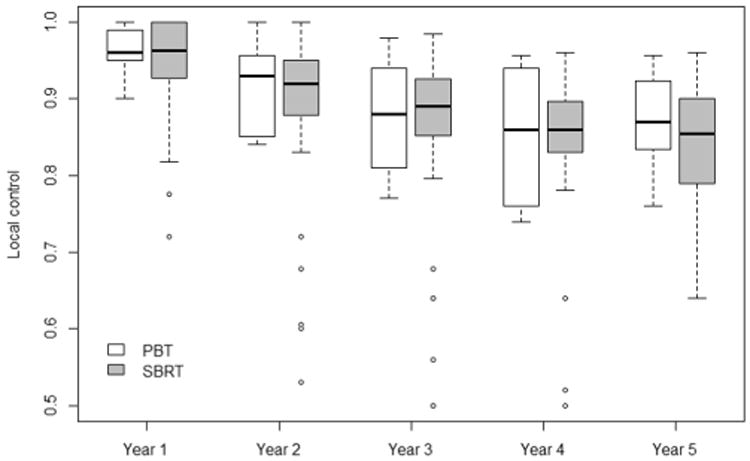
Local control following particle beam therapy (PBT) and photon stereotactic body radiation therapy (SBRT) for node-negative, early stage non-small cell lung cancer.
Treatment-related toxicity
The incidence of severe (grade 3–5) toxicity per Common Terminology Criteria for Adverse Events (CTCAE) following either PBT or SBRT was low (Table 2). However, significantly higher incidence of severe toxicity was observed following SBRT in the weighted-regression analysis (p = 0.05). No statistically significant difference in the incidence of grade 4–5 toxicity was observed between PBT and SBRT populations. The overall incidence of ≥grade 3 chest wall (CW) toxicity and radiation pneumonitis (RP) was low. The incidence of ≥grade 3 RP following PBT vs. SBRT was 0.9% (95% CI 0.4-1.9%) vs. 3.4% (95% CI 2.9–4.0%), p < 0.001. On the contrary, the incidence of >grade 3 CW toxicity following PBT vs. SBRT was 1.9% (95% CI 1.1–3.3%) vs. 0.9% (95% CI 0.6-1.3%), p = 0.03. The overall incidence of rib fractures for PBT vs. SBRT was 13% (95% CI 11–16%) vs. 3.2% (95% CI 2.7-3.8%), p < 0.001. Only the difference in the incidence of rib fractures reached statistical significance in the meta-analysis (Table 3).
Table 2.
Treatment-related severe toxicities and rib fractures of any grade following PBT and SBRT (meta-regression model weighted by sample size described in Supplementary materials, using statistical software R with the statistical package “Meta” [18,19]).
| Toxicity type | PBT (N = 614) Events percentage (95% CI) | SBRT (N = 4805) Events percentage (95% CI) | p value |
|---|---|---|---|
| Grade 3–5 toxicity | 4.8% (3.4%, 6.7%) | 6.9% (6.1%, 7.9%) | 0.05 |
| Grade 5 | 0% (0%, 0.6%) | 0.2% (0.1%, 0.4%) | 0.41 |
| Grade 4 | 1.8% (1.0%, 3.1%) | 1.3% (1.0%, 1.6%) | 0.34 |
| Radiation Pneumonitis (≥grade 3) | 0.9% (0.4%, 1.9%) | 3.4 % (2.9%, 4.0%) | <0.001 |
| Chest wall toxicity (≥grade 3) | 1.9% (1.1%, 3.3%) | 0.9% (0.6%, 1.3%) | 0.03 |
| Rib fractures | 13% (11%, 16%) | 3.2% (2.7%, 3.8%) | <0.001 |
Table 3.
Relative risk of treatment related toxicities based on the meta-regression model weighted by sample size described in Supplementary materials, using statistical software R with the statistical package “Meta” [18,19].
| Toxicity type | PBT = 0 SBRT = 1 RR (95% CI) | P value |
|---|---|---|
| Grade 3–5 toxicity | 0.69 (0.22, 3.08) | 0.53 |
| Grade 5 | – | 0.38 |
| Grade 4 | 2.02 (0.38, 10 +) | 0.40 |
| Radiation Pneumonitis (≥grade 3) | 0.49 (0.06, 2.03) | 0.19 |
| Chest wall toxicity (≥grade 3) | 2.02 (0.83, 10+) | 0.14 |
| Rib fractures | 5.26 (1.91, 10+) | <0.001 |
RR: relative risk.
Patterns of failure
There was no statistically significant difference in the crude incidence of local failure, regional failure, or distant metastasis with the weighted-regression model. Local failure, regional failure, and distant metastasis for PBT vs. SBRT were 12% (95% CI 6–18%) vs. 10% (95% CI 8-12%), 13% (95% CI 0-56%) vs. 16% (95% CI 0– 32%), and 23% (95% CI 15-31%) vs. 20% (95% CI 18-22%). Among studies providing information based on T stage (T1 vs. T2), better 3-year LC and OS were associated with T1 tumors following either PBT or SBRT. As shown in Fig. 3, the relative risks (RRs) for 3-year LC were 3.19 (95% CI 1.93-5.28) and 2.29 (95% CI 1.63-3.20) for PBT and SBRT. The RRs for 3-year OS were 2.43 (95% CI 1.39– 4.25) and 1.85 (95% CI 1.41-2.41) for PBT and SBRT. Both favored T1 tumors. The local control and OS among patients with the same T stage were not influenced by treatment type (PBT or SBRT).
Fig. 3.
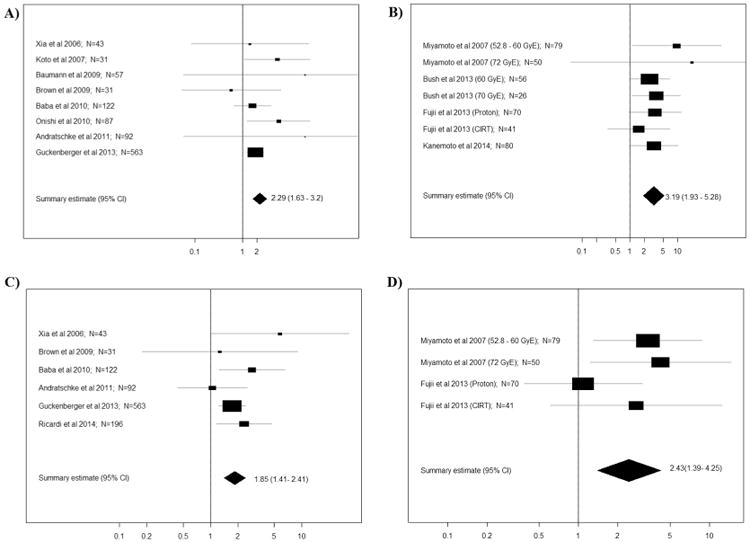
Forest plots of relative risk (RR) on: A) 3-year local control (SBRT). The summary RR = 2.29 with 95% CI (1.63, 3.20) between T2 and T1 groups, implying that local control is better for T1 tumors (95% CI does not cover 1); B) 3-year local control (PBT): the summary RR = 3.19 with 95% CI (1.93, 5.28) between T2 and T1 groups, implying that the local control is better in T1 group (95%CI does not cover 1); C) 3-year survival (SBRT): the summary RR = 1.85 with 95% CI (1.41, 2.41) between T2 and T1 groups, implying that the 3-year survival is better for T1 tumors (95% CI does not cover 1); D) 3-year survival (PBT): the summary RR = 2.43 with 95% CI (1.39, 4.25) between T2 and T1 groups, implying that the 3-year survival is better for T1 tumors (95% CI does not cover 1). The meta-analyses and forest plots were performed using the statistical package “Meta” in the R software [18,19].
Influence of study characteristics on survival endpoints
The influence of various study characteristics on study outcome was assessed with a multivariate analysis using the weighted-regression model. The factors assessed were age, functional performance status, tumor location (peripheral vs. central), T stage, treatment type (PBT vs. SBRT), and the BED at the tumor periphery. Among these factors, OS was significantly influenced by treatment type and functional performance status. Patients with ECOG score of 0-1 or its equivalent, and treatment with PBT were found to be associated with significantly better 2 and 3-year OS (p = 0.01 & 0.01, and 0.03 & 0.01). The survival benefit remained significant when treatment time period and PET/CT staging were added into the multivariate analysis (p < 0.05). However, this statistical significance was not observed (p = 0.11) after further adjusting for %Op (p = 0.02 for 2-year OS) in the final multivariate analysis (Suppl. Table 3). While improved LC was initially found to be significantly associated with tumor BED >100 Gy10 for the entire patient population (88% vs. 76% at 5 years, p = 0.03), BED's correlation with LC only trended toward statistical significance without, and with the 3 additional factors in the multivariate analysis (p = 0.09 & 0.07 for 2-year LC, respectively). LC was significantly influenced by treatment period, PET/CT staging, tumor T stage, age, and treatment modality; favoring more recent treatment periods (starting > 2005 and/or finishing > 2010), inclusion of PET/CT for staging, T1 lesions, younger age, and PBT in the final multivariate analysis that included the 3 additional factors (p < 0.05 for 3 year LC).
Discussion
Through a systematic review of the literature, data extracted from 72 studies on SBRT and 9 studies on hypo-fractionated PBT for ES NSCLC from North America, Europe and Asia were analyzed in a meta-analysis, which represents the most comprehensive review of the subject matter to date. Even with relatively more advanced tumors in size and T stage, patients treated with PBT were found to have significantly better OS and PFS than those treated with photon SBRT in the weighted multivariate analysis (p < 0.05). This survival benefit did not reach its statistical significance after adding %Op into the final multivariate analysis (p = 0.11). %Op appeared to be the strongest factor affecting patient survival among all study characteristics, implying significant influence of patients' functional performance and comorbidities on survival following high-dose irradiation in patients with ES NSCLC [100]; and the importance of identifying and adjusting for valid prognostic and predictive factors for survival and treatment-related toxicities following high dose irradiation when comparing the efficacy of different treatments [101–103]. These include patient, tumor, dosimetric, imaging characteristics, and biomarkers. Such adjustments are critical for the generation of meaningful data and the reduction of confounding in comparative effectiveness research (CER), which is important to guide treatment decision making especially in the absence of randomized controlled trials (RCTs) [104]. Different conclusions are often reached after such adjustments. This has been previously shown in a metaanalysis, which identified similar survival rates between patients who received SBRT and those who received surgery only after adjusting for age and %Op [102]. This finding has been corroborated in a pooled analysis of 2 RCTs, while unadjusted data still favored surgery [12,101,105]. The current study is limited by considerable selection bias due to that most studies included in the analysis were single- institutional, single-arm, observational studies from various continents of the world with great heterogeneity, including variation in quality. Ideally, controlling for various factors through randomization remains the most effective approach to generate meaningful data when comparing different treatments [106]. Confounding can be minimized by the analysis of individual patient data while controlling for various patient, tumor and treatment characteristics. Such a process may not totally eliminate confounding due to the presence of unknown confounding factors, and is not feasible with the current study. Therefore, our analyses may be biased with significant confounding due to the nature of the studies included, and our results are only exploratory and hypothesis-generating. However, they present useful information to guide the design of future RCTs.
Tumor BED has been previously shown to be correlated with local control and possibly patient survival [46,58,73,107]. Overall, local control following PBT or SBRT was significantly better with tumor BED > 100 Gy10. However, this association did not reach statistical significance after adjusting for various patient characteristics. The 3-year LC approached 90% following either PBT or SBRT. No statistically significant difference in LC was observed between PBT and SBRT cohorts without the inclusion of 3 additional factors into the multivariate analysis: treatment period, PET/CT staging, and %Op. However, the 3-year LC favored PBT after these factors were added into the multivariate analysis. This suggests a potential clinical improvement associated with PBT after multiple factors were adjusted for, which can only be discerned in a randomized trial as this can be affected by many unknown confounding factors. The 3-year LC was also significantly affected by treatment period, PET/CT staging, tumor T stage, and age. This suggests the importance of technical advances, rigorous quality assurance programs developed in recent years, and the accumulation of experience for accurate treatment delivery with high precision [104]. While smaller tumors have been known to be associated with better LC, older age should not prevent the utility of hypo-fractionated PBT or photon SBRT for ES NSCLC as they may lead to a survival benefit in elderly patients [108,109].
Low incidence of severe toxicity (grade 3–5) was observed following either PBT or SBRT. Overall, the most commonly reported severe toxicities were pulmonary or chest wall toxicities [12,22– 30,34,37,39,42,44,46,49,52–53,56,58–60,65,73,76–77,80–82,84,87, 89,92,94–98]. Severe radiation pneumonitis occurred significantly less, while severe chest wall toxicity and any-grade rib fracture were observed more frequently following PBT in the pooled analysis (p < 0.05). Only the difference in the incidence of rib fractures remained significant in the meta-analysis. The increased incidence of rib fractures was observed in studies on proton therapy or have the majority of patients treated with proton therapy [95–99]. Decreased dose to the lung parenchyma and other critical thoracic organs, such as the heart, spinal cord, and the esophagus, with PBT for ES NSCLC has been consistently shown in various dosimetric studies [110–117]. Although not statistically significant after controlling for multiple factors, the low incidence of radiation pneumonitis after PBT suggests its potential clinical benefit in OAR sparing. This may have an impact on patients' survival after irradiation as suggested in the correlation between cardiac dose and pulmonary toxicity, as well as cardiac dose and survival, especially for patients with significant co-morbidities and of older age [118– 121]. PBT may also help to expand the indications for hypo-fractionated radiotherapy in the treatment of ES NSCLC given its advantage in OAR sparing, such as treating lesions immediately adjacent to critical thoracic organs that cannot be safely treated with photon SBRT and recurrences or second primary lung cancer (SPLC) after surgery or previous high dose irradiation for maximal normal-tissue protection. No difference in the patterns of failure following PBT and that following SBRT was observed. The pattern of failure remains to be primarily distant following either treatment. Tumor stage, which is dictated by tumor size, has shown to be a significant prognostic factor for LC and OS among the studies which provided information based on T stage (Fig. 3). This corroborates with the newest (8th) edition of the AJCC lung cancer staging criteria, which places more emphasis on tumor size in ES NSCLC based on clinical evidence. It also suggests a need to individualize radiation dosing for ES NSCLC of different T stage, delivering higher doses to >T1 tumors. PBT may be especially advantageous for this, as it may further lower the normal tissue dose from what could be achieved with photon SBRT.
Passively scattered (PS) particle therapy (PT) has been most commonly used clinically worldwide. Despite reducing the dose at the distal end of the tumor target, the entry dose is often unmodulated. This leads to increased dose at the skin and the chest wall, especially for proton therapy. Thus, explaining why increased incidence of chest wall toxicity/rib fractures was observed following PBT in this meta-analysis. To reduce the proximal normal tissue dose, the layer-stacking method, which longitudinally sweeps the Bragg peak through the target and creating a series of short SOBPs for better dose conformity, has been commonly used in PS heavy-ion therapy [122]. Active scanning beams, which deliver pencil-thin particle beams to dose paint slices of the tumor target at various depths in a sequential manor, further enhance dose conformity to the shape of the PTV and decrease dose to the surrounding normal tissue. Scanning beams deliver significantly less dose at tissue depths superficial to the PTV, thus reducing the dose to the chest wall & skin and lowering the risk for chest wall injury and rib fractures [116]. Alternatively, increasing the number of beams may also help to mitigate the dose effect on the chest wall, while the sharper penumbra from carbon ion therapy may further reduce the chest wall dose, thus providing a clinical advantage over proton therapy in hypo-fractionated thoracic PBT.
Unlike PSPT, which delivers a highly homogeneous dose to a target volume at once, intensity modulated PT (IMPT) through beam scanning is very sensitive to internal tumor motion [123– 125]. This is mainly due to the necessity for precise and accurate dose deposition at specific voxels iso-energy layer by iso-energy layer longitudinally through a tumor target during IMPT. Commonly used motion management methods are shown in Suppl. Fig. 2. Gating and irradiation of an internal target volume (ITV) have been frequently used with PSPT in the studies analyzed, which did not select patients based on the amplitude of respiratory motion [91–99]. Gating reduces range uncertainties due to motion, and the amount of normal tissue irradiated with the prescription dose. Phase-controlled rescanning (PCR) through various isoenergy layers sequentially has led to improved dose conformity and OAR sparing during the irradiation of lung tumors with scanning CIRT, while fast rescanning can maximally suppress the interplay effect due to scanning motion and intra-fractional organ motion [126,127]. Irregularities in respiratory pattern and inconsistencies between the external marker position and the internal target motion remain challenges with gating [128,129]. These issues may be mitigated through the adaptation of amplitude-based gating and internal positional tracking with or without fiducial markers [125,130,131]. Alternative to gating, 4D planning may improve dose conformity and homogeneity, which warrants further clinical exploration [132].
Photon SBRT, which has been quickly adopted worldwide as a potentially curative treatment option for ES NSCLC, has been associated with excellent LC. However, how LC relates to survival and quality of life (QoL) remains unclear [100]. This is especially true for patients with significant co-morbidities, as they may not gain any survival or QoL benefit from SBRT. Clinical studies on SBRT for ES NSCLC with survival and QoL as primary endpoints, and how this treatment interacts with existing non-malignant co-morbidities are urgently needed to better define how to select patients with ES NSCLC for high dose irradiation and to avoid treating patients who will not benefit from it [100,104]. Secondly, prospective dose defining studies are lacking and the current dosing is largely based on retrospective data. This may not be appropriate for all tumor sizes and patients [100]. Lower than commonly accepted dose may be adequate for certain patients, while over-dosing may lead to worse survival [107,133]. Thus, how to individualize the dosing for ES NSCLC warrants further investigation. Other areas for future research include the validation of models of clinical diagnosis for patients who are unable to undergo a biopsy; identification and treatment of SPLC, and the treatment of ground glass opacities (GGOs) [104]. The roles of hypo-fractionated PBT in comparison to SBRT in addressing the questions raised above are largely unknown. Despite the lack of information on the clinical efficacy of hypo-fractionated PBT in comparison to SBRT in the absence of RCTs, our exploratory and hypothesis-generating study suggests that PBT's theoretical advantages may be of clinical significance in benefiting more patients with ES-NSCLC, even when no statistically significant survival benefit from PBT was observed.
Conclusion
Although hypo-fractionated PBT may lead to additional clinical benefit when compared with photon SBRT, no statistically significant survival benefit from PBT over SBRT was observed in the treatment of ES NSCLC in this hypothesis-generating meta-analysis after adjusting for potential confounding variables.
Supplementary Material
Acknowledgments
We would like to thank Ms. Jin H. Jiao for her significant contribution to the conduct of the systematic literature search.
Funding source: None
Footnotes
Conflict of interest: None
Appendix A. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2017.05.007.
References
- 1.Cancer Research UK; [accessed in July, 2016]. Worldwide cancer statistics. www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer. [Google Scholar]
- 2.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. J Clin Oncol. 2013;31:1002–8. doi: 10.1200/JCO.2012.43.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 5.Walter JE, Heuvelmans MA, de Jong PA, Vliegenthart R, van Ooijen PMA, Peters RB, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol. 2016;17:907–16. doi: 10.1016/S1470-2045(16)30069-9. [DOI] [PubMed] [Google Scholar]
- 6.Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24:201–8. doi: 10.1097/CEJ.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callister MEJ, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British thoracic society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(2):ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 9.Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic reviewof the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94:1–11. doi: 10.1016/j.radonc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011;81:1352–8. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 11.Verstegen NE, Oosterhuis JWA, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24:1543–8. doi: 10.1093/annonc/mdt026. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi A, Nguyen NP, Komaki R. The potential role of respiratory motion management and image guidance in the reduction of severe toxicities following stereotactic ablative radiation therapy for patients with centrally located early stage non-small cell lung cancer or lung metastases. Front Oncol. 2014;4:151. doi: 10.3389/fonc.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linz U. Physical and biological rationale for using ions in therapy. In: Linz U, editor. Ion Beam Therapy: Fundamentals, Technology, Clinical Applications. Springer; Heidelberg, Dordrecht, London, New York: 2012. pp. 45–59. [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLos Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seber GAF. Linear regression analysis. New York: Wiley; 1977. [Google Scholar]
- 17.Arends LR, Hunink MGM, Stijnen T. Meta-analysis of summary survival data. Statist Med. 2008;27:4381–96. doi: 10.1002/sim.3311. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–5. https://cran.r-project.org/doc/Rnews/Rnews_2007-3.pdf. [Google Scholar]
- 19.Schwarzer G, Carpenter JR, Rücker G. Switzerland: Springer International Publishing; 2015. Meta-analysis with R (Use-R!) http://www.springer.com/gp/book/9783319214153. [Google Scholar]
- 20.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmerman RD, Hu C, Michalski J, Straube W, Galvin J, Johnstone D, et al. Long-term results of RTOG 0236: a phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with medically inoperable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90:S30. [Google Scholar]
- 22.Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, et al. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG Oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93:757–64. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg K, Nyman J, Källskog VR, Hoyer M, Lund JǺ, Lax I, et al. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT – the Nordic experience. Acta Oncol. 2015;54:1096–104. doi: 10.3109/0284186X.2015.1020966. [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Martin A, Aso S, Cacicedo J, Arnaiz M, Navarro V, Rosales S, et al. Phase II trial of SBRT for stage I NSCLC: survival, local control, and lung function at 36 months. J Thorac Oncol. 2016 doi: 10.1016/j.jtho.2016.03.021. In press. [DOI] [PubMed] [Google Scholar]
- 27.Bral S, Gevaert T, Linthout N, Versmessen H, Collen C, Engels B, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:1343–9. doi: 10.1016/j.ijrobp.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–7. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–96. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 30.Koto M, Takai Y, Ogawa Y, Matsushita H, Takeda K, Takahashi C, et al. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2007;85:429–34. doi: 10.1016/j.radonc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Marino K, et al. Clinical outcomes of stereotactic radiotherapy for stage I non-small cell lung cancer using a novel irradiation technique: patient self-controlled breath-hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer. 2004;45:45–55. doi: 10.1016/j.lungcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Onimaru R, Shirato H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV** 100 cc using a continual reassessment method (JCOG0702) Radiother Oncol. 2015;116:276–80. doi: 10.1016/j.radonc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Chiang A, Thibault I, Warner A, Rodrigues G, Palma D, Soliman H, et al. A comparison between accelerated hypofractionation and stereotactic ablative radiotherapy (SABR) for early-stage non-small cell lung cancer (NSCLC): results of a propensity score-matched analysis. Radiother Oncol. 2016;118:478–84. doi: 10.1016/j.radonc.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Taremi M, Hope A, Dahele M, Pearson S, Fung S, Purdie T, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82:967–73. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Mak RH, Hermann G, Lewis JH, Aerts HJWL, Baldini EH, Chen AB, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer. 2015;16:24–32. doi: 10.1016/j.cllc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebright MI, Russo GA, Gupta A, Subramaniam RM, Fernando HC, Kachnic LA. Positron emission tomography combined with diagnostic chest computed tomography enhances detection of regional recurrence after stereotactic body radiation therapy for early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;145:709–15. doi: 10.1016/j.jtcvs.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Park HS, Harder EM, Mancini BR, Decker RH. Central versus peripheral tumor location: influence on survival, local control, and toxicity following stereotactic body radiotherapy for primary non-small-cell lung cancer. J Thorac Oncol. 2015;10:832–7. doi: 10.1097/JTO.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 38.Spratt DE, Wu AJ, Adeseye V, Din SU, Shaikh F, Woo KM, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: implications for surveillance. Clin Lung Cancer. 2015;17:177–83. doi: 10.1016/j.cllc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parashar B, Port J, Arora S, Christos P, Trichter S, Nori D, et al. Analysis of stereotactic body radiation vs. wedge resection vs. wedge resection plus cesium-131 brachytherapy in early stage lung cancer. Brachytherapy. 2015;14:648–54. doi: 10.1016/j.brachy.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Kelley KD, Benninghoff DL, Stein JS, Li JZ, Byrnes RT, Potters L, et al. Medically inoperable peripheral lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2015;10:120. doi: 10.1186/s13014-015-0423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vu CC, Matthews R, Kim B, Franceschi D, Bilfinger TV, Moore WH. Prognostic value of metabolic tumor volume and total lesion glycolysis from 18F-FDG PET/CT in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Nucl Med Commun. 2013;34:959–63. doi: 10.1097/MNM.0b013e32836491a9. [DOI] [PubMed] [Google Scholar]
- 42.Horne ZD, Clump DA, Vargo JA, Shah S, Beriwal S, Burton SA, et al. Pretreatment SUVmax predicts progression-free survival in early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2014;9:41. doi: 10.1186/1748-717X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahdat S, Oermann EK, Collins SP, Yu X, Abedalthagafi M, DeBrito P, et al. Cyberknife radiosurgery for inoperable stage IA non-small cell lung cancer: 18F-fluorodeoxyglucose positron emission tomography/computed tomography serial tumor response assessment. J Hem Oncol. 2010;3:6. doi: 10.1186/1756-8722-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunlap NE, Larner JM, Read PW, Kozower BD, Lau CL, Sheng K, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cnacers treated with stereotactic body radiation therapy (SBRT) J Thorac Cardiovasc Surg. 2010;140:583–9. doi: 10.1016/j.jtcvs.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 45.Brown WT, Wu X, Fayad F, Fowler JF, García S, Monterroso MI, et al. Application of robotic stereotactic radiotherapy to peripheral stage I non-small cell lung cancer with curative intent. Clin Oncol. 2009;21:623–31. doi: 10.1016/j.clon.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Grills IS, Hope AJ, Guckenberger M, Kestin LL, Werner-Wasik M, Yan D, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol. 2012;7:1382–93. doi: 10.1097/JTO.0b013e318260e00d. [DOI] [PubMed] [Google Scholar]
- 47.Videtic GM, Stephans KL, Woody NM, Reddy CA, Zhuang T, Magnelli A, et al. 30 Gy or 34 Gy? Comparing 2 single-fraction SBRT dose schedules for stage I medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90:203–8. doi: 10.1016/j.ijrobp.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Burdick MJ, Stephans KL, Reddy CA, Djemil T, Srinivas SM, Videtic GM. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1033–9. doi: 10.1016/j.ijrobp.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 49.Fischer-Valuck BW, Boggs H, Katz S, Durci M, Acharya S, Rosen LR. Comparison of stereotactic body radiation therapy for biopsy-proven versus radiographically diagnosed early-stage non-small lung cancer: a single-institution experience. Tumori. 2015;10:287–93. doi: 10.5301/tj.5000279. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Liu H, Balter P, Allen PK, Komaki R, Pan T, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:1558–65. doi: 10.1016/j.ijrobp.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shultz DB, Trakul N, Abelson JA, Murphy JD, Maxim PG, Le QT, et al. Imaging features associated with disease progression after stereotactic ablative radiotherapy for stage I non-small-cell lung cancer. Clin Lung Cancer. 2014;15:294–301. doi: 10.1016/j.cllc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Trakul N, Chang CN, Harris J, Chapman C, Rao A, Shen J, et al. Tumor volume-adpated dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys. 2012;84:231–7. doi: 10.1016/j.ijrobp.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 53.Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer. 2016;17:39–46. doi: 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Nath SK, Sandhu AP, Kim D, Bharne A, Nobiensky PD, Lawson JD, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol. 2011;99:12–7. doi: 10.1016/j.radonc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Olsen JR, Robinson CG, El Naqa I, Creach KM, Drzymala RE, Parikh PJ, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e299–303. doi: 10.1016/j.ijrobp.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 56.Richardi U, Frezza G, Filippi AR, Badellino S, Levis M, Navarria P, et al. Stereotactic ablative radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung cancer. 2014;84:248–53. doi: 10.1016/j.lungcan.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552–8. doi: 10.3109/0284186X.2013.813635. [DOI] [PubMed] [Google Scholar]
- 58.Guckenberger M, Allgäuer M, Appold S, Dieckmann K, Ernst I, Ganswindt U, et al. Safety and efficacy of stereotactic body radiotherapy for stage I non-small-cell lung cancer in routine clinical practice. J Thorac Oncol. 2013;8:1050–8. doi: 10.1097/JTO.0b013e318293dc45. [DOI] [PubMed] [Google Scholar]
- 59.Andratschke N, Zimmermann F, Boehm E, Schill S, Schoenknecht C, Thamm R, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol. 2011;101:245–9. doi: 10.1016/j.radonc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Kopeck N, Paludan M, Petersen J, Hansen AT, Grau C, Høyer M. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–7. doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Nyman J, Johansson KA, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer – mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Kastelijn EA, El Sharouni SY, Hofman FN, Van Putte BP, Monninkhof EM, Van Vulpen M, et al. Clinical outcomes in early-stage NSCLC treated with stereotactic body radiotherapy versus surgical resection. Anticancer Res. 2015;35:5607–14. [PubMed] [Google Scholar]
- 63.Peulen H, Belderbos J, Rossi M, Sonke JJ. Mid-ventilation based PTV margins in stereotactic body radiotherapy (SBRT): a clinical evaluation. Radiother Oncol. 2014;110:511–6. doi: 10.1016/j.radonc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Senthi S, Lagerwaard FJ, Haasbeek CJA, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–9. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 65.Van der Voort van Zyp NC, Prévost JB, Hoogeman MS, Praag J, van der Holt B, Levendag PC, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Lovinfosse P, Janvary ZL, Coucke P, Jodogne S, Bernard C, Hatt M, et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging. 2016;43:1453–60. doi: 10.1007/s00259-016-3314-8. [DOI] [PubMed] [Google Scholar]
- 67.Janssen S, Kaesmann L, Rudat V, Rades D. Stereotactic body radiotherapy (SBRT) with lower doses for selected patients with stage I non-small-cell lung cancer (NSCLC) Lung. 2016;194:291–4. doi: 10.1007/s00408-016-9849-4. [DOI] [PubMed] [Google Scholar]
- 68.Duncker-Rohr V, Nestle U, Momm F, Prokic V, Heinemann F, Mix M, et al. Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity Strahlenther Onkol. 2013;189:33–40. doi: 10.1007/s00066-012-0224-y. [DOI] [PubMed] [Google Scholar]
- 69.Fritz P, Kraus HJ, Blaschke T, Mühlnickel W, Strauch K, Engel-Riedel W, et al. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008;60:193–9. doi: 10.1016/j.lungcan.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Hof H, Muenter M, Oetzel D, Hoess A, Debus J, Herfarth K. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC) Cancer. 2007;110:148–55. doi: 10.1002/cncr.22763. [DOI] [PubMed] [Google Scholar]
- 71.Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys. 2004;60:186–96. doi: 10.1016/j.ijrobp.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 72.Scorsetti M, Navarria P, Facoetti A, Lattuada P, Urso G, Mirandola A, et al. Effectiveness of stereotactic body radiotherapy in the treatment of inoperable early-stage lung cancer. Anticancer Res. 2007;27:3615–20. [PubMed] [Google Scholar]
- 73.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 74.Tsurugai Y, Kozuka T, Ishizuka N, Oguchi M. Relationship between the consolidation to maximum tumor diameter ratio and outcomes following stereotactic body radiotherapy for stage I non-small-cell lung cancer. Lung Cancer. 2016;92:47–52. doi: 10.1016/j.lungcan.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Hayashi S, Tanaka H, Hoshi H. Imaging characteristics of local recurrences after stereotactic body radiation therapy for stage I non-small cell lung cancer: evaluation of mass-like fibrosis. Thorac Cancer. 2015;6:186–93. doi: 10.1111/1759-7714.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Miyakawa A, Murai T, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer. J Thorac Oncol. 2015;10:960–4. doi: 10.1097/JTO.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T, Kadoya N, Shirata Y, Koto M, Sato K, Matsushita H, et al. Impact of tumor attachment to the pleura measured by a pretreatment CT image on outcome of stage I NSCLC treated with stereotactic body radiotherapy. Radiat Oncol. 2015;10:35. doi: 10.1186/s13014-015-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshitake T, Nakamura K, Shioyama Y, Sasaki T, Ohga S, Shinoto M, et al. Stereotactic body radiation therapy for primary lung cancers clinically diagnosed without pathological confirmation: a single-institution experience. Int J Clin Oncol. 2015;20:53–8. doi: 10.1007/s10147-014-0698-y. [DOI] [PubMed] [Google Scholar]
- 79.Satoh Y, Onishi H, Nambu A, Araki T. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270:275–81. doi: 10.1148/radiol.13130652. [DOI] [PubMed] [Google Scholar]
- 80.Yamashita H, Haga A, Takahashi W, Takenaka R, Imae T, Takenaka S, et al. Volumetric modulated arc therapy for lung stereotactic radiation therapy can achieve high local control rates. Radiat Oncol. 2014;9:243. doi: 10.1186/s13014-014-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue T, Katoh N, Onimaru R, Shimizu S, Tsuchiya K, Suzuki R, et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage I non-small cell lung cancer. Radiat Oncol. 2013;8:69. doi: 10.1186/1748-717X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuo Y, Shibuya K, Nagata Y, Takayama K, Norihisa Y, Mizowaki T, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–11. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 83.Hamamoto Y, Kataoka M, Yamashita M, Shinkai T, Kubo Y, Sugawara Y, et al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in four fractions: in comparison with primary lung cancer. Jpn J Clin Oncol. 2010;40:125–9. doi: 10.1093/jjco/hyp129. [DOI] [PubMed] [Google Scholar]
- 84.Shen ZT, Wu XH, Li B, Zhu XX. Clinical outcomes of cyberknife stereotactic body radiotherapy for peripheral stage I non-small cell lung cancer. Med Oncol. 2015;32:55. doi: 10.1007/s12032-015-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu D, Zhu H, Tang H, Li C, Xu F. Clinical analysis of stereotactic body radiation therapy using extracranial gamma knife for patients with mainly bulky inoperable early stage non-small cell lung carcinoma. Radiat Oncol. 2011;6:84. doi: 10.1186/1748-717X-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng AW, Tung SY, Wong VY. Hypofractionated stereotactic radiotherapy for medically inoperable stage I non-small cell lung cancer-report on clinical outcome and dose to critical organs. Radiother Oncol. 2008;87:24–8. doi: 10.1016/j.radonc.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 87.Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–25. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Kim MJ, Yeo SG, Kim ES, Min CK, Se An P. Intensity-modulated stereotactic body radiotherapy for stage I non-small cell lung cancer. Oncol Lett. 2013;5:840–4. doi: 10.3892/ol.2012.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song SY, Choi W, Shin SS, Lee SW, Ahn SD, Kim JH, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 90.Baba F, Shibamoto Y, Ogino H, Murata R, Sugie C, Iwata H, et al. Clinical outcomes of stereotactic body radiotherapy for stage I non-small cell lung cancer using different doses depending on tumor size. Radiat Oncol. 2010;5:81. doi: 10.1186/1748-717X-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bush DA, Cheek G, Zaheer S, Wallen J, Mirshahidi H, Katerelos A, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys. 2013;86:964–8. doi: 10.1016/j.ijrobp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–26. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 93.Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, et al. Curative treatment of stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–8. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 94.Karube M, Yamamoto N, Nakajima M, Yamashita H, Nakagawa K, Miyamoto T, et al. Single-fraction carbon-ion radiation therapy for patients 80 years of age and older with stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:542–8. doi: 10.1016/j.ijrobp.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 95.Westover KD, Seco J, Adams JA, Lanuti M, Choi NC, Engelsman M, et al. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol. 2012;7:1021–5. doi: 10.1097/JTO.0b013e31824de0bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hatayama Y, Nakamura T, Suzuki M, Azami Y, Ono T, Yabuuchi T, et al. Clinical outcomes and prognostic factors of high-dose proton beam therapy for peripheral stage I non-small-cell lung cancer. Clin Lung Cancer. 2015 doi: 10.1016/j.cllc.2015.11.013. in press. [DOI] [PubMed] [Google Scholar]
- 97.Kanemoto A, Okumura T, Ishikawa H, Mizumoto M, Oshiro Y, Kurishima K, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non-small-cell lung cancer. Clin Lung Cancer. 2014;15:e7–12. doi: 10.1016/j.cllc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 98.Fujii O, Demizu Y, Hashimoto N, Takagi M, Terashima K, Mima M, et al. Particle therapy for clinically diagnosed stage I lung cancer: comparison with pathologically proven non-small cell lung cancer. Acta Oncol. 2015;54:315–21. doi: 10.3109/0284186X.2014.974828. [DOI] [PubMed] [Google Scholar]
- 99.Fujii O, Demizu Y, Hashimoto N, Araya M, Takagi M, Terashima K, et al. A retrospective comparison of proton therapy and carbon ion therapy for stage I non-small cell lung cancer. Radiother Oncol. 2013;109:32–7. doi: 10.1016/j.radonc.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 100.Brada M, Pope A, Baumann M. SABR in NSCLC – the beginning of the end or the end of the beginning? Radiother Oncol. 2015;114:135–7. doi: 10.1016/j.radonc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 101.Louie AV, Haasbeek CJA, Mokhles S, Rodrigues GB, Stephans KL, Lagerwaard FJ, et al. Predicting overall survival after stereotactic ablative radiation therapy in early-stage lung cancer: development and external validation of the Amsterdam prognostic model. Int J Radiat Oncol Biol Phys. 2015;93:82–90. doi: 10.1016/j.ijrobp.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90:603–11. doi: 10.1016/j.ijrobp.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 103.Ebert N, Baumann M, Troost EGC. Radiation-induced lung damage-clinical risk profiles and predictive imaging on their way to risk-adapted individualized treatment planning? Radiother Oncol. 2015;117:1–3. doi: 10.1016/j.radonc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Louie AV, Palma DA, Dahele M, Rodrigues G, Senan S. Management of early-stage non-small cell lung cancer using stereotactic ablative radiotherapy: controversies, insights, and changing horizons. Radiother Oncol. 2015;114:138–47. doi: 10.1016/j.radonc.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 105.Deng HY, Wang YC, Ni PZ, Li G, Yang XY, Lin YD, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2016:1–8. doi: 10.1093/ejcts/ezw272. [DOI] [PubMed] [Google Scholar]
- 106.Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63:64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang J, Yang F, Li B, Li H, Liu J, Huang W, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis Int J Radiat Oncol Biol Phys. 2011;81:e305–316. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 108.Haasbeek CJA, Palma D, Visser O, Lagerwaard FJ, Slotman B, Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743–7. doi: 10.1093/annonc/mds081. [DOI] [PubMed] [Google Scholar]
- 109.Louie AV, Rodrigues G, Hannouf M, Lagerwaard F, Palma D, Zaric GS, et al. Withholding stereotactic radiotherapy in elderly patients with stage I non-small cell lung cancer and co-existing COPD is not justified: outcomes of a markov model analysis. Radiother Oncol. 2011;99:161–5. doi: 10.1016/j.radonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 110.Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–96. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 111.Auberger T, Seydl K, Futschek T, Sztankay A, Sweeney RA, Lukas P. Photons or protons: precision radiotherapy of lung cancer. Strahlenther Onkol. 2007;183:3–6. doi: 10.1007/s00066-007-2002-9. [DOI] [PubMed] [Google Scholar]
- 112.Georg D, Hillbrand M, Stock M, Dieckmann K, Pötter R. Can protons improve SBRT for lung lesions? Dosimetric considerations. Radiother Oncol. 2008;88:368–75. doi: 10.1016/j.radonc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 113.Macdonald OK, Kruse JJ, Miller JM, Garces YI, Brown PD, Miller RC, et al. Proton beam radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: a comparative dosimetric analysis. Int J Radiat Oncol Biol Phys. 2009;75:950–8. doi: 10.1016/j.ijrobp.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 114.Hoppe BS, Huh S, Flampouri S, Nichols RC, Oliver KR, Morris CG, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 2010;97:425–30. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 115.Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;80:1015–22. doi: 10.1016/j.ijrobp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seco J, Gu G, Marcelos T, Kooy H, Willers H. Proton arc reduces range uncertainty effects and improves conformality compared with photon volumetric modulated arc therapy in stereotactic body radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:188–94. doi: 10.1016/j.ijrobp.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 117.Ebara T, Shimada H, Kawamura H, Shirai K, Saito J, Kawashima M, et al. Dosimetric analysis between carbon ion radiotherapy and stereotactic body radiotherapy in stage I lung cancer. Anticancer Res. 2014;34:5099–104. [PubMed] [Google Scholar]
- 118.Cella L, D'Avino V, Palma G, Conson M, Liuzzi R, Picardi M, et al. Modeling the risk of radiation-induced lung fibrosis: Irradiated heart tissue is an important as irradiated lung. Radiother Oncol. 2015;117:36–43. doi: 10.1016/j.radonc.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 119.Tucker SL, Liu A, Gomez D, Tang LL, Allen P, Yang J, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119:495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 120.Nalbantov G, Kietselaer B, Vandecasteele K, Oberije C, Berbee M, Troost E, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol. 2013;109:100–6. doi: 10.1016/j.radonc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 121.van den Bogaard VAB, Ta BDP, van der Schaaf A, Bouma AB, Middag AMH, Bantema-Joppe EJ, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.69.8480. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Futami Y, Kanai T, Fujita M, Tomura H, Higashi A, Matsufuji N, et al. Broad-beam three-dimensional irradiation system for heavy-ion radiotherapy at HIMAC. Nuc Instrum Meth A. 1999;430:143–53. [Google Scholar]
- 123.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-Ito H, Tomura H, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–10. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 124.Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol. 2011;56:R113–144. doi: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- 125.Kubiak T. Particle therapy of moving targets-the strategies for tumour motion monitoring and moving target irradiation. Br J Radiol. 2016;89:20150275. doi: 10.1259/bjr.20150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takahashi W, Mori S, Nakajima M, Yamamoto N, Inaniwa T, Furukawa T, et al. Carbon-ion scanning lung treatment planning with respiratory-gated phase-controlled rescanning: simulation study using 4-dimensional CT data. Radiat Oncol. 2014;9:238. doi: 10.1186/s13014-014-0238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Furukawa T, Hara Y, Mizushima K, Saotome N, Tansho R, Saraya T, et al. Development of NIRS pencil beam scanning system for carbon ion radiotherapy. Nuc Instrum Meth. 2016 In press. [Google Scholar]
- 128.Dobashi S, Mori S. Evaluation of respiratory pattern during respiratory-gated radiotherapy. Australas Phys Eng Sci Med. 2014;37:731–42. doi: 10.1007/s13246-014-0310-9. [DOI] [PubMed] [Google Scholar]
- 129.Korreman SS, Juhler-Nøttrup T, Boyer AL. Respiratory gated beam delivery cannot facilitate margin reduction, unless combined with respiratory correlated image guidance. Radiother Oncol. 2008;86:61–8. doi: 10.1016/j.radonc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 130.Mori S, Inaniwa T, Furukawa T, Takahashi W, Nakajima M, Shirai T, et al. Amplitude-based gated phase-controlled rescanning in carbon-ion scanning beam treatment planning under irregular breathing conditions using lung and liver 4DCTs. J Radiat Res. 2014;55:948–58. doi: 10.1093/jrr/rru032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mori S, Karube M, Shirai T, Tajiri M, Takekoshi T, Miki K, et al. Carbon-ion pencil beam scanning treatment with gated markerless tumor tracking: an analysis of positional accuracy. Int J Radiat Oncol Biol Phys. 2016;95:258–66. doi: 10.1016/j.ijrobp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 132.Graeff C. Robustness of 4D-optimized scanned carbon ion beam therapy against interfractional changes in lung cancer. Radiother Oncol. 2017;122:387–92. doi: 10.1016/j.radonc.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 133.van Baardwijk A, Tomé WA, van Elmpt W, Bentzen SM, Reymen B, Wanders R, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill? A systematic review Radiother Oncol. 2012;105:145–9. doi: 10.1016/j.radonc.2012.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


