Abstract
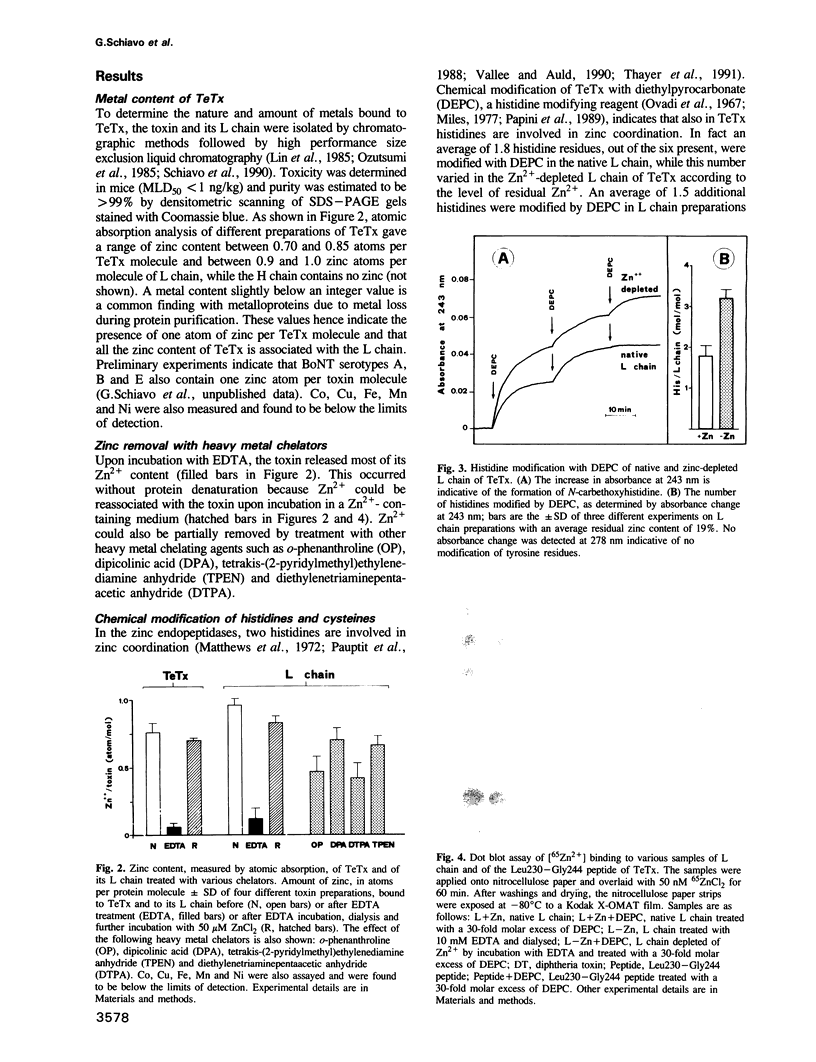
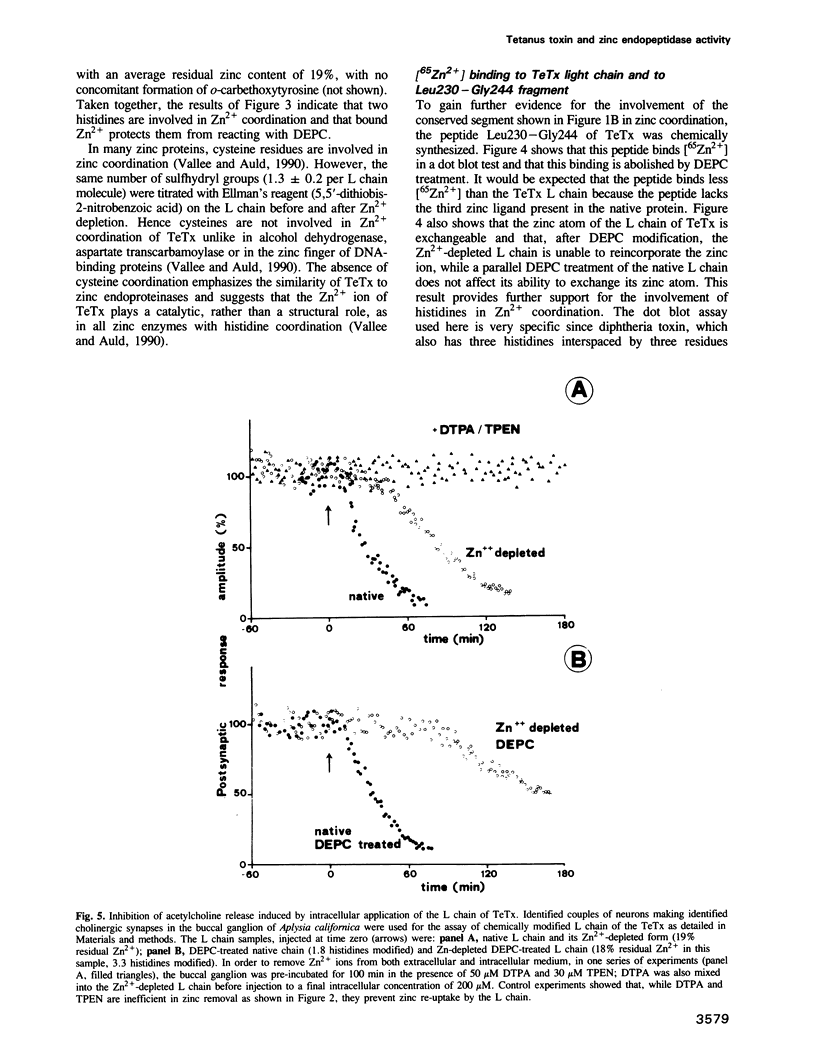
Tetanus and botulinum neurotoxins are the most potent toxins known. They bind to nerve cells, penetrate the cytosol and block neurotransmitter release. Comparison of their predicted amino acid sequences reveals a highly conserved segment that contains the HexxH zinc binding motif of metalloendopeptidases. The metal content of tetanus toxin was then measured and it was found that one atom of zinc is bound to the light chain of tetanus toxin. Zinc could be reversibly removed by incubation with heavy metal chelators. Zn2+ is coordinated by two histidines with no involvement in cysteines, suggesting that it plays a catalytic rather than a structural role. Bound Zn2+ was found to be essential for the tetanus toxin inhibition of neurotransmitter release in Aplysia neurons injected with the light chain. The intracellular activity of the toxin was blocked by phosphoramidon, a very specific inhibitor of zinc endopeptidases. Purified preparations of light chain showed a highly specific proteolytic activity against synaptobrevin, an integral membrane protein of small synaptic vesicles. The present findings indicate that tetanus toxin, and possibly also the botulinum neurotoxins, are metalloproteases and that they block neurotransmitter release via this protease activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Bagetta G., Nisticò G., Bowery N. G. Characteristics of tetanus toxin and its exploitation in neurodegenerative studies. Trends Pharmacol Sci. 1991 Aug;12(8):285–289. doi: 10.1016/0165-6147(91)90576-e. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F., Bähler M., Jahn R., Greengard P. Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J Cell Biol. 1989 May;108(5):1863–1872. doi: 10.1083/jcb.108.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F. Actin: protein structure and filament dynamics. J Biol Chem. 1991 Jan 5;266(1):1–4. [PubMed] [Google Scholar]
- De Camilli P., Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Suda H., Aoyagi T., Takeuchi T., Umezawa H. Studies on inhibitory effect of phosphoramidon and its analogs on thermolysin. Arch Biochem Biophys. 1975 Dec;171(2):727–731. doi: 10.1016/0003-9861(75)90085-5. [DOI] [PubMed] [Google Scholar]
- Krieglstein K., Henschen A., Weller U., Habermann E. Arrangement of disulfide bridges and positions of sulfhydryl groups in tetanus toxin. Eur J Biochem. 1990 Feb 22;188(1):39–45. doi: 10.1111/j.1432-1033.1990.tb15368.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Strittmatter W. J. Cellular functions of metallo-endoproteinases. Biochim Biophys Acta. 1991 Jul 22;1071(2):149–158. doi: 10.1016/0304-4157(91)90022-o. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Habig W. H., Hardegree M. C. Antibodies against the light chain of tetanus toxin in human sera. Infect Immun. 1985 Jul;49(1):111–115. doi: 10.1128/iai.49.1.111-115.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., Hawkins C., Mellanby H., Rawlins J. N., Impey M. E. Tetanus toxin as a tool for studying epilepsy. J Physiol (Paris) 1984;79(4):207–215. [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Mochida S., Poulain B., Eisel U., Binz T., Kurazono H., Niemann H., Tauc L. Exogenous mRNA encoding tetanus or botulinum neurotoxins expressed in Aplysia neurons. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7844–7848. doi: 10.1073/pnas.87.20.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozutsumi K., Sugimoto N., Matsuda M. Rapid, simplified method for production and purification of tetanus toxin. Appl Environ Microbiol. 1985 Apr;49(4):939–943. doi: 10.1128/aem.49.4.939-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E., Schiavo G., Sandoná D., Rappuoli R., Montecucco C. Histidine 21 is at the NAD+ binding site of diphtheria toxin. J Biol Chem. 1989 Jul 25;264(21):12385–12388. [PubMed] [Google Scholar]
- Pauptit R. A., Karlsson R., Picot D., Jenkins J. A., Niklaus-Reimer A. S., Jansonius J. N. Crystal structure of neutral protease from Bacillus cereus refined at 3.0 A resolution and comparison with the homologous but more thermostable enzyme thermolysin. J Mol Biol. 1988 Feb 5;199(3):525–537. doi: 10.1016/0022-2836(88)90623-7. [DOI] [PubMed] [Google Scholar]
- Poulain B., Baux G., Tauc L. Presynaptic transmitter content controls the number of quanta released at a neuro-neuronal cholinergic synapse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):170–173. doi: 10.1073/pnas.83.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain B., Mochida S., Wadsworth J. D., Weller U., Habermann E., Dolly J. O., Tauc L. Inhibition of neurotransmitter release by botulinum neurotoxins and tetanus toxin at Aplysia synapses: role of the constituent chains. J Physiol (Paris) 1990;84(4):247–261. [PubMed] [Google Scholar]
- Poulain B., Tauc L., Maisey E. A., Wadsworth J. D., Mohan P. M., Dolly J. O. Neurotransmitter release is blocked intracellularly by botulinum neurotoxin, and this requires uptake of both toxin polypeptides by a process mediated by the larger chain. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4090–4094. doi: 10.1073/pnas.85.11.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Schiavo G., Ferrari G., Rossetto O., Montecucco C. Tetanus toxin receptor. Specific cross-linking of tetanus toxin to a protein of NGF-differentiated PC 12 cells. FEBS Lett. 1991 Sep 23;290(1-2):227–230. doi: 10.1016/0014-5793(91)81266-b. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Papini E., Genna G., Montecucco C. An intact interchain disulfide bond is required for the neurotoxicity of tetanus toxin. Infect Immun. 1990 Dec;58(12):4136–4141. doi: 10.1128/iai.58.12.4136-4141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. M., Flaherty K. M., McKay D. B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J Biol Chem. 1991 Feb 15;266(5):2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Matthews B. W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J Mol Biol. 1977 Jul;114(1):119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]
- Weller U., Mauler F., Habermann E. Tetanus toxin: biochemical and pharmacological comparison between its protoxin and some isotoxins obtained by limited proteolysis. Naunyn Schmiedebergs Arch Pharmacol. 1988 Aug;338(2):99–106. doi: 10.1007/BF00174855. [DOI] [PubMed] [Google Scholar]





