Abstract
Alzheimer's disease (AD) is the most common dementia with dramatic consequences. The research in structural and functional neuroimaging showed altered brain connectivity in AD. In this study, we investigated the whole-brain resting state functional connectivity (FC) of the subjects with preclinical Alzheimer's disease (PAD), mild cognitive impairment due to AD (MCI) and mild dementia due to Alzheimer's disease (AD), the impact of APOE4 carriership, as well as in relation to variations in core AD CSF biomarkers. The synchronization in the whole-brain was monotonously decreasing during the course of the disease progression. Furthermore, in AD patients we found widespread significant decreases in functional connectivity (FC) strengths particularly in the brain regions with high global connectivity. We employed a whole-brain computational modeling approach to study the mechanisms underlying these alterations. To characterize the causal interactions between brain regions, we estimated the effective connectivity (EC) in the model. We found that the significant EC differences in AD were primarily located in left temporal lobe. Then, we systematically manipulated the underlying dynamics of the model to investigate simulated changes in FC based on the healthy control subjects. Furthermore, we found distinct patterns involving CSF biomarkers of amyloid-beta (Aβ1 − 42) total tau (t-tau) and phosphorylated tau (p-tau). CSF Aβ1 − 42 was associated to the contrast between healthy control subjects and clinical groups. Nevertheless, tau CSF biomarkers were associated to the variability in whole-brain synchronization and sensory integration regions. These associations were robust across clinical groups, unlike the associations that were found for CSF Aβ1 − 42. APOE4 carriership showed no significant correlations with the connectivity measures.
Keywords: Resting state fMRI, Dynamic functional connectivity, Computational modeling, Alzheimer's disease, Biomarkers
Highlights
-
•
Whole-brain resting state functional connectivity (FC) is investigated in subjects with preclinical Alzheimer’s disease (PAD), mild cognitive impairment due to AD (MCI) and mild dementia due to Alzheimer’s disease (AD).
-
•
Mean and standard deviation of the whole-brain synchronization reduce during the progression of AD. Regional FC shows widespread decreases in AD group, whereas whole-brain computational modeling approach reveals the left temporal lobe as the core of these alterations.
-
•
Systematically manipulating the dynamical regime of the model in healthy control subjects generates simulated FC patterns that match to the empirically observed FC in clinical groups (PAD, MCI, AD).
-
•
Amyloid-beta CSF biomarker primarily reflects the connectivity alterations in clinical groups with respect to the healthy control group.
-
•
Total tau and phosphorylated tau CSF biomarkers show distinct regional associations that are present also across clinical groups.
1. Introduction
Alzheimer's disease (AD), being the most prevalent dementia, became a major concern in developed countries as a consequence of increasing life expectancy (Blennow et al., 2006, Plassman et al., 2007). During the past two decades advancements in genetics, neurobiology and neuroimaging techniques allowed researchers to study the mechanisms behind the underlying causes of AD. In particular, resting state functional Magnetic Resonance Imaging (rs-fMRI) became a widely used tool to study the alterations in brain activity of AD patients as well as many other clinical conditions (Greicius, 2008). Furthermore, cerebrospinal fluid (CSF) biomarkers have been shown to serve as a proxy to monitor in vivo the neuropathological hallmarks of AD, namely amyloid-β and tau tangles (José Luis Molinuevo et al., 2014).
Various rs-fMRI studies showed altered functional connectivity in AD (Brier et al., 2014, Dennis and Thompson, 2014, Filippi and Agosta, 2011). The studies that used seed-based approach showed widespread decreases in hippocampal (Allen et al., 2007; W. Li et al., 2012, Wang et al., 2006) and posterior cingulate functional connectivity (Bai et al., 2011, Zhang et al., 2009) in AD. In addition, some of these studies reported increased FC between prefrontal cortex and hippocampus (Wang et al., 2006), and between prefrontal cortex and posterior cingulate (Bai et al., 2011, Zhang et al., 2009) in AD. The increased connectivity in prefrontal cortex was interpreted as a compensation mechanism during the initial stages of the disease (Dickerson et al., 2004, Filippi and Agosta, 2011, Sanz-Arigita et al., 2010). The studies based on independent component analysis (ICA) showed decreased activation of default mode network (DMN) (Agosta et al., 2012, Koch et al., 2010, Qi et al., 2010, Sorg et al., 2007) and increased activation of fronto-parietal network (FPN) (Agosta et al., 2012). Various other studies found impaired deactivation of DMN during task in AD and dementia (Celone et al., 2006, Greicius et al., 2004, Lustig et al., 2003, Petrella et al., 2007, Rombouts et al., 2009, Rombouts et al., 2005).
Another powerful approach to study AD comprised the relationship between CSF biomarkers and the progression of AD. For example, amyloid-β plaques are known to accumulate decades before the onset of the first disease symptoms in individuals with prolonged phase of “preclinical AD” (Price and Morris, 1999). The analysis of CSF Aβ1 − 42 concentrations has been shown to closely correlate with cerebral pathology. Furthermore, to identify the functional manifestations of CSF biomarkers, rs-fMRI has been proposed as a promising approach (Barkhof et al., 2014). Several studies showed an overlap between the spatial pattern of the DMN and that of Aβ1–42 accumulation that happens in this preclinical phase of AD (Buckner et al., 2008, Hedden et al., 2009). Furthermore, DMN connectivity was decreased in cognitively normal individuals with augmented cerebral amyloid load (Sheline et al., 2010b, Hedden et al., 2009, Oh et al., 2011). In addition to Aβ1 − 42, altered functional connectivity in the DMN have also been associated to abnormal levels of phosphorylated Tau181 (p-tau) in CSF (Wang et al., 2013) as well as the ratio Aβ1–42/p-tau and the AD CSF Index (Jose Luis Molinuevo et al., 2013), both of which constitute well-established markers of disease progression (X. Li et al., 2013). Reduced DMN functional connectivity has also been reported in amyloid-free carriers of at least one copy of the APOE4 allele, which is the strongest genetic risk factor for AD (Sheline, Morris, et al., 2010a). These findings suggested that differences in functional connectivity might even precede amyloid deposition (Sheline and Raichle, 2013).
Despite robust findings addressing altered DMN connectivity in AD, the mechanisms behind this alteration are not clear. Furthermore, dysfunction of DMN is the most common finding in many other mental disorders (Broyd et al., 2009). Therefore, it is crucial to understand the relationship between structure, function and CSF biomarkers in AD (Ramirez et al., 2014, Filippi and Agosta, 2011).
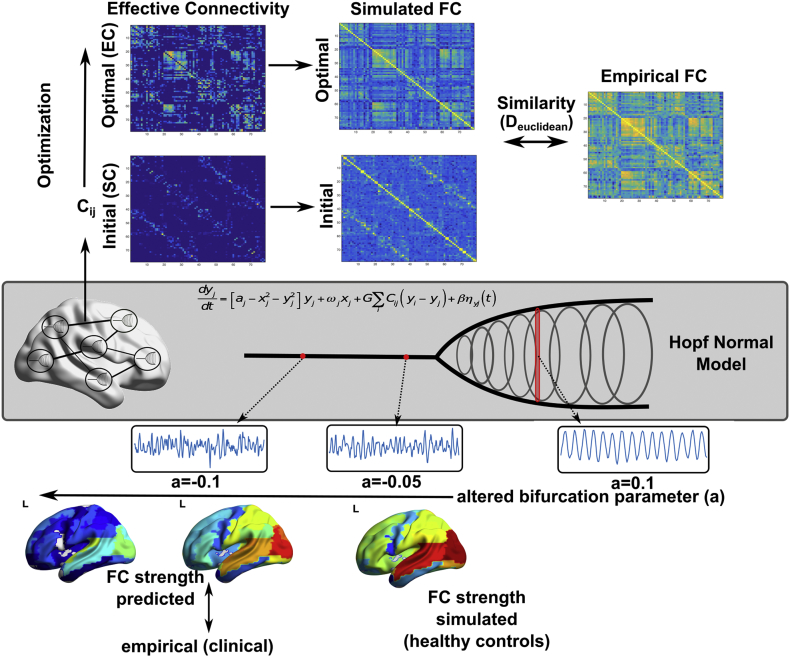
In this study, we investigated the rs-FC alterations in preclinical Alzheimer's disease (PAD), mild cognitive impairment due to AD (MCI) and mild dementia due to Alzheimer's disease (AD). First, we studied the whole-brain connectivity in each group based on the fluctuations in global synchronization level between all brain regions. Then, to understand the role of distinct brain regions, we characterized the rs-FC of each region to the rest of the brain by adapting a previously employed technique to parcellated data (Cole et al., 2010). Moreover, we proposed whole-brain computational model to provide mechanistic understanding of the connectivity alterations in each group. We performed two experiments using the model: First, to understand the role of long-range interactions between regions on the rs-FC alterations in clinical groups, we estimated the effective connectivity in the model. Effective connectivity (EC) refers to the optimal connection strengths between the regions in the model that generate the observed FCs. Second, to test whether a global shift in the optimal dynamics explains the rs-FC alterations, we investigated the predicted changes in rs-FC by manipulating the model parameters in healthy control subjects (Fig. 1). Furthermore, we studied the association between core AD CSF biomarkers and described connectivity measures.
Fig. 1.
Overview of the model. Top panel illustrates the optimization of the effective connectivity (EC). Middle panel illustrates Hopf normal model. Bottom panel illustrates the effect of local bifurcation parameter (a) and the overview of the computational experiment.
2. Materials and methods
2.1. Subjects
A total of 109 participants (58 HC, 12 PAD, 23 MCI and 16 AD) were recruited at the Alzheimer's disease and other cognitive disorders unit, from the Hospital Clinic of Barcelona. All subjects underwent clinical and neuropsychological assessment, MRI scanning and were submitted to a lumbar puncture to quantify the content of Aβ1–42, p-tau and t-tau in CSF. CSF biomarker quantitation was done at the local laboratory by means of ELISA (Enzyme-Linked ImmunoSorbent Assay kits, Innogenetics, Ghent, Belgium). An interdisciplinary clinical committee formed by two neurologists and one neuropsychologist established the diagnoses. HC and PAD presented no evidence of cognitive impairment on any of the administered neuropsychological tests, but PAD presented an abnormal level of CSF Aβ1–42 (below 500 pg/ml). MCI and AD presented signs of dementia. MCI patients had an objective memory deficit, defined as an abnormal score on the total recall measure of the Free and Cued Selective Reminding Test (FCRST) (over 1.5 × standard deviation), impairment on one or more of the other cognitive tests or preserved activities of daily living, as measured by the Functional Activities Questionnaire (FAQ score < 6). The NINCDS-ADRDA criteria were applied for probable AD diagnosis (Jack et al., 2011), taking into account clinical information and objective measures derived from the FAQ and neuropsychological results. AD patients were all in the mild stages of the disease (Global Deterioration Scale = 4). Diagnostic classification was made independent of CSF results. The local ethics committee approved the study and all participants gave written informed consent to participate in the study. Genomic DNA was extracted from peripheral blood of probands using the QIAamp DNA blood minikit (Qiagen AG, Basel, Switzerland). Apolipoprotein E genotyping was performed by polymerase chain reaction amplification and HhaI restriction enzyme digestion. Average demographic characteristics of the four diagnostic groups are shown in Table 1.
Table 1.
Demographics.
| HC | PAD | MCI | AD | |
|---|---|---|---|---|
| Age | 60.72 (6.99) | 69.00 (7.62) | 69.73 (7.77) | 65.00 (9.98) |
| Sex | 37 (M), 20 (F) | 9 (M), 3 (F) | 14 (M), 9 (F) | 9 (M), 7 (F) |
| APOE careers | 0.15 | 0.42 | 0.52 | 0.50 |
| CSF Biomarker Index | 0.44 (0.26) | 1.09 (0.55) | 1.50 (0.42) | 1.52 (0.43) |
| FR | 27.23 (6.92) | 21.25 (6.59) | 8.95 (7.14) | 6.23 (4.04) |
| TR | 43.14 (4.44) | 38.42 (7.06) | 21.77 (12.95) | 18.69 (12.48) |
| FDR | 10.43 (2.34) | 8.58 (3.18) | 2.55 (3.20) | 2.00 (2.52) |
| TDR | 14.84 (1.26) | 13.33 (2.46) | 6.32 (5.25) | 5.69 (4.87) |
Key: AD, Alzheimer's Disease; HC, healthy controls; PAD, preclinical Alzheimer's disease; MCI, mild cognitive impairment; APOE, apolipoprotein E allele; CSF, cerebrospinal fluid; FR, free recall; TR, total recall; FDR, free differed recall; TDR, total differed recall.
2.2. Image acquisition and preprocessing
Subjects were examined on a 3 T MRI scanner (Magnetom Trio Tim, Siemens, Erlangen, Germany) at the image core facilities of IDIBAPS (Barcelona, Spain). MRI session included a high-resolution three-dimensional structural T1-weighted image (sagittal MPRAGE; TR = 2300 ms, TE = 2.98 ms; matrix size = 256 × 256 × 240; isometric voxel 1 × 1 × 1 mm3), a 10 min resting state fMRI (rs-fMRI; 300 volumes, TR = 2000 ms, TE = 16 ms, 128 × 128 × 40 matrix, voxel size = 1.72 × 1.72 × 3 mm3) and two sets of diffusion weighted images (DWI; 30 no-collinear directions with a b value of 1000 s/mm2 and one volume with a b value of 0; TR = 7700 ms, TE = 89 ms; matrix size = 122 × 122 × 60; voxel size 2.05 × 2.05 × 2 mm3).
The pre-processing pipeline of rs-fMRI consisted in the slice-timing correction, the realignment and re-slice, smoothing with a Gaussian kernel (FWHM = 5 mm), second order de-trending and regressing out Volterra expanded parameters of movement (24 parameters), mean white matter (WM) signal, mean CSF signal and nulling regressors for bad volumes (Lemieux et al., 2007). Global signal regression (GSR) was not performed, because the known issues about GSR (Murphy et al., 2009) have a prominent impact on the analysis of whole-brain connectivity (Yang et al., 2014). Movement and other nuisance regressions were performed to alleviate the global artifacts. The quality criteria to consider a volume wrong and to override it by a nulling regressor, was that its correlation coefficient (cc) with the mean image of its series were beyond three standard deviations (cc < 0.991) from the mean cc of all the images from all subjects to their corresponding mean image (mean cc = 0.995). No subjects presented > 15% of bad volumes, being the average percentage of bad volumes of 1.6% and the standard deviation of 3.7%. To obtain the time series of each region from the Anatomical Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002), AAL atlas was adapted to every subject native space by co-registering it to the T1 structural image by mean of Advanced Normalization Tools (ANTs; UPENN, UVA and UIowa, USA; http://stnava.github.io/ANTs/). We kept the data in native space, because bringing the data to standard space slightly alters the data by interpolations. Moreover, we did not perform a voxel-wise analysis, which would require spatially normalized images. AAL maps in native space were resliced to fMRI resolution using nearest neighbor interpolation and masked with the gray mater (GM) mask. GM mask was constructed for every subject from the tissue probability maps resulted from segmentation of T1 images. The mask was formed by those voxels whose probability of belonging to GM was bigger than the probability of belonging to any other tissue. GM masks were dilated one voxel to include edges and to fill noise-related small gaps and, finally, resliced to fMRI resolution. Time series were obtained by averaging the fMRI signal in the each area of the GM-masked AAL atlas in native space (Tzourio-Mazoyer et al., 2002). The software used for the whole fMRI pre-processing, apart from the above mentioned ANTs, was a homemade MATLAB (Mathworks, Sherborn, MA, USA) scripts mostly formed by functions from SPM package (Wellcome Trust Center for Neuroimaging; UCL, UK; http://www.fil.ion.ucl.ac.uk/spm/).
DWI were first corrected for eddy current distortions using FMRIB Software Library (FSL) package (Jenkinson et al., 2012). Data was denoised using the overcomplete local principal component analysis (PCA) method described in (Manjón et al., 2013). Similarly, T1-weighted images were denoised using a non-local mean filter (Coupe et al., 2008) and then corrected for the usual acquisition bias with the N4 method from ANTs package (Tustison et al., 2010). Anatomical images were then segmented with VBM8 toolbox (Ashburner and Friston, 2000) to create GM, WM and CSF probabilistic maps. Bias-corrected T1 images were then co-registered to the non-gradient diffusion image and to the MNI template using ANT's (Avants et al., 2011). Brain regions from AAL template (Tzourio-Mazoyer et al., 2002) were then resampled to the diffusion space of each subject. Finally, FSL's Bedpostx and Probtrackx tractography was performed between AAL regions using default parameters. Cerebellum was not considered resulting in a 90 × 90 structural connectivity matrix.
2.3. Whole-brain connectivity measures
We quantified the measure of whole-brain connectivity based on level of synchronization in the BOLD time series of each group. Hilbert transform converts the narrowband signal as a(t) = A(t)cos(φ(t)), where A(t) is the instantaneous amplitude (or envelope), and A(t), and φ(t) is the instantaneous phase. The first and last 20 s (10 TR) of the transformed BOLD signal was then removed. The global coherence and metastability of the time-series were computed based on the Kuramoto Order Parameter (KOP) (Kuramoto, 1986, Shanahan, 2010, Cabral et al., 2012, Hellyer et al., 2014): , where N is the number of ROIs and φ(t) is the instantaneous phase of each region estimated using Hilbert Transform. The temporal average of Kuramoto Order Parameter defined as the coherence (mean synchronization), whereas the standard deviation of Kuramoto Order Parameter defined as the metastability (i.e. the variation in synchronization over time).
Since all the analyses relies on the choice of narrowband, to test the robustness of the proposed measures across frequency bands, we estimated the group differences in coherence and metastability in 7 narrowbands with 0.03 Hz bandwidth and frequency onsets varying between 0.01 Hz and 0.07 Hz (one-way ANOVA). We found significant group differences for both measures in frequency bands between onsets of 0.03 and 0.07 (i.e. 0.03–0.07 Hz to 0.07–0.1 Hz). The differences were significant except 0.01–0.04 Hz narrowband (Supplementary Fig. 1). Due to high computational demand of the modeling framework, for the rest of the analyses we focused on 0.06–0.09 Hz narrowband in which the group differences were high for both coherence and metastability. Furthermore, the 0.06 Hz frequency onset is also consistent with a previous study that showed the highest discriminative power for classification of MCI and AD was achieved between 0.054 and 0.068 Hz range (Wee et al., 2012). An alternative approach to define the narrowband would be to choose 0.04–0.07 Hz range, in which the BOLD signal is relatively less affected by known artifacts (Glerean et al., 2012). We repeated the group comparison and CSF biomarker correlation analyses based on whole-brain and FC (Supplementary Fig. 2–3). Most of the results remained unchanged when 0.04–0.07 Hz narrowband was used with some exceptions: group differences in coherence was insignificant after regressing out age, group differences in FC between AD-HC were more symmetric across hemispheres (Supplementary Fig. 2), and CSF biomarker correlations were absent in right prefrontal cortex (Supplementary Fig. 3).
The FC was computed as the Pearson's correlation between the time-series of different brain regions. Then, as a measure of regional connectivity, we used functional connectivity (FC) strength, which was calculated as the sum of FCs of each region to the rest of the regions. Previously global brain connectivity (GBC) was proposed to measure the regional variations in FC (Cole et al., 2010). Here, we preferred the term FC strength, used in graph theory, to emphasize that the measure was based on parcellated data.
2.4. Computational model
We modeled the whole-brain rs-fMRI BOLD signals using 78 nodes, excluding subcortical regions. The role of subcortical regions in whole-brain computational models is not well studied and subcortical regions are often excluded in these studies. The list of the regions and abbreviations were provided in Supplementary Table 1. Each node was coupled with each other via effective connectivity (EC) matrix. We described the local dynamics of each individual node using normal form of a supercritical Hopf bifurcation. The advantage of this model is that it allows transitions between asynchronous noise activity and oscillations. Where ω is the intrinsic frequency of each node, a is the local bifurcation parameter, η is additive Gaussian noise with standard deviation β, the temporal evolution of the activity, z, in node j is given in complex domain as:
and,
This system shows a supercritical bifurcation at aj = 0. Being specific, if aj is smaller than 0, the local dynamics has a stable fixed point at zj = 0, and for aj values larger than 0, there exists a stable limit cycle oscillation with a frequency f = ω/2π. Finally, the whole-brain dynamics is described by the following coupled equations:
where Cij is the Effective Connectivity (EC) between nodes i and j, G is the global coupling factor, and the standard deviation of Gaussian noise, β = 0.02. The natural frequency (f) of each region was taken as the peak frequency in the given narrowband of the corresponding region in the empirical time-series. The simulated activity corresponds to the BOLD signal of each node. The simulations were run for 30,000 s, sampled at 2 s, if not stated otherwise. Both the empirical and simulated BOLD signals were band-pass filtered in narrowband 0.06–0.09 Hz, since the group differences in coherence and metastability were optimal in this narrowband. The intrinsic frequency of each node was estimated as the peak frequency in the associated narrowband of the empirical BOLD signals of each brain region.
2.5. Optimization of effective connectivity
We implemented a heuristic approach to infer the most likely connectivity matrix (i.e. Effective Connectivity) that maximizes the similarity between empirical and simulated functional connectivity. As an initial guess, we used DWI-derived structural connectivity matrices (Fig. 1). First, we adjusted the global coupling parameter (G) to prevent overflow during the optimization procedure, and to ensure the stability of the system of equations. Where Ksim and Kemp are simulated and empirical coherences (average Kuramoto order parameter), we updated global coupling parameter as: G = G exp(Ksim − Kemp), until the desired condition, | Ksim − Kemp | < 0.1, was satisfied.
We evaluated both zero-time-lag Functional Connectivity (FC0) and time-lagged FC (FCτ) for both empirical and simulated BOLD signals. Time-lagged FC measure was chosen for two reasons. First, it provides an additional constraint ensuring that the optimal solution is unique. Second, time-lagged correlations allow inference on the directionality of the connections. The defined the distance metric as Euclidean Distance between simulated and empirical FC values for both FC0 and FCτ:
Then, where E is the average error between empirical and simulated FC measures;
where Sij is the anatomical connectivity matrix, N is the number of regions, and Λ denoted inter-hemispheric links; we updated the effective connectivity between i and j = (1, …, N) according to:Cijupdate = Cijcurrent + 0.01Eij, if Sij > 0 , or Sij ∈ {Λ}Cijupdate = 0, if Sij = 0 , or Cijcurrent + 0.01Eij < 0
In other words, we updated the EC based on the average error between empirical and simulated FC measures for non-zero connections and inter-hemispheric connections. Negative weights were not allowed, and they all set to zero during the update procedure.
We accepted the total distance between empirical and simulated FC measures, DT = D0 + Dτ, for updated EC is lower than the minimum total distance observed during the procedure. We repeated this procedure using 100 iterations, and the best solution (minimum D) was considered as EC for a given subject. The entire procedure was also repeated for bifurcation parameter a = {− 0.1, − 0.05, 0, 0.05, 1}. The best fit for the majority of the subjects was achieved at a = − 0.05 (Supplementary Fig. 4A). For consistency, we fixed the bifurcation parameter at a = − 0.05 for all the subjects. Finally, the EC strengths were computed as the sum of input and output weights of each node.
2.6. Computational experiment
Given the inferred EC matrices, we disrupted the dynamics in healthy controls based on the bifurcation parameter (a) of the supercritical Hopf Normal Model (Fig. 1). In brief, the local bifurcation parameter characterizes whether the underlying dynamics of each node are primarily asynchronous (a < 0) or oscillatory (a > 0). The model was discussed in detail in a recent paper (Deco et al., 2017). First, we computed the FC of the healthy controls at a = − 0.05, where the optimal similarity between simulated and empirical values was observed. Then, we decreased the bifurcation parameter by 0.001 at each iteration, spanning values between − 0.05 and − 0.15. The FC strengths (FCa) were computed for each a. Then, the distance between the average FC strength at each bifurcation parameter and the group averaged empirical FC strength for each clinical group (PAD, MCI, AD) was calculated. The distance was quantified as Euclidean distance between predicted and empirical FC. Finally, we used principal component analysis to investigate the main effects on FC strength across manipulated bifurcation parameters.
2.7. Statistical analyses
The group comparisons (for coherence, metastability, FC strength, EC strength) were done using permutation t-test (10,000 permutations), and multiple comparisons were corrected using FDR approach with Benjamini & Hochberg algorithm if necessary (Hochberg and Benjamini, 1990). Prior to group comparisons, we regressed out subject's age from each measure. The networks were visualized using BrainNet Viewer toolbox in Matlab (Xia et al., 2013). Correlations between CSF biomarkers (APOE-4, Aβ-42, t-tau and p-tau) and the measures (coherence, metastability, FC strength, EC strength) were estimated as the partial correlations controlled for age, gender and education level. APOE was quantified as the carriers and non-carriers of the gene.
3. Results
3.1. Whole-brain connectivity
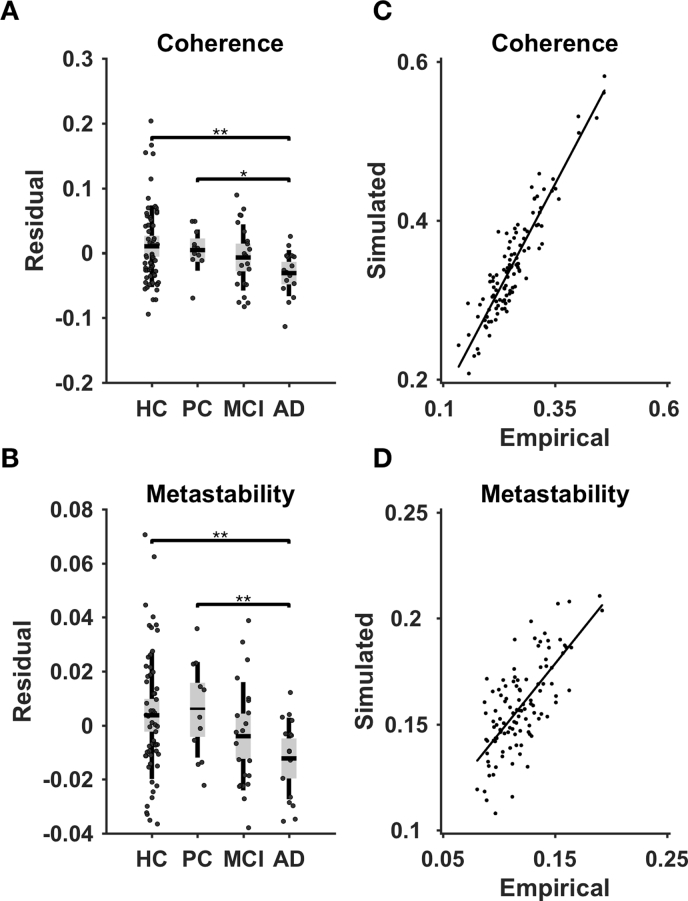
We characterized the whole-brain connectivity of each group using mean (coherence) and standard deviation (metastability) of Kuramoto order parameter, which quantifies the level of synchronization between brain regions across time. We found significant correlations between diagnostic category and coherence (r = − 0.37, p-value < 0.01), and metastability (r = − 0.33, p-value < 0.01). After regressing out age as confounding variable, we compared coherence and metastability across HC, PAD, MCI, and AD groups using permutation t-test (10,000 permutations, p-value < 0.05). The coherence and metastability in the AD group were significantly lower than those in HC group (coherence: T-statistic = 3.4, p-value < 0.01; metastability: T-statistic = 3.27, p-value < 0.01) and those in PAD group (coherence: T-statistic = 2.77, p-value < 0.05; metastability: T-statistic = 2.84, p-value < 0.01) (Fig. 2A–B). We found no significant differences in coherence and metastability across the rest of the groups (Supplementary Table 2). These results showed that the magnitude and variability of whole-brain connectivity is decreasing along with the progression of the disease, which reaches statistical significance in AD.
Fig. 2.
A Group differences in coherence (average Kuramoto order parameter). B Group differences in metastability (standard deviation of Kuramoto order parameter). The results are shown for 0.06–0.09 Hz narrowband signal. The comparisons were done using permutation t-test (10,000 permuations; ⁎p < 0.05, ⁎⁎p < 0.01) after regressing out age as a confounding variable. C The correspondence between empirical and simulated coherence. D The correspondence between empirical and simulated metastability.
3.2. Computational model
We proposed a whole-brain computational approach to model the whole-brain connectivity of each subject. Based on the similarity between empirical and simulated FC, we estimated the effective connectivity (EC) between brain regions. The average correlation coefficient between empirical and simulated FC was r = 0.81 (std = 0.03). Furthermore, the correlation coefficients between empirical and simulated coherence and metastability were r = 0.93 (p-value < 0.001) and r = 0.72 (p-value < 0.001), respectively (Fig. 2C–D). Therefore, the proposed model not only showed high similarity to the empirical FC, but also accounted for the variations in coherence and metastability.
3.3. Regional connectivity
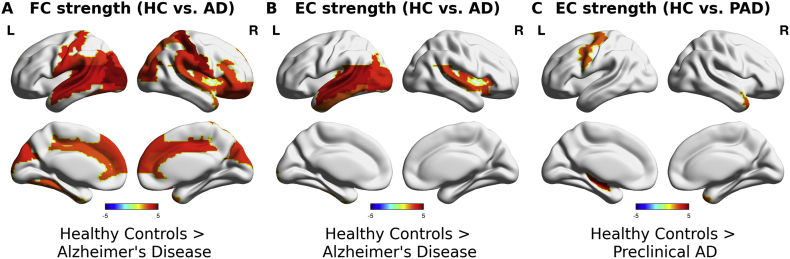
To investigate the regional variations in whole-brain connectivity, we compared the strengths of functional connectivity (FC) and strengths of effective connectivity (EC) of PAD, MCI and AD groups with those of HC group. AD group exhibited significantly decreased FC strengths widespread across the cortex (Fig. 3A) (Table 2). No significant differences were found in FC strengths of PAD and MCI groups. In contrast, significant decreases in EC strengths were located in left temporal lobe in AD group (Fig. 3B). Furthermore, EC strengths in PAD group were significantly decreased in left-precentral gyrus (T-statistic = 2.82, FDR adjusted p-value < 0.05), left hippocampus (T-statistic = 3.88, FDR adjusted p-value < 0.05) and right temporal pole (T-statistic = 2.92, FDR adjusted p-value < 0.05) (Fig. 3C). No significant differences were found in MCI group. These results showed that the model-based estimate of EC restricted the regional alterations to the left temporal lobe.
Fig. 3.
Group differences in FC and EC strengths. A FC strength of healthy control group vs. Alzheimer's disease (AD) group. B EC strength of healthy control group vs. Alzheimer's disease (AD) group. C EC strength of healthy control group vs. preclinical Alzheimer's disease subjects (PAD). The comparisons were done using permutation t-test (10,000 permuations) after regressing out age as a confounding variable. Colorbars indicate T-statistic, where hot colors indicate higher values in healthy control group. Only the regions that showed significant differences after FDR correction (adjusted p-value < 0.05) were shown.
Table 2.
Significant differences in FC and EC strengths.
| FC Strength |
(HC vs. AD) |
EC Strength |
(HC vs. AD) |
|---|---|---|---|
| ROI | T-statistic | ROI | T-statistic |
| l-MOG | 4.6237 | l-MTG | 4.2007 |
| r-PoCG | 4.5427 | l-IOG | 3.9683 |
| l-STG | 4.0204 | l-STG | 3.5094 |
| l-MTG | 3.9036 | l-HES | 3.4922 |
| l-HES | 3.7915 | l-MOG | 3.3404 |
| l-IOG | 3.6295 | r-INS | 3.2251 |
| l-PoCG | 3.5312 | r-STG | 3.0947 |
| r-MOG | 3.5258 | l-ITG | 2.7366 |
| l-ROL | 3.4757 | EC Strength | (HC vs. PC) |
| r-DCG | 3.4686 | ROI | T-Statistic |
| l-CUN | 3.4414 | l-HIP | 3.8781 |
| r-ROL | 3.4013 | r-TPOmid | 2.9248 |
| r-SMG | 3.3754 | l-PreCG | 2.8196 |
| r-ORBinf | 3.3300 | ||
| r-SFGmed | 3.3281 | ||
| r-ORBmid | 3.3205 | ||
| l-SOG | 3.3086 | ||
| r-CUN | 3.2861 | ||
| r-ORBsup | 3.2579 | ||
| r-INS | 3.2364 | ||
| l-SMG | 3.2118 | ||
| l-SFGmed | 3.1908 | ||
| l-ANG | 3.1634 | ||
| r-ACG | 3.1387 | ||
| r-IFGtriang | 3.0819 | ||
| r-STG | 3.0325 | ||
| r-SOG | 3.0077 | ||
| l-FFG | 2.9761 | ||
| l-DCG | 2.9755 | ||
| r-SPG | 2.8759 | ||
| l-ACG | 2.8703 | ||
| r-TPOmid | 2.8015 |
FDR-adjusted p-values < 0.05.
3.4. APOE4 and CSF biomarkers
We investigated the relationship between whole-brain/regional connectivity and APOE4 allele carrier status and CSF biomarkers. We calculated the partial correlations between each whole-brain/regional connectivity measure and APOE4 carrier status and CSF biomarkers (Aβ1–42, t-tau, p-tau) controlling for age, gender and education level. We performed the analysis across clinical groups (i.e. PAD, MCI, and AD subjects) and across all subjects (i.e. HC, PAD, MCI and AD subjects), separately.
Regarding the whole-brain connectivity measures, APOE4 allele carrier status showed no significant correlations with coherence and metastability (Table 3). Across all subjects, coherence showed a significant correlation with Aβ1–42 (rho = 0.23, p-value < 0.05). However, across clinical groups there were no significant correlations between Aβ1–42 and coherence (Table 3). Coherence showed no significant correlations with t-tau and p-tau (Table 3). In contrast, we found no correlations between metastability and Aβ1–42. Metastability was significantly correlated with t-tau (across all subjects, rho = − 0.27, p-value < 0.01; across clinical groups, rho = − 0.31, p-value < 0.05) and p-tau (across all subjects, rho = − 0.27, p-value < 0.01; across clinical groups, rho = − 0.36, p-value < 0.05) (Table 3). These results showed that Aβ1–42 CSF levels reflect the attenuation in coherence (average synchronization) due to its prevalence, but these levels do not correlate with the progression of AD. In contrast, the correlations between t-tau/p-tau CSF biomarkers and metastability (variability in synchronization) are also consistent with the progression of AD.
Table 3.
Partial correlations (rho) between CSF biomarkers and whole-brain connectivity measures.
| Across all subjects |
||||
|---|---|---|---|---|
| APOE-4 allelle carrier status | Aβ-42 | t-tau | p-tau | |
| Coherence | − 0.1264 | 0.2343⁎ | − 0.1221 | − 0.1307 |
| Metastability | − 0.0631 | 0.1349 | − 0.2664⁎⁎ | − 0.2738⁎⁎ |
| Across clinical groups |
||||
|---|---|---|---|---|
| APOE-4 allelle carrier status | Aβ-42 | t-tau | p-tau | |
| Coherence | − 0.0398 | − 0.0004 | − 0.0079 | − 0.0965 |
| Metastability | − 0.019 | 0.0207 | − 0.3097⁎ | − 0.3647⁎ |
p-value < 0.05.
p-value < 0.01.
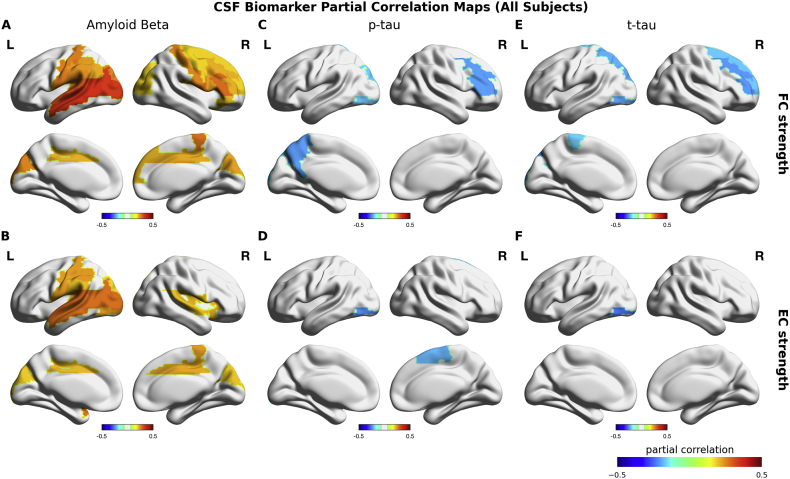
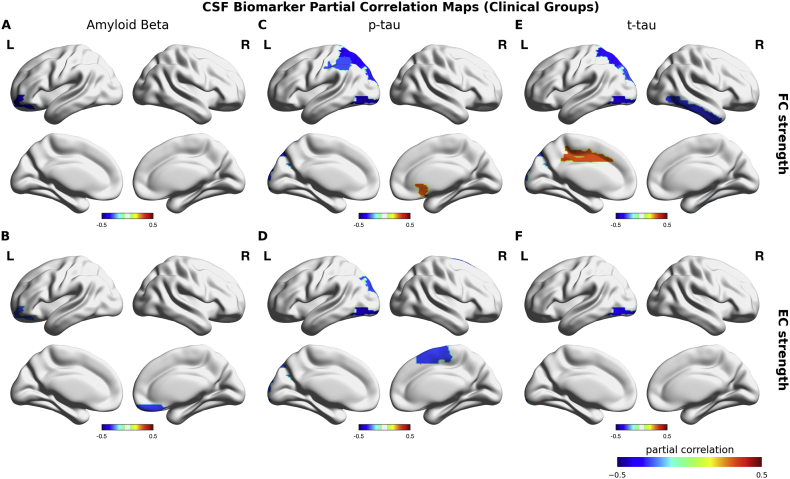
Across all subjects, CSF Aβ1–42 showed significant positive correlations with FC strength and EC strength (Fig. 4A–B) (Table 4). The spatial maps of these correlations were highly overlapping with the group differences between AD and HC subjects (Fig. 4A–B). For p-tau and t-tau CSF biomarkers, we found significant negative correlations with FC strengths in frontal, parietal and occipital regions, and with EC strengths in occipital and parietal regions (Fig. 4C–E) (Table 5). Across clinical groups, CSF Aβ1–42 showed no positive correlations with FC and EC strengths. In contrast, we found significant negative correlations between CSF Aβ1–42 and left orbital frontal FC strength (rho = − 0.38, p-value < 0.01) and EC strength (rho = − 0.30, p-value < 0.05), and right rectus EC strength (r = − 0.31, p-value < 0.05) (Fig. 5A–B). The spatial maps of the correlations of p-tau and t-tau CSF biomarkers were relatively consistent across clinical groups with those found across all subjects (Fig. 5C–E). In particular, superior parietal gyrus, superior and inferior occipital gyri in left hemisphere were the most consistent regions and the magnitude of the correlations in these regions were substantially higher across clinical than those across all subjects (Table 5). Furthermore, FC strength showed significant positive correlations between t-tau CSF biomarker in left dorsal cingulate and between CSF p-tau in right olfactory cortex (Table 5). The results suggested distinct roles of Aβ1–42 and tau CSF biomarkers in AD.
Fig. 4.
CSF biomarker partial correlation maps across all subjects. Upper row shows the relationship between FC strength and each CSF biomarker. Bottom row shows the relationship between FC strength and each CSF biomarker. Colorbars indicate the partial correlation coefficient (rho) between Aβ-42 and FC (A), between Aβ-42 and EC (B), between p-tau and FC (C), between p-tau and EC (D), between t-tau and FC (E), between t-tau and EC (F) that were calculated across all subjects controlled for age, gender and education level. Only significant correlations were colored on cortical surface plots.
We found similar trends in regional connectivity measures (FC and EC strengths) (this figure and Fig. 5) (Supplementary Tables 3–6). We found no significant correlations between APOE4 allele carrier status and FC strengths (across all subjects and across clinical groups) and EC strengths (across all subjects). Across clinical groups, left superior frontal EC strength showed a significant correlation with APOE-4 allele carrier status (rho = − 0.30, p-value < 0.05) (Supplementary Tables 3–6).
Table 4.
Partial correlations between Aβ-42 CSF biomarker and FC-EC strengths.
| FC strength |
EC strength |
||
|---|---|---|---|
| ROI | rho | ROI | rho |
| l-MTG | 0.3402⁎⁎ | l-STG | 0.2887⁎⁎ |
| l-MOG | 0.3293⁎⁎ | l-MTG | 0.2785⁎⁎ |
| l-STG | 0.2946⁎⁎ | l-MOG | 0.2773⁎⁎ |
| r-IFGtriang | 0.2817⁎⁎ | l-TPOsup | 0.2759⁎⁎ |
| r-PCL | 0.2789⁎⁎ | l-ROL | 0.2528⁎⁎ |
| r-ROL | 0.2682⁎⁎ | l-IPL | 0.2435⁎ |
| l-PoCG | 0.2664⁎⁎ | l-IOG | 0.2409⁎ |
| l-CUN | 0.2615⁎⁎ | l-PoCG | 0.2405⁎ |
| l-SOG | 0.2594⁎⁎ | r-PCL | 0.2400⁎ |
| r-PoCG | 0.2574⁎⁎ | r-DCG | 0.2301⁎ |
| l-SMG | 0.2545⁎⁎ | r-INS | 0.2225⁎ |
| l-ROL | 0.2538⁎⁎ | r-ROL | 0.2210⁎ |
| l-HES | 0.2502⁎ | l-DCG | 0.2191⁎ |
| l-IPL | 0.2411⁎ | r-STG | 0.2150⁎ |
| r-IFGoperc | 0.2380⁎ | l-SMG | 0.2137⁎ |
| r-CUN | 0.2358⁎ | r-CUN | 0.2109⁎ |
| r-DCG | 0.2304⁎ | l-HES | 0.2061⁎ |
| l-ANG | 0.2284⁎ | l-CUN | 0.2053⁎ |
| r-MFG | 0.2257⁎ | l-SOG | 0.1982⁎ |
| l-DCG | 0.2252⁎ | ||
| r-SFGmed | 0.2195⁎ | ||
| r-ORBinf | 0.2183⁎ | ||
| r-SFGfor | 0.1991⁎ | ||
| r-MOG | 0.1981⁎ | ||
| r-SOG | 0.1973⁎ | ||
| r-PreCG | 0.1971⁎ | ||
p-value < 0.05.
p-value < 0.01.
Table 5.
Partial correlations between t-tau and p-tau CSF biomarkers and FC-EC strengths.
| ROI | FC strength |
|||
|---|---|---|---|---|
| Across all subjects |
Across clinical groups |
|||
| t-tau | p-tau | t-tau | p-tau | |
| l-SOG | − 0.2282⁎ | − 0.2011⁎ | − 0.2944⁎ | − 0.3287⁎ |
| l-IOG | − 0.2264⁎ | − 0.2045⁎ | − 0.4215⁎⁎ | − 0.4529⁎⁎ |
| l-SPG | − 0.2187⁎ | − 0.188 | − 0.3371⁎ | − 0.3730⁎ |
| l-PCL | − 0.1946⁎ | − 0.1473 | − 0.2451 | − 0.2146 |
| r-SFGfor | − 0.2005⁎ | − 0.1928 | − 0.1437 | − 0.1788 |
| r-MFG | − 0.2359⁎ | − 0.2322⁎ | − 0.199 | − 0.2253 |
| r-IFGtriang | − 0.1744 | − 0.2309⁎ | − 0.1101 | − 0.2818 |
| l-PCUN | − 0.1865 | − 0.2320⁎ | − 0.1773 | − 0.2869 |
| r-ITG | − 0.1626 | − 0.1079 | − 0.3212⁎ | − 0.276 |
| l-IPL | − 0.1891 | − 0.1566 | − 0.2615 | − 0.2935⁎ |
| l-DCG | − 0.0236 | − 0.0268 | 0.3080⁎ | 0.2415 |
| r-OLF | 0.0597 | 0.0399 | 0.2894 | 0.3082⁎ |
| ROI | EC strength | |||
| Across all subjects | Across clinical groups | |||
| t-tau | p-tau | t-tau | p-tau | |
| l-IOG | − 0.2638⁎⁎ | − 0.2438⁎ | − 0.3817⁎⁎ | − 0.4382⁎⁎ |
| r-SMA | − 0.1903 | − 0.2124⁎ | − 0.2237 | − 0.3048⁎ |
| l-SOG | − 0.1557 | − 0.1605 | − 0.2364 | − 0.2986⁎ |
p-value < 0.05.
p-value < 0.01.
Fig. 5.
CSF biomarker partial correlation maps across clinical groups. Upper row shows the relationship between FC strength and each CSF biomarker. Bottom row shows the relationship between FC strength and each CSF biomarker. Colorbars indicate the partial correlation coefficient (rho) between Aβ-42 and FC (A), between Aβ-42 and EC (B), between p-tau and FC (C), between p-tau and EC (D), between t-tau and FC (E), between t-tau and EC (F) that were calculated across PC, MCI and AD groups controlled for age, gender and education level. Only significant correlations were colored on cortical surface plots.
3.5. Computational experiment
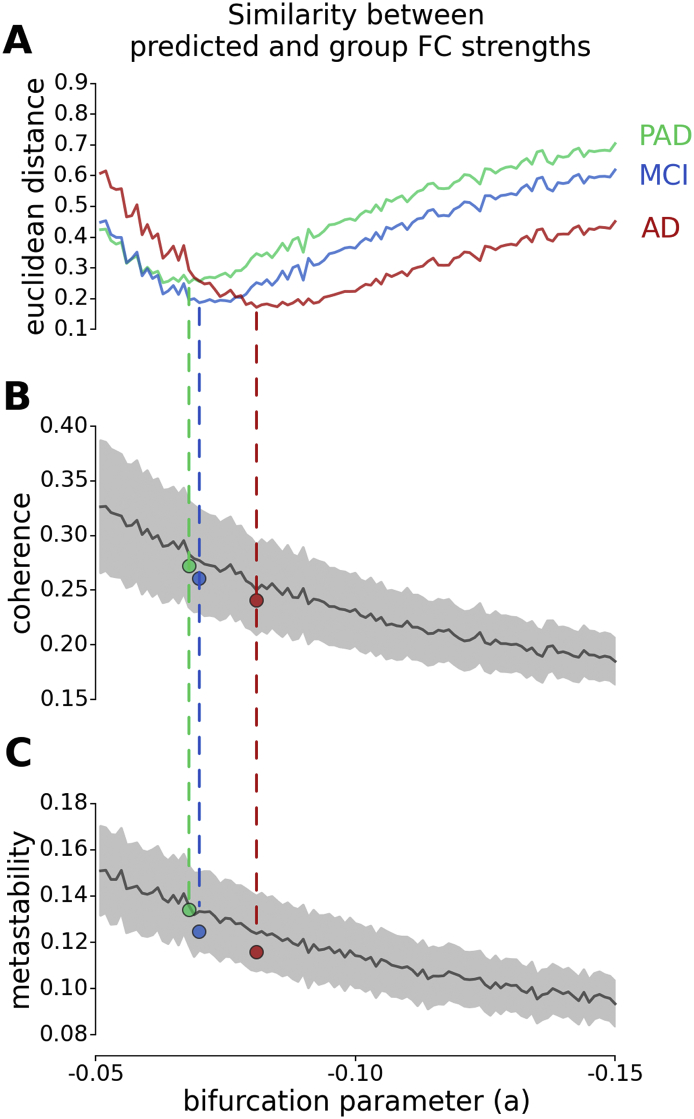
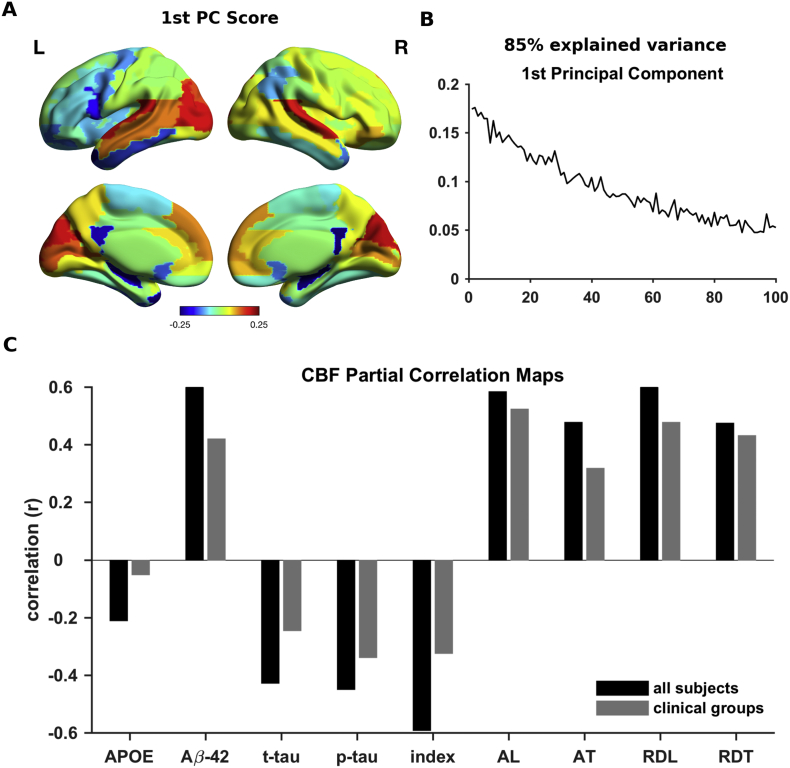
To link the altered whole-brain and regional connectivity, we conducted a computational experiment. Based on the proposed model, we systematically manipulated the local bifurcation parameter (a) in healthy control subjects to characterize the alterations in whole-brain and regional connectivity due to a global shift in optimal dynamics. Being specific, we disrupted the local dynamics of all regions in favor of noisy dynamics in healthy control subjects and checked the effects of this manipulation on simulated FC strengths. We found that the Euclidean distances between empirical FC strengths of each clinical group (PAD, MCI and AD) and predicted FC strengths were minimized at distinct bifurcation parameter values consistent with the hypothesis that the divergence from optimal dynamics aligns with the progression of AD (Fig. 6A). Furthermore, coherence and metastability at the predicted bifurcation parameter values were consistent with the empirically observed values in each clinical group (Fig. 6B–C). We used principal component analysis (PCA) on predicted FC strengths along the manipulated bifurcation parameter. Then, we extracted the spatial map based on the factor weights (scores) of the first principal component (PC) reflecting the main effect of the manipulation with 85% of the explained variance (Fig. 7A–B). We found that the spatial map of the first PC was correlated with the FC strengths of HC group (r = 0.97, p-value < 0.001). Furthermore, the spatial map of the first PC showed significant correlations with those of CSF biomarkers and cognitive performance scores (Fig. 7C). These results showed that the attenuation of the FC strength in each region is proportional to its initial magnitude. Furthermore, the empirically observed alterations in FC strengths primarily reflect these proportional attenuations due to the disintegration of the whole-brain connectivity.
Fig. 6.
Computational experiment. FC strengths were calculated after performing simulations with manipulated local bifurcation parameters (within range − 0.05 and − 0.15) based on healthy control subjects. A The Euclidean distance between simulated FC strengths and group averaged empirical FC strengths of PAD (green), MCI (blue) and AD (red) subjects. Dashed lines indicate the minimum distance between simulated FC strengths and group averaged empirical FC strengths of each group, colored accordingly. B The simulated coherence with respect to manipulated local bifurcation parameters. C The simulated metastability with respect to manipulated local bifurcation parameters. Gray shadings show the standard deviations of each parameter across subjects. Colored dots indicate the average empirical coherence and metastability of PAD (green), MCI (blue) and AD (red) groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Principal Component Analysis (PCA) of the simulated FC strengths across manipulated local bifurcation parameters. A The spatial map of the first principal component scores. The spatial map indicates the most and the least affected regions due to local bifurcation parameter manipulation in hot and cold colors, respectively. B The first principal component reflecting the FC alterations due to the divergence from optimal bifurcation parameter, explaining 85% of the total variance. C The correlation coefficient between the spatial map of first principal component and that of the relationship between FC strength and APOE-4 allelle carier status, CSF biomarkers and behavioral performance measures (i.e. partial correlation coefficients between FC strength and each measure). Black and gray bars indicate across all subject and across clinical group spatial maps, respectively.
4. Discussion
In this paper, we studied the changes in whole-brain and regional connectivity in PAD, MCI and AD. We treated the condition as a continuum based on severity comprising PAD, MCI and AD. We found that the whole-brain connectivity decreased through the continuum reaching significance only in AD group. Decreased whole-brain synchronization was also reported recently in AD (Córdova-Palomera et al., 2017). The alterations in regional connectivity were also consistent with this trend. We proposed a whole-brain computational model to explore the mechanisms behind these alterations.
We showed that the strongly connected brain regions were affected more severely than other brain regions. Confirming the previous findings on FC impairments in AD, these regions were overlapping with default mode network (DMN). However, alterations in DMN were also reported in many other mental disorders (Broyd et al., 2009). This is an expected result, because the regions forming DMN repeatedly found as the structural hubs of the brain (Hagmann et al., 2008, Margulies et al., 2016). We hypothesized that the core alterations in connectivity might be obscured in FC due to the propagation of impaired activity. To reveal the connectivity changes in a deeper level, we used model-based inference of effective connectivity (EC). Although these two measures are related as one is derived from the other, they are not equivalent. First, unlike FC, EC is explicitly constrained by the anatomical connectivity. Second, in FC the contribution of direct and indirect connections between brain regions is unknown, whereas EC seeks the optimal configuration in which the direct connections sufficiently explain FC. It is important to note that in this study EC inference does not rely on generative models of underlying neural activity (Friston et al., 2003) or statistical models (Rogers et al., 2010). Instead, we defined EC as the parameter space optimized for the given dynamical system that lumps together various biophysical features of the underlying neural activity as well as hemodynamic properties of observed BOLD signal. Confirming our hypothesis, we found decreased EC strength in AD primarily affecting left temporal lobe, as opposed to the widespread decreases in FC strength. These results are more consistent with the previous findings on the spatial distribution and the lateralization in early stages of AD (Thompson et al., 2003, Singh et al., 2006). We did not find significant FC differences in PAD and MCI groups. PAD subjects exhibited decreased EC strength in several regions including the left hippocampus, whereas no significant EC differences were found in the MCI group. Previous studies reported mixed results regarding FC alterations in the early stages of the disease. These results were often interpreted as a compensation mechanism to maintain cognitive function under the pathology related insults in the preclinical stage of AD (Dickerson et al., 2004, Filippi and Agosta, 2011, Sanz-Arigita et al., 2010). When the brain cannot compensate any longer, cognition levels cannot be sustained and decline below normal ranges.
We confirmed the relationship between whole-brain and regional connectivity by conducting a computational experiment. Based on the inferred EC in healthy control subjects, we calculated the changes in FC strength with manipulated the local bifurcation parameters. Being specific, we systematically diverged dynamical system from the bifurcation point such that each node exhibited noise-driven dynamics and the functional integration was disrupted. The severity of the predicted decrease in FC strength of each node was determined by its FC strength at the origin. This result demonstrated that the connectivity hubs in the brain are more vulnerable to the perturbations in local dynamics. The spatial patterns associated to the perturbations were also correlated with those associated to the CSF biomarkers. Furthermore, the bifurcation parameter maximizing the similarity between predicted and observed FC strengths of each clinical group was consistent with the diagnosis (i.e. divergence from optimal bifurcation parameter was higher in AD than in MCI, and higher in MCI than in PAD). These findings support the view that abnormal synchronization in neural circuits may underlie the development of clinical symptoms in AD (Palop and Mucke, 2016). An unexpected prediction of the computational model was the emergence of spatially structured increases in FC strengths for slight deviations in bifurcation parameter (Supplementary Fig. 9). A rapid decrease in variance of the hub regions might be responsible for these FC increases, which were prominent in cingulate cortex and precuneus in right hemisphere. Future studies are needed to investigate counter-intuitive network effects that are critical to interpret FC alterations.
We also investigated the role of genetic and CSF biomarkers during the progression of AD. We found distinct patterns for Aβ1–42 and tau CSF biomarkers. The correlations between CSF Aβ1–42 and whole-brain/regional connectivity were dramatically different across clinical groups than those found across all subjects. Across all subjects, CSF Aβ1–42 showed significant correlations with coherence (average synchronization) and FC strengths of the regions that were altered in AD. Furthermore, the correlations between CSF Aβ1–42 and whole-brain/regional connectivity were not present across clinical groups. These results are consistent with the findings that amyloid-β was associated with abnormal DMN connectivity in elders without dementia (Sheline et al., 2010b, Sperling et al., 2009). These findings also support the previous studies, which showed that cortical amyloid load is correlated with cognitive function in healthy individuals, but this relationship was absent in AD (Pike et al., 2007). Furthermore, disruption of the correlations across clinical groups supports the findings that Aβ1–42 is more relevant to the presence of connectivity alterations, but not the progression of the symptoms (Musiek and Holtzman, 2015).
In contrast, t-tau and p-tau CSF biomarkers were correlated with metastability (variability in synchronization) both across all subjects and across clinical groups. This finding also support the idea that metastability might be a more robust measure of functional integration in the brain (Deco and Kringelbach, 2014). Furthermore, tau CSF biomarkers were significantly correlated with FC/EC strengths in posterior and occipital brain regions. The regional correlation maps of CSF biomarkers were consistent with those of PET tau and Aβ topographies (Brier et al., 2016). Similar to the correlations with metastability, t-tau and p-tau correlations across clinical groups were relatively more consistent than Aβ1–42. The spatial patterns of these correlations were much more consistent in EC that those in FC. These results supported the findings that tau is a stronger predictor of cognition than Aβ (Brier et al., 2016). In addition, previous studies suggested that the impact of amyloid-β is functionally less relevant than that of tau tangles (Yoshiyama et al., 2013). Nevertheless, we found the highest significant correlations between t-tau/p-tau and FC/EC in sensory and attention related regions in occipital and parietal lobes. It is important to note that CSF biomarker regional correlation patterns do not necessarily correspond to the spatial patterns of amyloid/tau deposition. We speculate that the discrepancy in t-tau/p-tau CSF biomarker regional correlation patterns might indicate a breakdown of sensory integration on the development of cognitive impairments. Finally, APOE4 carriership showed no significant correlations with whole-brain/regional connectivity measures with the exception of left superior frontal EC strength.
This study had several limitations. One limitation rises from the optimization of EC. Given a highly non-linear model, the use of well-established techniques such as Bayesian inference is computationally infeasible. Heuristic approaches to infer EC are difficult to validate given the complexity of the model and the available data. We addressed some of these problems by constraining the parameter space to non-zero connections in structural connectivity (SC) and limiting the parameter range. Another limitation is that the model-based estimation of EC can be strongly influenced by the observed data. One approach to characterize the links between brain regions is the anatomical connectivity measures based on DWI. Nevertheless, DWI suffers from systematic biases (Van Essen et al., 2014), which may get aggravated in subjects with significant neurodegeneration as in AD. Indeed, DWI-based structural connectivity measures performed considerably worse (Supplementary Fig. 8). Furthermore, directed connectivity between cortical areas were shown to be abundant in primates (Markov et al., 2014), which cannot be captured by DWI. We found that the altered patterns of directed EC provided additional information regarding clinical groups including impaired prefrontal input strengths in PAD and MCI groups (Supplementary Figs. 5–7). However, we kept these results out of scope in this study as comprehensive theoretical analysis of the computational model is necessary to understand the role of directed connectivity.
This study showed that whole-brain connectivity alterations in AD progression involve gradual attenuation of overall synchronization primarily affecting the strongly connected brain networks such as DMN. The proposed computational modeling approach revealed that these alterations can be explained by disruptions in local dynamics and the underlying core connectivity changes might be distinct from those observed in FC. The results of this study provide insights to understand functional mechanisms of the progression of symptoms in AD.
Acknowledgments
Acknowledgements
GD is supported by the ERC Advanced Grant: DYSTRUCTURE (no. 295129), by the Spanish Research Project PSI2016-75688-P (AEI/FEDER) and by the European Union's Horizon 2020 research and innovation programme under grant agreement no. 720270 (HBP SGA1).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.08.006.
Appendix A. Supplementary data
Supplementary figures.
Supplementary tables.
References
- Agosta Federica, Pievani Michela, Geroldi Cristina, Copetti Massimiliano, Frisoni Giovanni B., Filippi Massimo. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging. 2012;33(8):1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Allen Greg, Barnard Holly, McColl Roderick, Hester Andrea L., Fields Julie A., Weiner Myron F., Wendy K. Ringe, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch. Neurol. 2007;64(10):1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avants Brian B., Tustison Nicholas J., Song Gang, Cook Philip A., Klein Arno, Gee James C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Jane P.F., Bell Robert, Buckman ShaAvhree, Burckart Gilbert J., Eichler Hans-Georg, Fang Kenneth C., Goodsaid Federico M. Translational biomarkers: from preclinical to clinical a report of 2009 AAPS/ACCP biomarker workshop. AAPS J. 2011;13(2):274–283. doi: 10.1208/s12248-011-9265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof Frederik, Haller Sven, Rombouts Serge A.R.B. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272(1):29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- Blennow Kaj, de Leon Mony J., Zetterberg Henrik. Alzheimer's disease. Lancet (London, England) 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Brier Matthew R., Thomas Jewell B., Ances Beau M. Network dysfunction in Alzheimer's disease: refining the disconnection hypothesis. Brain Connect. 2014;4(5):299–311. doi: 10.1089/brain.2014.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier M.R., Gordon B., Friedrichsen K., McCarthy J., Stern A., Christensen J., Owen C. Tau and A imaging, CSF measures, and cognition in Alzheimers disease. Sci. Transl. Med. 2016;8(338):338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd Samantha J., Demanuele Charmaine, Debener Stefan, Helps Suzannah K., James Christopher J., Sonuga-Barke Edmund J.S. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner Randy L., Andrews-Hanna Jessica R., Schacter Daniel L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(March):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabral Joana, Hugues Etienne, Kringelbach Morten L., Deco Gustavo. Modeling the outcome of structural disconnection on resting-state functional connectivity. NeuroImage. 2012;62(3):1342–1353. doi: 10.1016/j.neuroimage.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Celone Kim A., Calhoun Vince D., Dickerson Bradford C., Atri Alireza, Chua Elizabeth F., Miller Saul L., DePeau Kristina. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. Off. J. Soc. Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole Michael W., Pathak Sudhir, Schneider Walter. Identifying the Brain's most globally connected regions. NeuroImage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Córdova-Palomera Aldo, Kaufmann Tobias, Persson Karin, Alnæs Dag, Doan Nhat Trung, Moberget Torgeir, Lund Martina Jonette. Disrupted global metastability and static and dynamic brain connectivity across individuals in the Alzheimer's disease continuum. Sci Rep. 2017;7(January):40268. doi: 10.1038/srep40268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27(4):425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco Gustavo, Kringelbach Morten L. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84(5):892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Deco Gustavo, Kringelbach Morten L., Jirsa Viktor K., Ritter Petra. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-03073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Emily L., Thompson Paul M. Functional brain connectivity using fMRI in aging and Alzheimer's disease. Neuropsychol. Rev. 2014;24(1):49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson Bradford C., Salat David H., Bates Julianna F., Atiya Monika, Killiany Ronald J., Greve Douglas N., Anders M. Dale, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen Van, David C., Jbabdi Saad, Sotiropoulos Stamatios N., Chen Charles, Dikranian Krikor, Coalson Tim, Harwell John, Behrens Timothy E.J., Glasser Matthew F. Diffusion MRI. Second Edition. Academic Press; San Diego: 2014. Mapping connections in humans and non-human primates: aspirations and challenges for diffusion imaging; pp. 337–358.http://www.sciencedirect.com/science/article/pii/B9780123964601000160 [Google Scholar]
- Filippi Massimo, Agosta Federica. Structural and functional network connectivity breakdown in Alzheimer's disease studied with magnetic resonance imaging techniques. J. Alzheimers Dis. 2011;24(3):455–474. doi: 10.3233/JAD-2011-101854. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Glerean Enrico, Salmi Juha, Lahnakoski Juha M., Jaaskelainen Iiro P., Sams Mikko. Functional magnetic resonance imaging phase synchronization as a measure of dynamic functional connectivity. Brain Connect. 2012;2(2):91–101. doi: 10.1089/brain.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius Michael. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius Michael D., Srivastava Gaurav, Reiss Allan L., Menon Vinod. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann Patric, Cammoun Leila, Gigandet Xavier, Meuli Reto, Honey Christopher J., Wedeen Van J., Sporns Olaf. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7) doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden Trey, Van Dijk Koene R.A., Alex Becker J., Mehta Angel, Sperling Reisa A., Johnson Keith A., Buckner Randy L. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. Off. J. Soc. Neurosci. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer Peter J., Shanahan Murray, Scott Gregory, Wise Richard J.S., Sharp David J., Leech Robert. The control of global brain dynamics: opposing actions of frontoparietal control and default mode networks on attention. J. Neurosci. 2014;34(2):451–461. doi: 10.1523/JNEUROSCI.1853-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Yosef, Benjamini Yoav. More powerful procedures for multiple significance testing. Stat. Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Jack Clifford R., Albert Marilyn, Knopman David S., McKhann Guy M., Sperling Reisa A., Carillo Maria, Thies William, Phelps Creighton H. Introduction to revised criteria for the diagnosis of Alzheimer's disease: National Institute on Aging and the Alzheimer Association Workgroups. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson Mark, Beckmann Christian F., Behrens Timothy E.J., Woolrich Mark W., Smith Stephen M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Koch W., Teipel S., Mueller S., Buerger K., Bokde A.L.W., Hampel H., Coates U., Reiser M., Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51(1):280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kuramoto Y. 66 (7) Springer-Verlag; Berlin-Heidelberg-New York-Tokyo: 1986. Chemical Oscillations, Waves, and Turbulence; p. 296. [Google Scholar]
- Lemieux Louis, Salek-Haddadi Afraim, Lund Torben E., Laufs Helmut, Carmichael David. Modelling large motion events in fMRI studies of patients with epilepsy. Magn. Reson. Imaging. 2007;25(6):894–901. doi: 10.1016/j.mri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Li Wenjun, Antuono Piero G., Xie Chunming, Chen Gang, Jones Jennifer L., Douglas Ward B., Franczak Malgorzata B., Goveas Joseph S., Li Shi-Jiang. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12-week donepezil treatment. NeuroImage. 2012;60(2):1083–1091. doi: 10.1016/j.neuroimage.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Xiaozhen, Li Tie-Qiang, Andreasen Niels, Wiberg Maria Kristoffersen, Westman Eric, Wahlund Lars-Olof. Ratio of Aβ42/P-tau181p in CSF is associated with aberrant default mode network in AD. Sci Rep. 2013;3(February) doi: 10.1038/srep01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Cindy, Snyder Abraham Z., Bhakta Mehul, O'Brien Katherine C., McAvoy Mark, Raichle Marcus E., Morris John C., Buckner Randy L. Functional deactivations: change with age and dementia of the Alzheimer type. Proc. Natl. Acad. Sci. U. S. A. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón José V., Coupé Pierrick, Concha Luis, Antonio Buades, Louis Collins D., Robles Montserrat. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies Daniel S., Ghosh Satrajit S., Goulas Alexandros, Falkiewicz Marcel, Huntenburg Julia M., Langs Georg, Bezgin Gleb. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. 2016;113(44):12574–12579. doi: 10.1073/pnas.1608282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov N.T., Ercsey-Ravasz M.M., Ribeiro Gomes A.R., Lamy C., Magrou L., Vezoli J., Misery P. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb. Cortex. 2014;24(1):17–36. doi: 10.1093/cercor/bhs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo Jose Luis, Domingo Gispert Juan, Bruno Dubois, Heneka Michael T., Alberto Lleo, Sebastiaan Engelborghs, Jesús Pujol. The AD-CSF-index discriminates Alzheimer's disease patients from healthy controls: a validation study. J. Alzheimers Dis. 2013;1:67–77. doi: 10.3233/JAD-130203. [DOI] [PubMed] [Google Scholar]
- Molinuevo José Luis, Blennow Kaj, Dubois Bruno, Engelborghs Sebastiaan, Lewczuk Piotr, Perret-Liaudet Armand, Teunissen Charlotte E., Parnetti Lucilla. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's biomarkers standardization initiative. Alzheimers Dement. 2014;10(6):808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Murphy Kevin, Birn Rasmus M., Handwerker Daniel A., Jones Tyler B., Bandettini Peter A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek Erik S., Holtzman David M. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci. 2015;May:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Hwamee, Mormino Elizabeth C., Madison Cindee, Hayenga Amynta, Smiljic Andre, Jagust William J. β-Amyloid affects frontal and posterior brain networks in normal aging. NeuroImage. 2011;54(3):1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop Jorge J., Mucke Lennart. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016;17(12):777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella Jeffrey R., Wang Lihong, Krishnan Sriyesh, Slavin Melissa J., Prince Steven E., Tran Thanh-Thu T., Murali Doraiswamy P. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245(1):224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- Pike K.E., Savage G., Villemagne V.L., Ng S., Moss S.A., Maruff P., Mathis C.A., Klunk W.E., Masters C.L., Rowe C.C. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130(11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B., Burke J.R. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Morris J.C. Tangles and plaques in nondemented aging and ‘preclinical’ Alzheimer's disease. Ann. Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Qi Zhigang, Wu Xia, Wang Zhiqun, Zhang Nang, Dong Huiqing, Yao Li, Li Kuncheng. Impairment and compensation coexist in amnestic MCI default mode network. NeuroImage. 2010;50(1):48–55. doi: 10.1016/j.neuroimage.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Ramirez Maria J., Lai Mitchell K.P., Tordera Rosa M., Francis Paul T. Serotonergic therapies for cognitive symptoms in Alzheimer's disease: rationale and current status. Drugs. 2014;74(7):729–736. doi: 10.1007/s40265-014-0217-5. [DOI] [PubMed] [Google Scholar]
- Rogers Baxter P., Katwal Santosh B., Morgan Victoria L., Asplund Christopher L., Gore John C. Functional MRI and multivariate autoregressive models. Magn. Reson. Imaging. 2010;28(8):1058–1065. doi: 10.1016/j.mri.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts Serge A.R.B., Barkhof Frederik, Goekoop Rutger, Stam Cornelis J., Scheltens Philip. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts Serge A.R.B., Damoiseaux Jessica S., Goekoop Rutger, Barkhof Frederik, Scheltens Philip, Smith Stephen M., Beckmann Christian F. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum. Brain Mapp. 2009;30(1):256–266. doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Arigita Ernesto J., Schoonheim Menno M., Damoiseaux Jessica S., Rombouts Serge A.R.B., Maris Erik, Barkhof Frederik, Scheltens Philip, Stam Cornelis J. Loss of ‘small-world’ networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan Murray. Metastable chimera states in community-structured oscillator networks. Chaos (Woodbury, N.Y.) 2010;20(1):13108. doi: 10.1063/1.3305451. [DOI] [PubMed] [Google Scholar]
- Sheline Yvette I., Raichle Marcus E. Resting state functional connectivity in preclinical Alzheimer's disease. Biol. Psychiatry. 2013;74(5):340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Yvette I., Morris John C., Snyder Abraham Z., Price Joseph L., Yan Zhizi, D'Angelo Gina, Liu Collin. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Yvette I., Raichle Marcus E., Snyder Abraham Z., Morris John C., Head Denise, Wang Suzhi, Mintun Mark A. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Chertkow H., Lerch J.P., Evans A.C., Dorr A.E., Kabani N.J. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain. 2006;129(11):2885–2893. doi: 10.1093/brain/awl256. [DOI] [PubMed] [Google Scholar]
- Sorg Christian, Riedl Valentin, Mühlau Mark, Calhoun Vince D., Eichele Tom, Läer Leonhard, Drzezga Alexander. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling Reisa A., Laviolette Peter S., O'Keefe Kelly, O'Brien Jacqueline, Rentz Dorene M., Pihlajamaki Maija, Marshall Gad. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Paul M., Hayashi Kiralee M., de Zubicaray Greig, Janke Andrew L., Rose Stephen E., Semple James, Herman David. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 2003;23(3):994. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison Nicholas J., Avants Brian B., Cook Philip A., Zheng Yuanjie, Egan Alexander, Yushkevich Paul A., Gee James C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wang Liang, Zang Yufeng, He Yong, Liang Meng, Zhang Xinqing, Tian Lixia, Wu Tao, Jiang Tianzi, Li Kuncheng. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. NeuroImage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang Liang, Brier Matthew R., Snyder Abraham Z., Thomas Jewell B., Fagan Anne M., Xiong Chengjie, Benzinger Tammie L., Holtzman David M., Morris John C., Ances Beau M. Cerebrospinal fluid Aβ42, phosphorylated Tau181, and resting-state functional connectivity. JAMA Neurol. 2013;70(10):1242–1248. doi: 10.1001/jamaneurol.2013.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee Chong-Yaw, Yap Pew-Thian, Denny Kevin, Browndyke Jeffrey N., Potter Guy G., Welsh-Bohmer Kathleen A., Wang Lihong, Shen Dinggang. Resting-state multi-spectrum functional connectivity networks for identification of MCI patients. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Mingrui, Wang Jinhui, He Yong. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Genevieve J., Murray John D., Repovs Grega, Cole Michael W., Savic Aleksandar, Glasser Matthew F., Pittenger Christopher. Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Yasumasa, Lee Virginia M.Y., Trojanowski John Q. Therapeutic strategies for tau mediated neurodegeneration. J. Neurol. Neurosurg. Psychiatry. 2013;84(7):784–795. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Hong-Ying, Wang Shi-Jie, Xing Jiong, Liu Bin, Ma Zhan-Long, Yang Ming, Zhang Zhi-Jun, Teng Gao-Jun. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav. Brain Res. 2009;197(1):103–108. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.
Supplementary tables.