Abstract
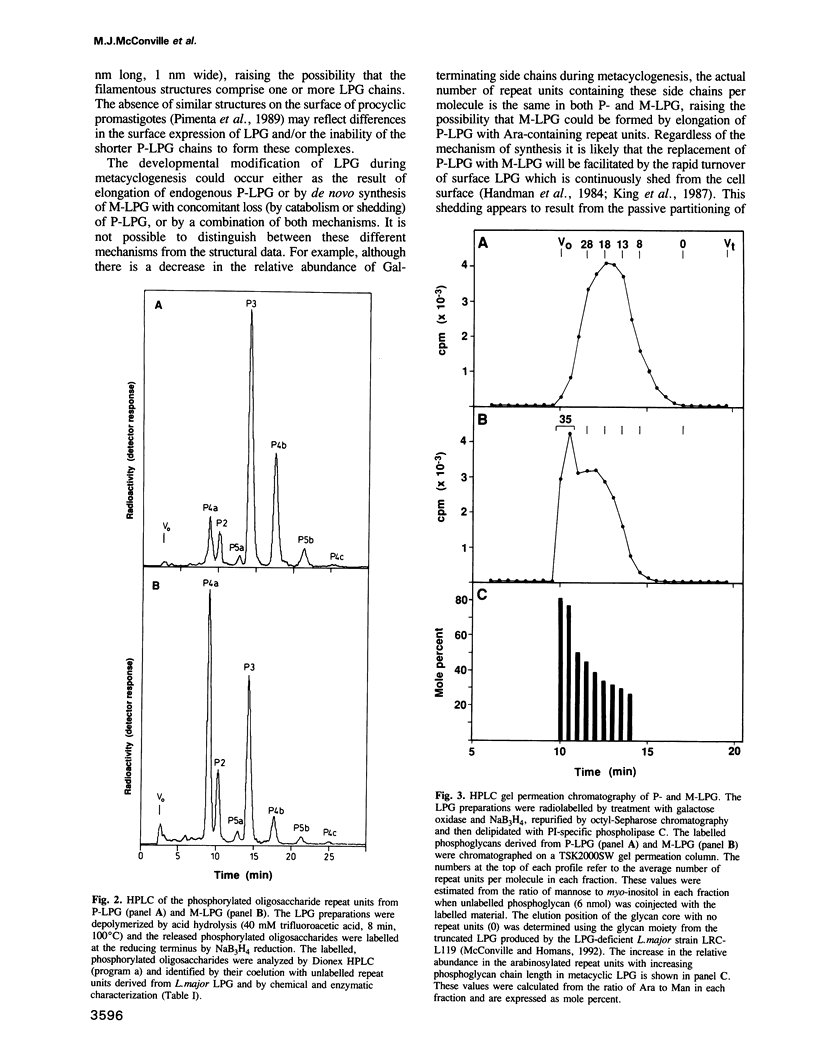
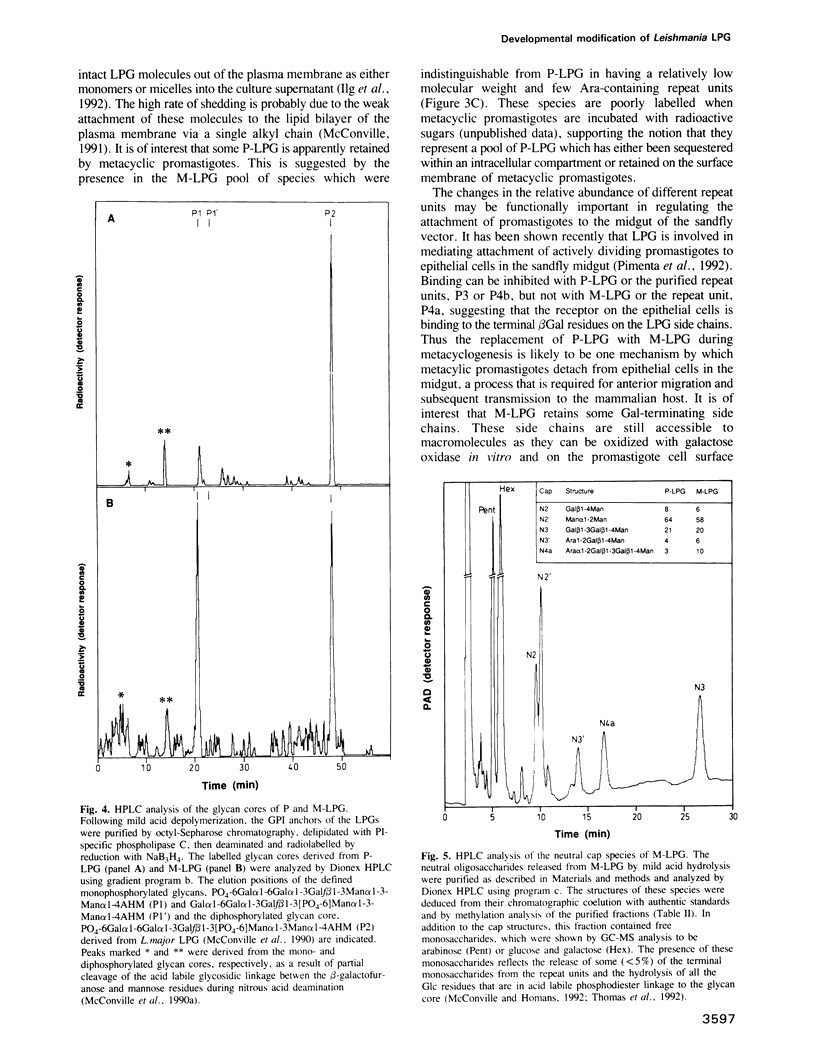
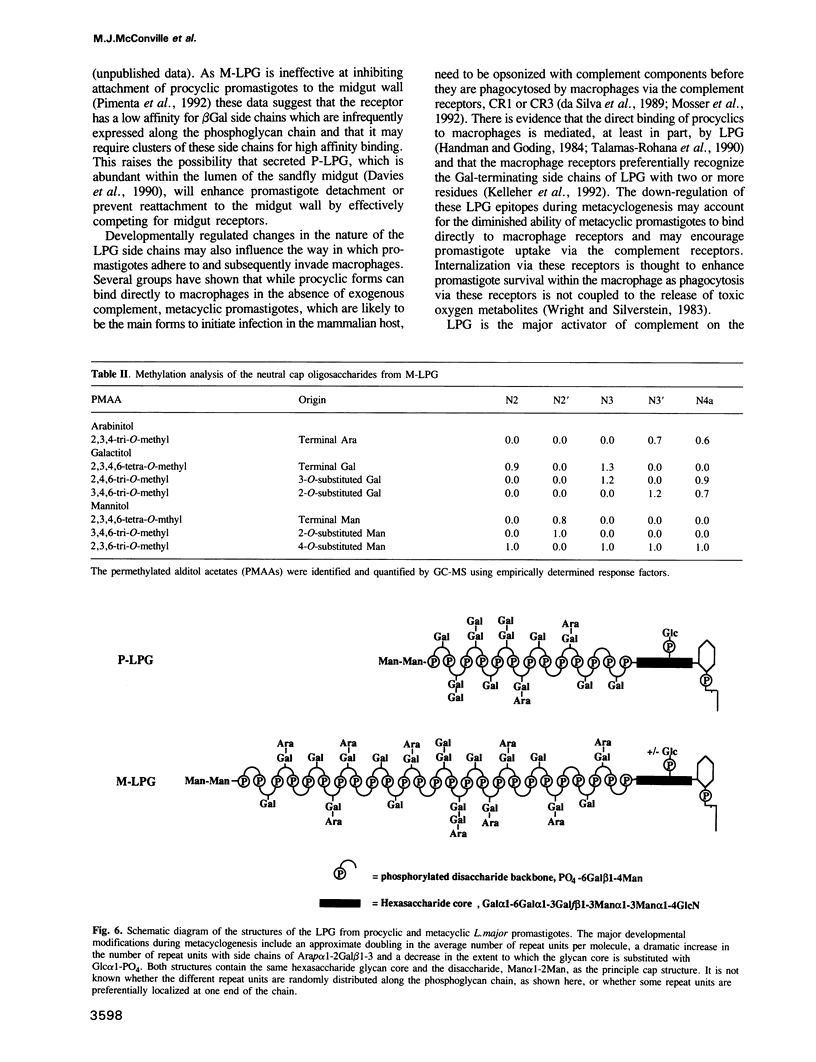
Protozoan parasites of the genus Leishmania produce the novel surface glycoconjugate, lipophosphoglycan (LPG), which is required for parasite infectivity. In this study we show that LPG structure is modified during the differentiation of L. major promastigotes from a less infectious form in logarithmic growth phase to a highly infectious 'metacyclic' form during stationary growth phase. In both stages, the LPGs comprise linear chains of phosphorylated oligosaccharide repeat units which are anchored to the membrane via a glycosyl-phosphatidylinositol glycolipid anchor. During metacyclogenesis there is (i) an approximate doubling in the average number of repeat units per molecule from 14 to 30, (ii) a pronounced decrease in the relative abundance of repeat units with side chains of beta Gal or Gal beta 1-3Gal beta 1-, and a corresponding increase in repeat units with either no side chains or with side chains of Arap alpha 1-2 Gal beta 1- and (iii) a decrease in the frequency with which the glycolipid anchor is substituted with a single glucose alpha 1-phosphate residue. While the majority of the LPG phosphoglycan chains are capped with the neutral disaccharide, Man alpha 1-2Man, a significant minority of the chains appeared to terminate in non-phosphorylated repeat units and may represent incompletely capped species. We suggest that the developmental modification of LPG may be important in modulating the binding of promastigotes to receptors in the sandfly midgut and on human macrophages and in increasing the resistance of metacyclic promastigotes to complement-mediated lysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- Davies C. R., Cooper A. M., Peacock C., Lane R. P., Blackwell J. M. Expression of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology. 1990 Dec;101(Pt 3):337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homans S. W., Mehlert A., Turco S. J. Solution structure of the lipophosphoglycan of Leishmania donovani. Biochemistry. 1992 Jan 28;31(3):654–661. doi: 10.1021/bi00118a004. [DOI] [PubMed] [Google Scholar]
- Ilg T., Etges R., Overath P., McConville M. J., Thomas-Oates J., Thomas J., Homans S. W., Ferguson M. A. Structure of Leishmania mexicana lipophosphoglycan. J Biol Chem. 1992 Apr 5;267(10):6834–6840. [PubMed] [Google Scholar]
- Kelleher M., Bacic A., Handman E. Identification of a macrophage-binding determinant on lipophosphoglycan from Leishmania major promastigotes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):6–10. doi: 10.1073/pnas.89.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. L., Chang Y. D., Turco S. J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987 May;24(1):47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- Lopes A. H., Iovannisci D., Petrillo-Peixoto M., McMahon-Pratt D., Beverley S. M. Evolution of nuclear DNA and the occurrence of sequences related to new small chromosomal DNAs in the trypanosomatid genus Endotrypanum. Mol Biochem Parasitol. 1990 May;40(2):151–161. doi: 10.1016/0166-6851(90)90037-m. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990 Jan 1;38(1):57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McConville M. J. Glycosylated-phosphatidylinositols as virulence factors in Leishmania. Cell Biol Int Rep. 1991 Sep;15(9):779–798. doi: 10.1016/0309-1651(91)90033-f. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Homans S. W. Identification of the defect in lipophosphoglycan biosynthesis in a non-pathogenic strain of Leishmania major. J Biol Chem. 1992 Mar 25;267(9):5855–5861. [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990 Nov 15;265(32):19611–19623. [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol. 1990 Apr 1;144(7):2745–2750. [PubMed] [Google Scholar]
- Mosser D. M., Springer T. A., Diamond M. S. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J Cell Biol. 1992 Jan;116(2):511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta P. F., Turco S. J., McConville M. J., Lawyer P. G., Perkins P. V., Sacks D. L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992 Jun 26;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Pimenta P. F., da Silva R. P., Sacks D. L., da Silva P. P. Cell surface nanoanatomy of Leishmania major as revealed by fracture-flip. A surface meshwork of 44 nm fusiform filaments identifies infective developmental stage promastigotes. Eur J Cell Biol. 1989 Apr;48(2):180–190. [PubMed] [Google Scholar]
- Puentes S. M., Da Silva R. P., Sacks D. L., Hammer C. H., Joiner K. A. Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol. 1990 Dec 15;145(12):4311–4316. [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Brodin T. N., Turco S. J. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):225–233. doi: 10.1016/0166-6851(90)90165-i. [DOI] [PubMed] [Google Scholar]
- Sacks D. L. Metacyclogenesis in Leishmania promastigotes. Exp Parasitol. 1989 Jul;69(1):100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., da Silva R. P. The generation of infective stage Leishmania major promastigotes is associated with the cell-surface expression and release of a developmentally regulated glycolipid. J Immunol. 1987 Nov 1;139(9):3099–3106. [PubMed] [Google Scholar]
- Talamás-Rohana P., Wright S. D., Lennartz M. R., Russell D. G. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J Immunol. 1990 Jun 15;144(12):4817–4824. [PubMed] [Google Scholar]
- Thomas J. R., McConville M. J., Thomas-Oates J. E., Homans S. W., Ferguson M. A., Gorin P. A., Greis K. D., Turco S. J. Refined structure of the lipophosphoglycan of Leishmania donovani. J Biol Chem. 1992 Apr 5;267(10):6829–6833. [PubMed] [Google Scholar]
- Turco S. J., Orlandi P. A., Jr, Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989 Apr 25;264(12):6711–6715. [PubMed] [Google Scholar]
- Turco S. J. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990 Feb;70(2):241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]



