Abstract
Mitoxantrone (MXT) is an androstenedione that is used to treat cancers and progressive forms of multiple sclerosis; however, its use is limited by its cardiotoxicity. Pituitary adenylate cyclase activating polypeptide (PACAP) is a member of the secretin/growth hormone-releasing hormone/vasoactive intestinal peptide family and has many functions, including cytoprotection and immunosuppression. We tested the hypothesis that PACAP can protect against MXT-induced cardiotoxicity in mice. Female BALB/c mice were treated once weekly for 4 weeks with saline (n = 14) or MXT (3 mg/kg, i.p.; n = 14). Half of the mice in each group received PACAP (10 μg, i.p.) 1 h before and 24 and 48 h after MXT, while the remaining mice received injections of saline on the same schedule. Echocardiography was used to assess cardiac structure and function. In mice treated with MXT and saline, body weight was significantly reduced after the third dose of MXT. PACAP significantly attenuated the reduction in body weight; however, the weights did not return to control level. Compared to controls, MXT-treated mice had significantly increased left ventricular (LV) diameter and LV volume and decreased LV posterior wall thickness. Fractional shortening (FS) and ejection fraction (EF) were also significantly decreased. Treatment with PACAP prevented MXT-induced LV dilation and significantly attenuated the reductions in FS and EF, although FS and EF did not return to control level. PACAP38 did not prevent MXT-induced decreases in LV posterior wall thickness. MXT dose-dependently decreased the viability of cultured U937 (human leukemia) cells; PACAP did not protect cultured U937 cells from MXT-mediated cell death. In conclusion, PACAP can attenuate MXT-mediated LV dilation and dysfunction in mice.
Keywords: blood cancer, chemotherapy, cardioprotection, multiple sclerosis, pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal peptide
1. Introduction
Mitoxantrone (MXT) is an androstenedione chemotherapeutic agent that is used to treat acute nonlymphocytic leukemia, acute lymphoblastic leukemia, prostate cancer, metastatic breast cancer, and non-Hodgkin’s lymphoma [13, 14]. It is also approved by the Food and Drug Administration (FDA) for progressive/relapsing (also called worsening relapsing-remitting) and secondary progressive multiple sclerosis (MS) [7, 47]. Its chemical structure is similar to that of doxorubicin, a widely used anthracycline chemotherapeutic agent. MXT causes apoptosis in proliferating and non-proliferating cells by inhibiting DNA replication, DNA-dependent RNA synthesis and DNA repair by topoisomerase II [13]. The drug also has numerous effects on immune function, resulting in decreased lymphocyte proliferation, decreased cytokine release, suppression of T-cell and B-cell function, including antibody production [9, 13]. However, the therapeutic efficacy of MXT is limited by its irreversible cumulative dose-related cardiotoxicity [33, 60] and its potential to cause secondary leukemias [5, 13].
Pituitary adenylate cyclase-activating polypeptide (PACAP) was first isolated from the hypothalamus during a search for novel hypophysiotropic factors [48], but it rapidly became clear that it is a multifunctional peptide with potent anti-inflammatory and potent cytoprotective properties [69]. PACAP is a member of the secretin/growth hormone-releasing hormone/vasoactive intestinal peptide (VIP) family. It exists as two α-amidated peptides with 38 (PACAP38) or 27 (PACAP27) amino acids, and PACAP27 has 68% sequence identity with VIP. PACAP binds to three subtypes of Class II (secretin-type) G protein-coupled receptors that are called the PAC1, VPAC1 and VPAC2 receptors [23]. PACAP binds not only to the PAC1 receptor with high affinity, but it also binds to the VPAC1 (VIP1) and VPAC2 (VIP2) receptors with affinities comparable to or greater than VIP. On the other hand, VIP binds to the PAC1 receptor with an affinity 100–1,000 times lower than PACAP [24]. PACAP is most abundant in the brain, but there are high levels in other organs, including the thymus, spleen, lymph nodes, and duodenal mucosa [69]. The cytoprotective effects of PACAP have been most extensively studied in the brain and kidney [40, 69, 72]. PACAP has both direct and indirect cytoprotective effects in the brain and kidney [32, 72]. The direct protective effects of PACAP in the brain and kidney are mediated mainly via the cyclic AMP/protein kinase A signal transduction pathway, while the indirect protective effects of PACAP are mediated by multiple signal transduction pathways [32, 69, 72]. PACAP protects the brain, kidney and liver against ischemia/reperfusion injury [28, 31, 59, 68] and protects the brain and kidney from injury caused by a wide range of therapeutic agents [2, 32, 40, 41, 69]. Despite extensive library screens by major pharmaceutical companies over several decades, small molecule orthosteric agonists have not been found for the cognate receptors for any member of the secretin/growth hormone-releasing hormone/VIP family [24, 27].
PACAP/VIP receptors are found in the myocardium: the PAC1 receptor is the predominant receptor in cardiac myocytes and the VPAC2 receptor is the predominant receptor in cardiac fibroblasts [63]. The VPAC2 receptor is also the predominant PACAP/VIP receptor in vascular smooth muscle cells [63, 69]. PACAP has positive inotropic and chronotropic effects on the heart [61] and increases blood flow to most major organs [69]. PACAP has only a slight transient effect on systemic blood pressure [4, 39]. PACAP directly protects cardiomyocytes in vitro against oxidative stress and doxorubicin [18, 19, 56, 57]. In addition, the cardiotoxicity elicited by doxorubicin is exacerbated in PACAP-deficient mice and significantly reduced in PACAP-deficient mice by treatment with PACAP38 [49]. Mori and colleagues [49] did not determine whether PACAP can protect wild-type mice against doxorubicin. Whether PACAP can protect against MXT-induced cardiotoxicity has not been determined in vitro or in vivo. Therefore, the purpose of this study was to test the hypothesis that PACAP can block MXT-induced cardiotoxicity in mice. We show for the first time that PACAP significantly attenuates MXT-induced left ventricular dysfunction and remodeling in wild-type mice in vivo. However, the clinical utility of these observations would be limited if PACAP also blocked the therapeutic actions of MXT. Therefore, we also demonstrated that PACAP does not protect cultured human leukemia cells against MXT-induced cell death. PACAP has already been shown by several laboratories to be efficacious in preclinical in vivo models for MS [15, 20, 30, 66, 67].
2. Materials and methods
2.1. Animals
All studies were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals. The animal experiments used 12-week-old female BALB/c mice (Harlan, Indianapolis, IN). The mice were group-housed in a pathogen-free, climate-controlled room with a 12-h light/dark cycle. The standard rodent chow and tap water were available ad libitum. After their arrival in the animal care facility, the mice were allowed 1 week to acclimate before beginning the experiments.
2.2 Peptide synthesis and purification
PACAP38 was prepared at the Peptide Research Laboratory (Tulane University, New Orleans, LA) by solid-phase synthesis using Fmoc chemistries on a CEM microwave-assisted automatic peptide synthesizer (Matthews, NC). Mass spectroscopic analysis of intermediate sequences revealed a tendency for incomplete couplings to occur near the center of the alpha-helical region of PACAP38 (residues 16–27) and peptide synthesizers were programmed to make many repeat couplings in this region. The crude PACAP38 was cleavage from the Rink amide resin with trifluoroacetic acid/triisopropylsilane/water (95:1:4) followed by precipitation with ether and filtration. The white powder was dissolved in dilute acetic acid and applied directly to a preparative chromatography column (5 × 50 cm) containing Vydac C-l8 silica (300-angstrom pore size) and eluted with a linear gradient (20–60%) of acetonitrile/0.1% trifluoroacetic acid. The fractions containing PACAP38 but no contaminating deletion sequences were identified using analytical high-performance liquid chromatography and matrix-assisted laser desorption/ionization mass spectrometry. These fractions were pooled and lyophilized several times from deionized water. The overall yield of PACAP38 with > 95% purity was ~10%. PACAP was reconstituted in sterile 0.9% NaCl (saline) prior to injection, or in distilled water for cell culture studies.
2.3. Design of animal experiments
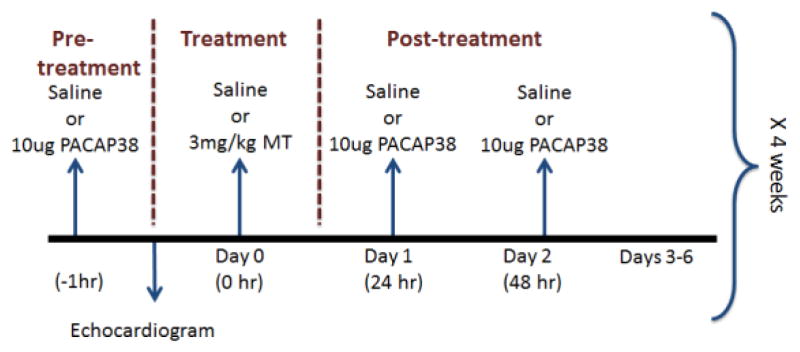
Four experimental groups were used: saline-treated mice (saline), mice treated with PACAP, mice treated with MXT, and mice treated with MXT plus PACAP (MXT+PACAP). All solutions were injected intraperitoneally in a volume of 100 μl. On day 0, the saline (n = 7) and MXT (n = 7) groups were injected with saline, while the PACAP (n = 7) and PACAP+MXT (n = 7) groups were injected with PACAP (10 μg/mouse). One hour later, echocardiograms were recorded for each mouse under light isoflurane anesthesia (~1%). Immediately after completing the echocardiogram, the mice in the saline and PACAP groups were given injections of saline, while the mice in the MXT and PACAP+MXT groups were injected with MXT (3 mg/kg; Tocris Bioscience, Ellisville, MO) dissolved in saline. This dose was chosen because it produced cardiac toxicity in female mice [3]. Twenty-four and 48 h later, the saline and MXT groups received saline injections, while the PACAP and the PACAP+MXT groups received injections of PACAP (10 μg/mouse). This regimen of PACAP injections was chosen because a similar regimen prevented cisplatin-induced renal failure in mice [41]. The 3-day dosing protocol was repeated on experimental days 7, 14 and 21 (Fig. 1). The echocardiograms were repeated on days 14 and 21, with a final echocardiogram performed 1 week after the last saline or MXT treatment (day 28). After the last echocardiogram, the mice were deeply anesthetized and the heart removed, rinsed, patted dry, and weighed.
Figure 1.
Schematic drawing of the experimental protocol. The weekly dosing of MXT in mice was intended to mimic the monthly dosing of MXT in human cancer patients [13,14]. The order of the weekly injections for the Saline Group was saline, saline, saline, and saline; the order of the weekly injections for the PACAP Group was PACAP, saline, PACAP, and PACAP; the order of the weekly injections for the MXT Group was saline, MXT, saline, and saline; and the order of the weekly injections for the PACAP+MXT Group was PACAP, MXT, PACAP, and PACAP.
2.4. Echocardiography protocol
Echocardiograms were performed (Vevo 770; VisualSonics, Toronto, Canada) under isoflurane anesthesia as described previously [44]. M-mode echocardiographic measurements of the left ventricular posterior wall (LVPW;d), intraventricular septum (IVS;d), left ventricular internal diameter during diastole (LVID;d) and left ventricular volume during diastole (LV Vol;d) were made in the parasternal short axis view at the level of the papillary muscles. Left ventricular systolic function was assessed by changes in percent fractional shortening (%FS = [LVID;d-LVID;s]/LVID;d × 100) and ejection fraction (%EF= [LV Vol;d− LV Vol;s]/LV Vol;d × 100).
2.5. Cell culture methods, treatments and assays
U937 human leukemia cells (ATCC, Manassas, VA; CRL-1593.2) were cultured in RPMI 1640 medium with 10% fetal bovine serum and 50 μg/ml of gentamicin. At the conclusion of the study, the authenticity of the U937 cells was verified (100% match) by ATCC using short tandem repeat profiling. Sterile water was used as the vehicle for MXT and PACAP stock solutions. Equal numbers of cells were placed in 96-well plates and treated with MXT (0–25 μM) for 24 h to determine the concentration-response relationship between MXT and U937 cell viability. Cell viability was assessed using the resazurin (Alamar blue) reduction assay [52]. Briefly, after 24 h of treatment, the cells were incubated with resazurin for 2 h at 37°C, the developed fluorescence was recorded using a plate reader (SpectraMax M2; excitation = 550 nm and emission = 585 nm) and used to determine the number of live cells. Based on the concentration-response studies, the viability experiments were repeated using MXT (1 μM) and PACAP (10 nM-1 μM), alone or in combination. All experiments were performed in fully-supplemented growth medium (n = 3–8).
2.6. Western blot analysis
Following MXT and/or PACAP treatments, U937 cell samples were centrifuged for 10 min at 125 RCF and the treatment media aspirated. U937 cellular proteins were extracted with 1x RIPA lysis buffer for 15 min at 4°C and centrifuged for 10 min at 10,000 RCF to yield cell lysate supernatants. The protein concentration in each sample was quantified using the BCA (bicinchoninic acid) protein assay. Equal amounts of protein were loaded onto gels (4–20% TGX, Bio-Rad, Hercules, CA), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then wet transferred onto polyvinylidene difluoride membranes. Blots were stained with Ponceau S and imaged to confirm equal protein loading and uniform blotting across the sample lanes. Blots were blocked in Tris-buffered saline with 0.05% Tween 20 containing 3% bovine serum albumin (TBST) for 1 h. Western blots were probed with a primary antibody against cleaved caspase 3 (#9664; Cell Signaling, Boston, MA) overnight, washed three times with TBST, probed with a secondary antibody against rabbit IgG (#NA934V; GE Healthcare, Pittsburgh, PA) for 1 h, and washed three more times with TBST. Finally, the blots were developed using the Amersham ECL imaged/quantified using the reagent and LAS4000 ImageQuant imager (both from GE Healthcare).
2.7. Statistical analyses
All data are reported as the mean ± standard error. Statistical analysis was carried out using the Prism software package (GraphPad, La Jolla, CA). Echocardiographic parameters were compared using a two-way repeated measure analysis of variance and group means were subsequently compared using Bonferroni multiple comparison tests. Cell viability and Western blot data were analyzed using a one-way analysis of variance and Bonferroni multiple comparison tests. Differences were considered statistically significant at p < 0.05.
3. Results
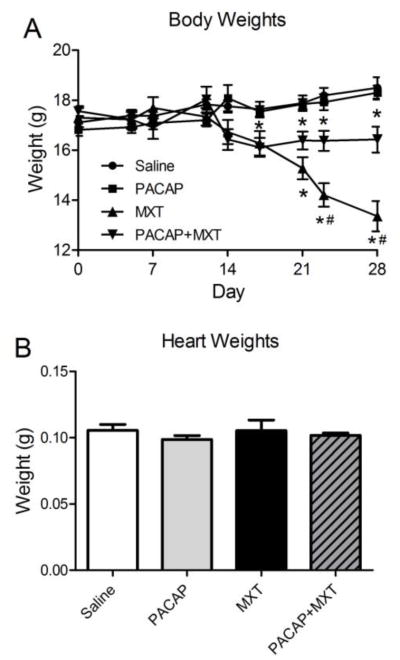
The average body weights for each group of mice over the course of the study are shown in Figure 2A. The average body weights of the mice in the saline control group and PACAP group increased slightly during the study. In contrast, starting at day 17, the body weights of the mice treated only with MXT decreased significantly compared to the control and PACAP groups. During the fourth week of the study, two of the mice in the MXT group died. While PACAP significantly attenuated the MXT-induced reduction in body weight, from day 17 onward, the body weights of the mice treated with both PACAP and MXT were significantly lower than in the control groups. In contrast to the changes in body weight, there were no significant differences in heart weight among the four groups (Fig. 2B). Fat pads were not found in the MXT-treated mice sacrificed on day 28.
Figure 2.
Body weights (A) and heart weights (B) of mice treated with vehicle (saline, n = 7), PACAP (10 μg, i.p., n = 7), MXT (3 mg/kg, i.p., n = 5–7) or PACAP+MXT (n = 7) on days 1, 7, 14, and 21. PACAP was also given to the PACAP and PACAP+MXT groups 24 and 48 h after the first dose of PACAP. MXT, mitoxantrone; PACAP, pituitary adenylate cyclase activating polypeptide. * p < 0.05 versus the saline group. # p < 0.05 MXT group versus the PACAP+MXT group.
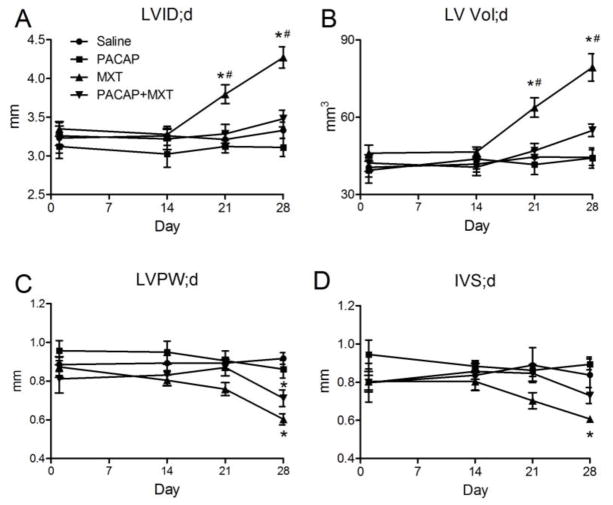
Serial echocardiograms were used to monitor cardiac structure and function in the mice. Over the course of the study, there were no significant changes in LVID;d, LV Vol;d, LVPW;d, or IVS;d in the saline or the PACAP groups (Fig. 3A–D). By day 21 of the study, echocardiography revealed that treatment with MXT alone significantly increased LVID;d and LV Vol;d (Fig. 3A and B) compared to saline-treated mice. By day 28, treatment with MXT also significantly decreased LVPW;d and IVS;d (Fig. 3C and D) compared to the saline group. Treatment with PACAP blocked the MXT-induced increases in LVID;d and LV Vol;d (Fig. 3A and B). Treatment with PACAP did not significantly attenuate the MXT-induced decreases in LVPW;d (Fig. 3C); however, it did attenuate the MXT-induced decrease in IVS;d, because IVS;d did not differ significantly between the saline and the PACAP+MXT groups (Fig. 3D).
Figure 3.
Serial echocardiographic measures of left ventricular structure. (A–D) Treatment with MXT significantly increased LVID;d and LV Vol;d while significantly decreasing LVPW;d and IVS;d. PACPA reversed the increases in VLID;d and LV Vol;d and attenuated the decreases in IVS;d (A, B, D), but not the decrease in LVPW;d (C). LV; left ventricle, LVID;d, LV diameter during diastole, LV Vol;d, LV volume during diastole, LVPW;d, LV posterior wall thickness during diastole, IVS;d, intraventricular septal thickness during diastole. n = 7 mice per group, except for the MXT group (n = 5–7). * p < 0.05 versus the saline group. # p < 0.05 MXT group versus the PACAP+MXT group.
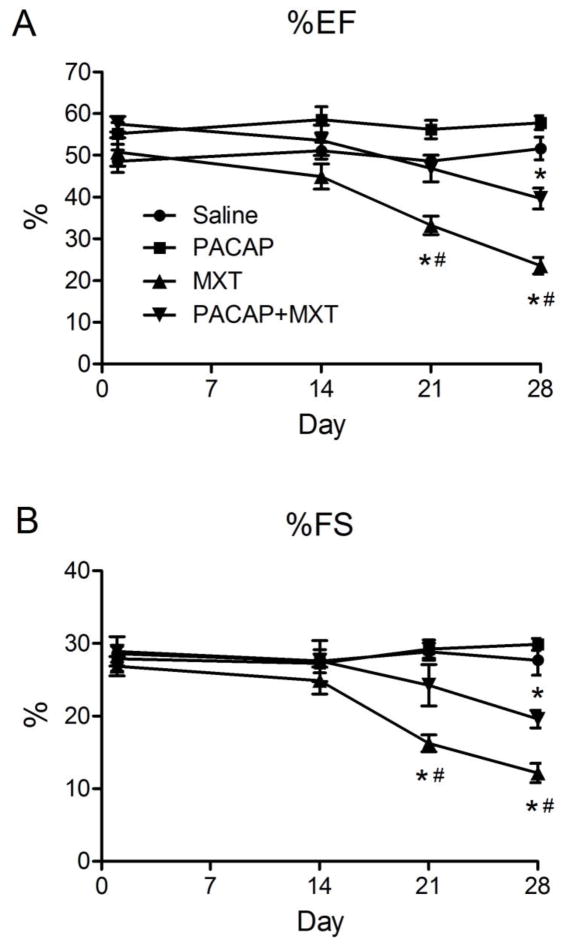
When compared to saline- and PACAP-treated mice, treatment with MXT alone significantly reduced %EF and %FS (Fig 4A and B). PACAP significantly attenuated the MXT-induced reductions in %EF and %FS; however, these functional parameters in the PACAP+MXT group were still significantly reduced compared to the saline control group (Fig. 4A and B).
Figure 4.
Serial echocardiographic measures of left ventricular function. The (A) ejection fraction (%EF) and (B) percent fractional shortening (%EF) in mice treated with vehicle, PACAP, MXT or PACAP+MXT. n = 7 mice per group, except for the MXT group (n = 5–7). * p < 0.05 versus the saline group. # p < 0.05 MXT group versus the PACAP+MXT group.
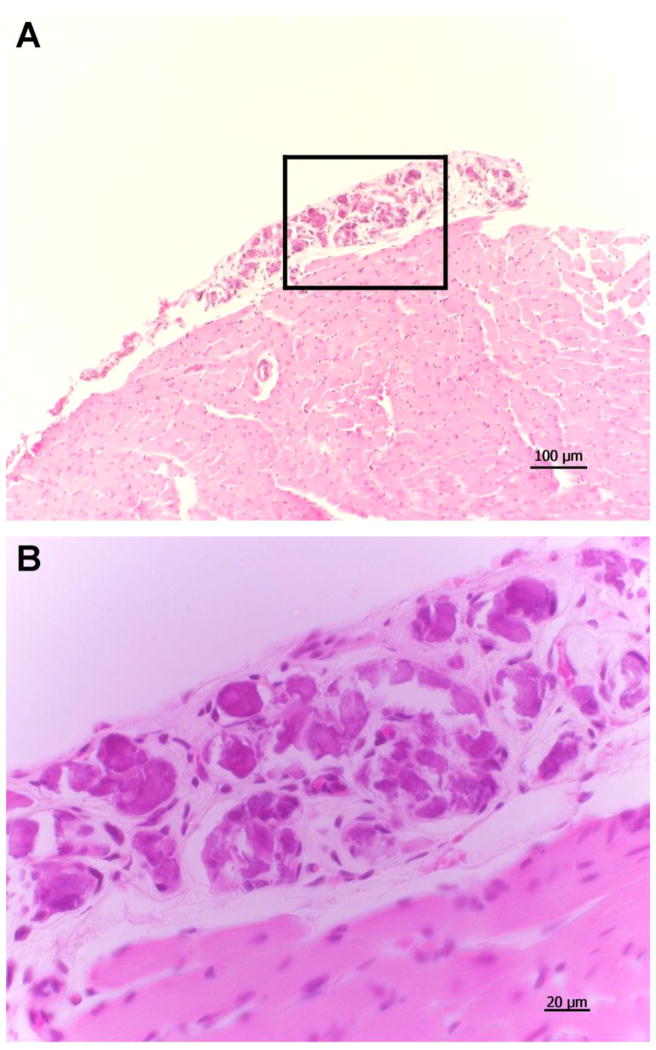
Histological examination of the mouse hearts failed to show gross evidence of inflammation (e.g., lymphocytic infiltration or myocyte disruption) or fibrosis (Fig. 5). The hearts of mice treated with MXT alone had what appeared to be multiple, large calcified lesions scattered over the epicardial surface (Fig. 5A and 4B). These lesions were observed in all five of the hearts examined from the MXT group. Similar lesions were not observed in any of the hearts from the mice treated with saline (n = 7), PACAP alone (n = 7) or PACAP+MXT (n = 7).
Figure 5.
Histological sections showing a typical epicardial lesion on the left ventricle of a mouse treated with MXT. A: low-power image (10X) of left ventricle and lesion. B: high-power (40X) image of the area bounded by the rectangle in A.
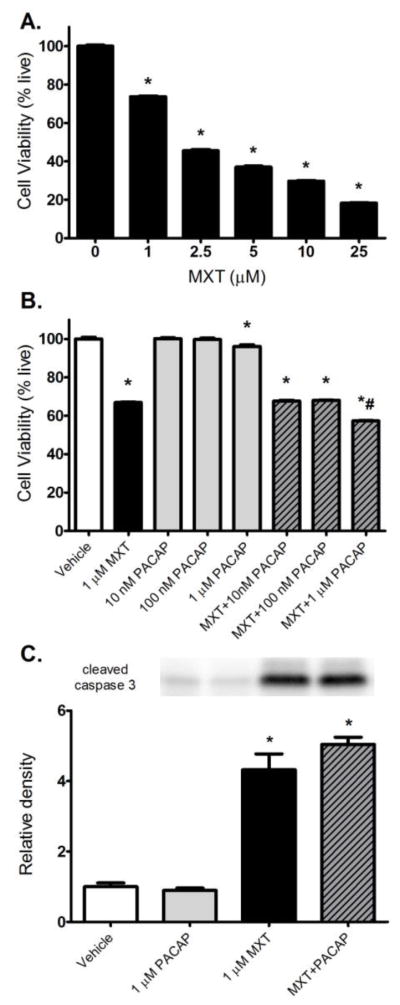
The 24-h treatment with MXT (0.1–25 μM) produced dose-related decreases in the viability of U937 cells, a human leukemia cell line (Fig. 6A). Except at the highest concentration tested (1 μM), which produced a small, but significant increase in cell death, the 24-h treatment with PACAP alone did not reduce the viability of the U937 cells (Fig. 6B). PACAP did not protect U937 cells from MXT-induced cell death after a 24-h co-treatment and even significantly enhanced the MXT-induced cell death of the U937 cells at the highest concentration tested (Fig. 6B). MXT-induced cell death in U937 cells was accompanied by significant increases in the levels of cleaved caspase 3 (Fig. 6C). The increase in cleaved caspase 3 was not reduced by co-treatment with PACAP. PACAP treatment by itself did not affect caspase 3 cleavage relative to control cells (Fig. 6C).
Figure 6.
Effects of MXT and PACAP on the viability of U937 cells. (A) The dose-response relationship between MXT concentration and U937 cell viability after a 24-h treatment. (B) The effect of MXT and PACAP, alone and in combination, on U937 cell viability after a 24-h treatment. (C) Summary of Western blot analysis showing that the MXT-induced increase in cleaved caspase 3 is not attenuated by a 24-h co-treatment with PACAP. * p < 0.05 versus the vehicle group. # p < 0.05 MXT group versus the MXT + 1 μM PACAP group.
4. Discussion
4.1. Mitoxantrone-induced cardiac injury
This study is the first to demonstrate that repeated administration of MXT produces eccentric LV dilation and dysfunction in mice in vivo. More important, this study also provides the first evidence that this cardiac toxicity can be significantly attenuated by the administration of PACAP. Treatment with PACAP also improved the overall health and survival of the MXT-treated mice over the duration of this study.
Although early reports suggested that MXT was less cardiotoxic than doxorubicin, it is now generally accepted that MXT can still produce an irreversible cardiomyopathy, decrease LV function (decreased %EF) and cause heart failure in animals and humans [3, 36, 43, 45, 55]. In some cases, these cardiotoxic effects may occur years after the cessation of treatment. MXT administration can be associated with electrocardiographic abnormalities [25]. In the present study, serial echocardiography revealed that the repeated administration of MXT significantly increased LV diameter and volume, and decreased the thickness of the posterior wall and septum after the third dose of MXT. These structural changes are indicative of eccentric dilation and were accompanied by significant decreases in LV function (e.g., decreases in %EF and %FS). The fact that changes in body weight and in cardiac structure and function did not occur until after the third dose of MXT suggests that, as in humans, the toxicity of this drug in mice occurs after a specific cumulative dose. For this reason, the FDA recommends strict limits on the lifetime doses of anthracycline and androstenedione chemotherapeutic agents administered to humans. Repeated administration of MXT also produces a degenerative cardiomyopathy in rodents as evidenced micro- and macro vacuolization, intercellular edema, atrophy, inflammatory infiltrates, aberrant mitochondria, myolysis of cardiomyocytes, and interstitial fibrosis [1, 3, 62]. None of these studies reported functional or structural changes in the heart; however, Rossato and colleagues [62] reported increases in cardiac weight in rats 28 days after administering 3 doses of MXT (2.5 mg/kg) on days 0, 10 and 20. There were no significant differences in heart weight among our four groups of BALB/c mice. This observation may reflect the fact that MXT produced eccentric dilation (increased chamber size and thinning of the chamber walls) rather than concentric hypertrophy in our animals. However, it is possible that we would have observed increases in heart weight as a result of a more severe hypertrophy if we had extended the duration of our study.
4.2. PACAP-mediated cardiac protection
The co-treatment of mice with both MXT and PACAP prevented the MXT-mediated increases in LVID;d and LV Vol;d as well as the thinning of the ventricular septum. While there was a tendency for PACAP to reduce the thinning of the posterior LV wall, LVPW;d was not significantly different between the mice treated only with MXT and those treated with both MXT and PACAP. Why PACAP did not prevent the thinning of the posterior LV wall is unknown. In addition to preventing MXT-induced eccentric dilation, PACAP also significantly attenuated the decreases in %EF and %FS produced by MXT. Whether higher doses of PACAP or increases in the frequency or duration of PACAP administration after MXT would have returned %EF and/or %FS to control levels remains to be determined. It is also possible that MXT-induced cardiotoxicity involves multiple pathways, only some of which are sensitive to PACAP. For example, the observed decrease in LV function may involve a combination of changes in LV structure that were prevented by PACAP and myocyte death/dysfunction that was only partially prevented by PACAP.
The precise mechanism(s) by which PACAP protects the heart from the actions of MXT is largely unknown. Early reports suggested that MXT, like doxorubicin, produces cardiac injury by inducing oxidative stress, ultimately leading to myocyte apoptosis [11, 43, 70]. In fact, several studies have reported that PACAP protects rat neonatal cardiac myocytes from oxidative stress-induced apoptosis [18, 56]. These protective actions were attributed to the inhibition of MAP kinase-dependent pathways and the phosphorylation of Akt and protein kinase A, leading to the inhibition of Bad and increases in Bcl-xL and 14-3-3 protein. However, more recent investigations using in vivo models have shown that MXT does not increase lipid peroxidation, serum lipids, or alter the antioxidant status of the heart, indicating that oxidative stress does not play a major role in MXT-induced cardiac damage [1, 35, 62]. Rossato and colleagues concluded that MXT causes cardiac dysfunction and ultimately failure by causing mitochondrial dysfunction and energy depletion [62]. These authors speculated that the mitochondrial damage results from MXT-mediated DNA and RNA strand breaks and inhibition of RNA and DNA synthesis.
4.3. Potential clinical applications
PACAP has been given to healthy human volunteers by several academic laboratories in the European Union [6, 16, 21, 50, 71] and to a patient with multiple myeloma under a FDA-approved single-patient protocol [39] without any hint of serious adverse reactions. The safety of PACAP and its ability to attenuate the cardiotoxic effects of MXT without attenuating its intended therapeutic effects, strongly suggests that PACAP would be a valuable adjunctive therapy for patients receiving MXT for the treatment of blood cancers or autoimmune disorders.
4.3.1. Blood cancers
While PACAP provided cardiac protection in vivo, it did not protect cultured U937 human leukemia cells from the cytotoxic actions of MXT, suggesting that PACAP could be a useful cardioprotective adjuvant when MXT is used to treat certain blood cancers. In fact, PACAP inhibited the proliferation of HEL myeloid leukemia cells [26, 37], Jurkat T-cell leukemia cells [8] and multiple myeloma cells [38]. PACAP also inhibited the secretion of IL-2 by activated Jurkat cells [8] and the secretion of immunoglobulin light chains by multiple myeloma cells [39]. Therefore, the addition of PACAP to MXT regimens for some blood cancers may not only reduce the cardiotoxicity of MXT but could also enhance the efficacy of the therapeutic regimen. Whether PACAP can also significantly reduce the cardiotoxicity of other commonly used classes of anticancer agents such as alkylating agents (e.g., cyclophosphamide), antimetabolites (e.g., 5-fluorouracil) and tyrosine kinase inhibitors (e.g., dasatinib) should be determined. Cyclosphosphamide has also been used for the treatment of MS [46] and other autoimmune diseases (e.g., 64).
4.3.2. Multiple Sclerosis
PACAP has also been shown by several laboratories to be beneficial in preclinical in vivo models for MS [15, 20, 30, 66, 67] and other autoimmune disorders, including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and type I diabetes [J29, 54, 69].
Simply reducing the cardiac toxicity of MXT would allow physicians to increase the lifetime cumulative dose of MXT, thus allowing for longer treatment regimens, which would be especially beneficial for chronic conditions such as progressive/relapsing and secondary progressive MS. However, because treatment with PACAP alone has significant efficacy in preclinical in vivo models of MS, the addition of PACAP to a standard MXT regimen for MS should simultaneously increase the TD50 and decrease the ED50 of the therapeutic index.
4.3.3. Alzheimer’s disease
The extracellular accumulation of amyloid β-proteins (Aβ), especially insoluble aggregates of Aβ42, in the brain plays a crucial role in the pathogenesis of Alzheimer’s disease [65]. Eleuteri et al. [12] screened a library of FDA-approved drugs for in vitro inhibition of seeding-mediated Aβ42 aggregation and identified three potent inhibitors: hexachlorophene, bithionol and mitoxantrone. Only bithionol and mitoxantrone inhibited the histopathology in a transgenic mouse model of Alzheimer’s disease. Bithionol was withdrawn from the market by the FDA in 1967 [17]. The intracellular accumulation of insoluble neurofibrillary tangles composed mainly of hyperphosphorylated tau in the brain also plays a critical role in the pathogenesis of Alzheimer’s disease (73). MXT has been shown to reduce the synthesis of the pathogenic splice variants of tau in vitro (42).
PACAP protected neuronal cells against Aβ42 toxicity in vitro [22, 53] and stimulated the non-amyloid processing of amyloid precursor protein in neuronal cells in vitro [34]. PACAP was beneficial in preclinical in vivo models of Alzheimer’s disease [10, 51, 58]. Therefore, dual therapy with both MXT and PACAP38 could have a much higher therapeutic index for the treatment of Alzheimer’s disease than monotherapy with either MXT or PACAP38 alone.
4.4. Conclusions
Chronic treatment with MXT caused LV dilation, decreased EF, decreased FS, and body weight loss in mice.
Overlapping treatment with PACAP38 could reverse the effects of MXT on LV volume, EF, FS, and body weight in mice.
Dual therapy with PACAP38 and MXT could be beneficial for the treatment of some blood cancers, MS and Alzheimer’s disease.
Highlights.
Treatment with mitoxantrone once a week caused a dilated cardiomyopathy in mice.
Treatment with mitoxantrone once a week caused a large loss of body weight in mice.
Co-treatment with PACAP38 reduced the mitoxantrone-induced dilated cardiomyopathy.
Co-treatment with PACAP38 reduced the mitoxantrone-induced loss of body weight.
Co-treatment with PACAP38 could increase the clinical safety of mitoxantrone.
Acknowledgments
This work was supported in part by the National Institutes of Health (5P30 GM106392) and the Akira Arimura Foundation.
Abbreviations
- d
diastole
- EF
ejection fraction
- FDA
Food and Drug Administration
- FS
fractional shortening
- i.p
intraperitoneal
- IVS
intraventricular septum
- LV
left ventricle
- LVID
internal diameter of the left ventricle
- LVPW
thickness of the posterior wall of the left ventricle
- MS
multiple sclerosis
- MXT
mitoxantrone
- PACAP
pituitary adenylate cyclase-activity polypeptide
- s
systole
- TBST
Tris-buffered saline with 0.05% Tween 20 containing 3% bovine serum albumin
- VIP
vasoactive intestinal peptide
- Vol
volume
Footnotes
Conflict of interest
David H. Coy and Jerome L. Maderdrut are Inventors on multiple World (PCT) patents about the properties and uses of PACAP and PACAP analogs. All of these patents have been assigned to the Administrators of the Tulane Educational Fund. The other authors have no conflicts of interest.
Author contributions
Dr. Subramanian wrote major portions of the paper and conducted most of the animal experiments. He was assisted by Dr. Burn and Dr. Xia. Dr. Bradley assisted with the echocardiographic measurements. Dr. Chuang conducted the cell culture experiments. Dr. Coy synthesized and purified the PACAP38. Dr. Maderdrut wrote major portions of the paper. Drs. Varner and Maderdrut designed and supervised the experiments, and edited the final version of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alderton PM, Gross J, Green MD. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res. 1992;52:194–201. [PubMed] [Google Scholar]
- 2.Aubert N, Vaudry D, Falluel-Morel A, Desfeux A, Fisch C, Ancian P, de Jouffrey S, Le Bigot JF, Couvineau A, Laburthe M, Fournier A, Laudenbach V, Vaudry H, Gonzalez BJ. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: involvement of the mitochondrial apoptotic pathway. Neurobiol Dis. 2008;32:66–80. doi: 10.1016/j.nbd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, Sala F. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: Comparative studies against doxorubicin and mitoxantrone. Invest New Drugs. 2007;25:187–195. doi: 10.1007/s10637-007-9037-8. [DOI] [PubMed] [Google Scholar]
- 4.Champion HC, Santiago JA, Garrison EA, Cheng DY, Coy DH, Murphy WA, Ascuitto RJ, Ross-Ascuitto NT, McNamara DB, Kadowitz PJ. Analysis of cardiovascular responses to PACAP-27, PACAP-38, and vasoactive intestinal polypeptide. Ann N Y Acad Sci. 1996;805:429–441. doi: 10.1111/j.1749-6632.1996.tb17502.x. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Lo-Coco F. Mitoxantrone-related acute leukemia in MS: An open or closed book? Neurology. 2013;80:1529–1533. doi: 10.1212/WNL.0b013e31828cf891. [DOI] [PubMed] [Google Scholar]
- 6.Chiodera P, Volpi R, Capretti L, Caffarri G, Magotti MG, Coiro V. Effects of intravenously infused pituitary adenylate cyclase-activation polypeptide on adrenohypophyseal hormone secretion in normal men. Neuroendocrinology. 1996;64:242–246. doi: 10.1159/000127124. [DOI] [PubMed] [Google Scholar]
- 7.Cocco E, Marrosu MG. The current role of mitoxantrone in the treatment of multiple sclerosis. Expert Rev Neurother. 2014;14:607–616. doi: 10.1586/14737175.2014.915742. [DOI] [PubMed] [Google Scholar]
- 8.Coy DH, Maderdrut JL, Li M, Batuman V. Use of pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP analogs as adjunctive treatments with inhibitors of calcineurin or inhibitors of the mammalian target of rapamycin (mTOR) complexes. 2011:131. PTC/US2011/023930. [Google Scholar]
- 9.Derwenskus J. Current disease-modifying treatment of multiple sclerosis. Mt Sinai J Med. 2011;78:161–175. doi: 10.1002/msj.20239. [DOI] [PubMed] [Google Scholar]
- 10.Dogrukol-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka N, Nakamachi T, Ohtaki H, Niehoff ML, Edwards JC, Shioda S, Morley JE, Banks WA. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29:411–422. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- 11.Duthie SJ, Grant MH. The role of reductive and oxidative metabolism in the toxicity of mitoxantrone, adriamycin and menadione in human liver derived Hep G2 hepatoma cells. Br J Cancer. 1989;60:566–571. doi: 10.1038/bjc.1989.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eleuteri S, Di Giovanni S, Rockenstein E, Mante M, Adame A, Trejo M, Wrasidlo W, Wu F, Fraering PC, Masliah E, Lashuel HA. Novel therapeutic strategy for neurodegeneration by blocking Aβ seeding mediated aggregation in models of Alzheimer’s disease. Neurobiol Dis. 2015;74:144–157. doi: 10.1016/j.nbd.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evison BJ, Sleebs BE, Watson KG, Phillips DR, Cutts SM. Mitoxantrone, more than just another topoisomerase II poison. Med Res Rev. 2016;36:248–299. doi: 10.1002/med.21364. [DOI] [PubMed] [Google Scholar]
- 14.Faulds D, Balfour JA, Chrisp P, Langtry HD. Mitoxantrone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs. 1991;41:400–449. doi: 10.2165/00003495-199141030-00007. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- 16.Filipsson K, Tornøe K, Holst J, Ahrén B. Pituitary adenylate cyclase-activating polypeptide stimulates insulin and glucagon secretion in humans. J Clin Endocrinol Metab. 1997;82:3093–3098. doi: 10.1210/jcem.82.9.4230. [DOI] [PubMed] [Google Scholar]
- 17.Fung M, Thornton A, Mybeck K, Wu JH, Hornbuckle K, Muniz E. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999. Drug Information J. 2001;35:293–317. [Google Scholar]
- 18.Gasz B, Racz B, Rőth E, Borsiczky B, Ferencz A, Tamas A, Cserepes B, Lubics A, Gallyas F, Jr, Tóth G, Lengvári I, Reglődi D. Pituitary adenylate cyclase activating polypeptide protects cardiomyocytes against oxidative stress-induced apoptosis. Peptides. 2006;2:87–94. doi: 10.1016/j.peptides.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Gasz B, Rácz B, Röth E, Borsiczky B, Tamás A, Boronkai A, Gallyas F, Tóth G, Reglödi D. PACAP inhibits oxidative stress-induced activation of MAP kinase-dependent apoptotic pathway in cultured cardiomyocytes. Ann N Y Acad Sci. 2006;1070:293–297. doi: 10.1196/annals.1317.029. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond PJ, Talbot K, Chapman R, Ghatei MA, Bloom SR. Vasoactive intestinal peptide, but not pituitary adenylate cyclase-activating peptide, modulates the responsiveness of the gonadotroph to LHRH in man. J Endocrinol. 1993;137:529–532. doi: 10.1677/joe.0.1370529. [DOI] [PubMed] [Google Scholar]
- 22.Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM, Shi J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol Aging. 2014;35:2064–2071. doi: 10.1016/j.neurobiolaging.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- 24.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartung H-P, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, Krapf H, Zwingers T. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 26.Hayez N, Harfi I, Lema-Kisoka R, Svoboda M, Corazza F, Sariban E. The neuropeptides vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) modulate several biochemical pathways in human leukemic myeloid cells. J Neuroimmunol. 2004;149:167–181. doi: 10.1016/j.jneuroim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Hollenstein K, de Graaf C, Bortolato A, Wang M-W, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35:12–22. doi: 10.1016/j.tips.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji H, Zhang Y, Shen X, Gao F, Huang CY, Abad C, Busuttil RW, Waschek JA, Kupiec-Weglinski JW. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation via cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimeno R, Gomariz RP, Gutierrez-Canas I, Martinez C, Juarranz Y, Leceta J. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol Cell Biol. 2010;88:734–745. doi: 10.1038/icb.2010.29. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 31.Khan A-M, Li M, Abdulnour-Nakhoul S, Maderdrut JL, Simon EE, Batuman V. Delayed administration of pituitary adenylate cyclase-activating polypeptide 38 ameliorates renal ischemia/reperfusion injury in mice by modulating Toll-like receptors. Peptides. 2012;38:395–403. doi: 10.1016/j.peptides.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Khan A-M, Maderdrut JL, Li M, Toliver HL, Coy DH, Simon EE, Batuman V. Pituitary adenylate cyclase-activating polypeptide prevents contrast-induced nephropathy in a novel mouse model. Physiol Rep. 2013;1:e00163. doi: 10.1002/phy2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingwell E, Koch M, Leung B, Isserow S, Geddes J, Rieckmann P, Tremlett H. Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology. 2010;74:1822–1826. doi: 10.1212/WNL.0b013e3181e0f7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojro E, Postina R, Buro C, Meiringer C, Gehrig-Burger K, Fahrenholz F. The neuropeptide PACAP promotes the α-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 2006;20:512–514. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- 35.Koutinos G, Stathopoulos GP, Dontas I, Perrea-Kotsarelis D, Couris E, Darayannacos PE, Deliconstantinos G. The effect of doxorubicin and its analogue mitoxantrone on cardiac muscle and on serum lipids: an experimental study. Anticancer Res. 2002;22:815–820. [PubMed] [Google Scholar]
- 36.Lefrak EA, Pit’ha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Lema-Kisoka R, Hayez N, Langer T, Robberecht P, Sariban E, Delporte C. Characterization of functional VIP/PACAP receptors in the human erythroleukemic HEL cell line. Peptides. 2001;22:2155–2162. doi: 10.1016/s0196-9781(01)00567-8. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Cortez S, Nakamachi T, Batuman V, Arimura A. Pituitary adenylate cyclase-activating polypeptide is a potent inhibitor of the growth of light chain-secreting human multiple myeloma cells. Cancer Res. 2006;66:8796–8803. doi: 10.1158/0008-5472.CAN-05-2809. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Maderdrut JL, Lertora JJL, Batuman V. Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: A case study. Peptides. 2007;28:1891–1895. doi: 10.1016/j.peptides.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Maderdrut JL, Lertora JJL, Arimura A, Batuman V. Renoprotection by pituitary adenylate cyclase-activating polypeptide in multiple myeloma and other kidney diseases. Regul Pept. 2008;145:24–32. doi: 10.1016/j.regpep.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Balamuthusamy S, Khan AM, Maderdrut JL, Simon EE, Batuman V. Pituitary adenylate cyclase-activating polypeptide prevents cisplatin-induced renal failure. J Mol Neurosci. 2010;43:58–66. doi: 10.1007/s12031-010-9394-1. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Peacey E, Dickson J, Donahue CP, Zheng S, Varani G, Wolfe MS. Mitoxantrone analogues as ligands for a stem-loop structure of tau pre-mRNA. J Med Chem. 2009;52:6523–6526. doi: 10.1021/jm9013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llesuy S, Milei J, Gonzalex Flecha BS, Boveris A. Myocardial damage induced by doxorubicins: hydroperoxide-initiated chemiluminescence and morphology. Free Radic Biol Med. 1990;8:259–264. doi: 10.1016/0891-5849(90)90071-p. [DOI] [PubMed] [Google Scholar]
- 44.Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. doi: 10.1093/cvr/cvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lores Arnaiz S, Llesuy S. Oxidative stress in mouse heart by antitumoral drugs: a comparative study of doxorubicin and mitoxantrone. Toxicology. 1993;29:31–38. doi: 10.1016/0300-483x(93)90135-f. [DOI] [PubMed] [Google Scholar]
- 46.Makhani N, Gorman MP, Branson HM, Stazzone L, Banwell BL, Chitnis T. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72:2076–2082. doi: 10.1212/WNL.0b013e3181a8164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinelli V, Radaelli M, Straffi L, Rodegher M, Comi G. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci. 2009;30:167–170. doi: 10.1007/s10072-009-0142-7. [DOI] [PubMed] [Google Scholar]
- 48.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 49.Mori H, Nakamachi T, Ohtaki H, Yofu S, Sato A, Endo K, Iso Y, Suzuki H, Takeyama Y, Shintani N, Hashimoto H, Baba A, Shioda S. Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on doxorubicin-induced cardiomyopathy in mice. Circ J. 2010;74:1183–1190. doi: 10.1253/circj.cj-09-1024. [DOI] [PubMed] [Google Scholar]
- 50.Murck H, Steiger A, Frieboes RM, Antonijevic IA. Pituitary adenylate cyclase-activating peptide affects homeostatic sleep regulation in healthy young men. Am J Physiol Endocrinol Metab. 2007;292:E853–E857. doi: 10.1152/ajpendo.00152.2006. [DOI] [PubMed] [Google Scholar]
- 51.Nonaka N, Farr SA, Nakamachi T, Morley JE, Nakamura M, Shioda S, Banks WA. Intranasal administration of PACAP: uptake by brain and regional brain targeting with cyclodextrins. Peptides. 2012;36:168–175. doi: 10.1016/j.peptides.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 53.Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K. The neuropeptide PACAP attenuates β-amyloid (1–42)-induced toxicity in PC12 cells. Peptides. 2002;23:1471–1478. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 54.Onoue S, Hanato J, Kuriyama K, Mizumoto T, Yamada S. Development of PACAP38 analogue with improved stability: physicochemical and in vitro/in vivo pharmacological characterization. J Mol Neurosci. 2011;43:85–93. doi: 10.1007/s12031-010-9415-0. [DOI] [PubMed] [Google Scholar]
- 55.Perkins WE, Schroeder RL, Carrano RA, Imondi AR. Myocardial effects of mitoxantrone and doxorubicin in the mouse and guinea pig. Cancer Treat Rep. 1984;68:841–847. [PubMed] [Google Scholar]
- 56.Rácz B, Gasz B, Gallyas F, Jr, Kiss P, Tamás A, Szántó Z, Lubics A, Lengvári I, Tóth G, Hegyi O, Rőth E, Reglődi D. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept. 2008;145:105–115. doi: 10.1016/j.regpep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Racz B, Reglodi D, Horvath G, Szigeti A, Balatonyi B, Roth E, Weber G, Alotti N, Toth G, Gasz B. Protective effect of PACAP against doxorubicin-Induced cell death in cardiomyocyte culture. J Mol Neurosci. 2010;42:419–427. doi: 10.1007/s12031-010-9349-6. [DOI] [PubMed] [Google Scholar]
- 58.Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, Postina R, van Leuven F, Fahrenholz F, Kojro E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;9:3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- 60.Rommer PS, Zettl UK, Kieseier B, Hartung HP, Menge T, Frohman E, Greenberg BM, Hemmer B, Stüve O. Requirement for safety monitoring for approved multiple sclerosis therapies: an overview. Clin Exp Immunol. 2014;175:397–407. doi: 10.1111/cei.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross-Ascuitto NT, Ascuitto RJ, Ramage D, Kydon DW, Coy DH, Kadowitz PJ. Pituitary adenylate cyclase activating polypeptide: A neuropeptide with potent inotropic and coronary vasodilatory effects in neonatal pig hearts. Pediatr Res. 1993;34:323–328. doi: 10.1203/00006450-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Rossato LG, Costa VM, Dallegrave E, Arbo M, Silva R, Ferreira R, Amado F, Dinis-Oliveira RJ, Duarte JA, de Lourdes Bastos M, Palmeira C, Remião F. Mitochondrial cumulative damage induced by mitoxantrone: late onset cardiac energetic impairment. Cardiovasc Toxicol. 2013;14:30–40. doi: 10.1007/s12012-013-9230-2. [DOI] [PubMed] [Google Scholar]
- 63.Sano H, Miyata A, Horio T, Nishikimi T, Matsuo H, Kangawa K. The effect of pituitary adenylate cyclase activating polypeptide on cultured rat cardiocytes as a cardioprotective factor. Regul Pept. 2002;109:107–113. doi: 10.1016/s0167-0115(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt KJ, Fellermann K, Wellhöner P, Weitz G, Homann N, Herrlinger K, Lehnert H, Ludwig D, Büning J. Clinical trial: cyclophosphamide pulse therapy - a promising therapeutic alternative in refractory Crohn’s disease. Aliment Pharmacol Ther. 2009;29:1230–1239. doi: 10.1111/j.1365-2036.2009.03999.x. [DOI] [PubMed] [Google Scholar]
- 65.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO 2016; Mol Med. 8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Y-V, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan Y-V, Abad C, Wang Y, Lopez R, Waschek JA. Pituitary adenylate cyclase activating peptide deficient mice exhibit impaired thymic and extrathymic regulatory T cell proliferation during EAE. PLoS One. 2013;8:e61200. doi: 10.1371/journal.pone.0061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsung A, Nace GW, Geller DA. Pituitary adenylate cyclase-activating polypeptide: A neuromodulator of hepatic ischemia-reperfusion injury? Hepatology. 2013;57:878–880. doi: 10.1002/hep.25902. [DOI] [PubMed] [Google Scholar]
- 69.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 Years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 70.Vibet S, Mahéo K, Goré J, Dubois P, Bougnoux P, Chourpa I. Differential subcellular distribution of mitoxantrone in relation to chemosensitization in two human breast cancer cell lines. Drug Metab Dispos. 2007;35:822–828. doi: 10.1124/dmd.106.013474. [DOI] [PubMed] [Google Scholar]
- 71.Warren JB, Cockcroft JR, Larkin SW, Kajekar R, Macrae A, Ghatei MA, Bloom SR. Pituitary adenylate cyclase activation polypeptide is a potent vasodilator in humans. J Cardiovasc Pharmacol. 1992;20:83–87. [PubMed] [Google Scholar]
- 72.Waschek JA. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol. 2013;169:512–523. doi: 10.1111/bph.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfe MS. Tau mutations in neurodegenerative diseases. J Biol Chem. 2009;284:6021–6025. doi: 10.1074/jbc.R800013200. [DOI] [PubMed] [Google Scholar]