Abstract
Background
Ganglioside has a neuroprotective role in neonatal hypoxic–ischemic encephalopathy (HIE). This study aimed to evaluate the neurological outcomes of monosialoganglioside as adjuvant treatment for neonatal HIE by conducting a meta-analysis.
Methods
A comprehensive literature search was made in the Pubmed, EMBASE, Cochrane Library, Wanfang, CNKI, VIP databases through October 2016. Randomized controlled trials comparing monosialoganglioside with the usual treatment for newborns having HIE deemed eligible. Weighted mean difference (WMD) and risk ratio (RR) with 95% confidence interval (CI) were calculated for continuous and dichotomous data, respectively.
Results
Ten trials consisting of 787 neonates were included. Adjuvant treatment with monosialoganglioside significantly reduced major neurodevelopmental disabilities (RR = 0.35; 95% CI = 0.21–0.57), cerebral palsy (RR = 0.32; 95% CI = 0.12–0.87), mental retardation (RR = 0.31; 95% CI = 0.11–0.88) as well as improved the mental (WMD = 14.95; 95% CI = 7.44–22.46) and psychomotive (WMD = 13.40; 95% CI = 6.69–20.11) development index during the follow-up. Also, monosialoganglioside significantly improved Neonatal Behavioral Neurological Assessment scores (WMD = 2.91; 95% CI = 2.05–3.78) compared with the usual treatment. However, adverse effects associated with monosialoganglioside were poorly reported in the included trials.
Conclusion
Adjuvant treatment with monosialoganglioside had beneficial effects in improving neurological outcomes in neonatal HIE. However, these findings should be interpreted with caution because of methodological flaws in the included trials. Furthermore, safety of monosialoganglioside use should also be further evaluated.
Introduction
Neonatal hypoxic ischemic encephalopathy (HIE) is a common cause of brain damage secondary to perinatal asphyxia, affecting 1 to 8 per 1000 live full-term births [1]. Approximately 25%–30% of these suffering neonates developed permanent neurologic disabilities [2]. HIE can be classified into mild, moderate or severe in accordance with Sarnat’s criteria [3]. The majority of neonates with mild HIE are usually associated with normal outcomes [4]. Severe HIE consequently contributes to a higher risk of neonatal death as well as long-term neurologic disabilities, including cerebral palsy, mental retardation, learning disability, and epilepsy [5]. Therapeutic hypothermia has been considered as standard treatment for neonates with moderate to severe HIE but is only partially effective [6]. Currently, well–established effective therapies are lacking [7] and supportive medical therapies to maintain physiologic parameters remain the standard therapy. For high rates of neurologic morbidities caused by HIE, development of new therapeutic agents is needed for the management of neonatal HIE.
Gangliosides are sphingolipids located predominantly in the neuronal membranes [8]. Gangliosides perform key roles in maintaining membrane integrity and in regulating brain development [9]. Significant reduction in several gangliosides was observed after hypoxia–ischemia [10]. Experimental studies showed that monosialoganglioside can protect against neuronal apoptotic insults [11, 12] and attenuate brain damage [13]. These findings revealed that monosialoganglioside may act as a promising therapeutic option for neonatal HIE. Monosialoganglioside exhibited promising outcomes in the management of neonatal HIE in China [14, 15]. However, the effect of monosialoganglioside as an adjuvant therapy on neuronal outcomes in neonatal HIE remains under debate.
Considering that monosialoganglioside treatment is mainly used in China but considerably less often in Western countries, we therefore evaluate the neuroprotective effects of monosialoganglioside as an adjuvant therapy in neonatal HIE by conducting a meta-analysis of randomized controlled trials (RCTs) in the Chinese literature.
Materials and methods
Search strategy
This meta-analysis was performed in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (S1 Table) [16]. PubMed, Embase, and Cochrane Library, WanFang, VIP, and China National Knowledge Infrastructure databases were searched through October 2016 for RCTs that compared monosialoganglioside to controls in newborns with moderate or severe HIE.A combination of the following search terms and Medical Subject Headings was applied for each selected database: (monosialoganglioside OR ganglioside) AND (neonate OR newborn OR infant) AND (hypoxic-ischemic encephalopathy OR encephalopathies OR birth asphyxia) AND (randomized controlled trial OR RCT OR random) (S1 File). In addition, we manually searched the bibliographies of relevant study to identify any possible trial.
Study selection
Inclusion criteria for considering studies for this meta-analysis: 1) types of studies: RTCs; 2) types of participants: neonates with encephalopathy caused by perinatal asphyxia; 3) types of interventions: monosialoganglioside plus usual therapy versus usual therapy alone. Supportive care and other treatments were identical between two groups; and 4) outcome measures: primary endpoints were the incidence of major neurodevelopmental disabilities, cerebral palsy, mental retardation, and epilepsy. The secondary endpoints were Neonatal Behavioral Neurological Assessment (NBNA) scores at the end of treatment as well as mental development index (MDI), and psychomotive development index (PDI) during the longest follow-up. Usual therapy included control of seizures, reduction of encephalic pressure, elimination of brainstem symptoms, maintainance of normal ventilation, blood glucose, blood-gas or organ blood perfusion and symptomatic treatment. Trials included newborns with major congenital and hereditary abnormalities, congenital viral infections or other possible overt causes of neonatal encephalopathy.
Data extraction and quality assessment
Two investigators independently scanned the titles or abstracts and extracted data. A standardized form was used to extract the characteristics of the included trials. Extracted data included the first author’s name, year of publication, sample size, gender, type of HIE, monosialoganglioside intervention, intervention and follow-up duration, and outcome measures. The methodological quality of the included trials was evaluated using the risk of bias tool of the Cochrane Handbook for Systematic Reviews of Interventions, including random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias.
Statistical methods
Dichotomous data were calculated by the risk ratio (RR) with 95% confidence interval (CI) and continuous data were expressed as weighted mean difference (WMD) with 95% CI. Heterogeneity across the included trials was assessed using the I2 test and Cochrane Q statistic. A I2-value exceeding 50% was considered to indicate significant heterogeneity. We selected a random effects model when statistical heterogeneity was present; otherwise, a fixed-effect model was adopted. Potential publication bias was explored using the Egger's linear regression test and Begg's rank correlation test when the number of trials included in the analysis was exceeded 10 trials [17]. Sensitivity analyses were conducted by sequentially omitting anyone study each time. Subgroup analyses were conducted by treatment duration (>14 days versus ≤14 days), follow-up duration (≥12 months versus <12 months) and type of HIE (moderate and severe versus all types). All analyses were conducted using STATA 12.0 software (STATA Corp LP, College Station, TX, USA).
Results
Description of included trials
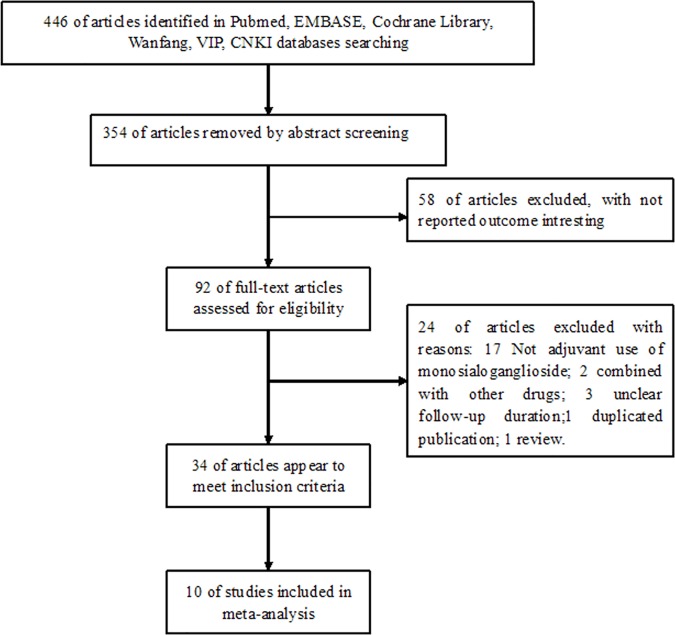
Fig 1 presents the details of literature search and trial selection process. The PRISMA flow chart is shown in S1 Fig. In brief, a total of 446 potentially relevant publications were identified from the preliminary search. After the title and abstract were reviewed, 354 publications were excluded mainly due to being preclinical studies and reviews or lack of interesting outcomes. Among the 92 remaining records, 82 full-text articles were further removed after our predefined inclusion and exclusion criteria were applied. Thus, 10 RCTs [18–27] were finally included in the meta-analysis.
Fig 1. Flow chart of trial selection process.
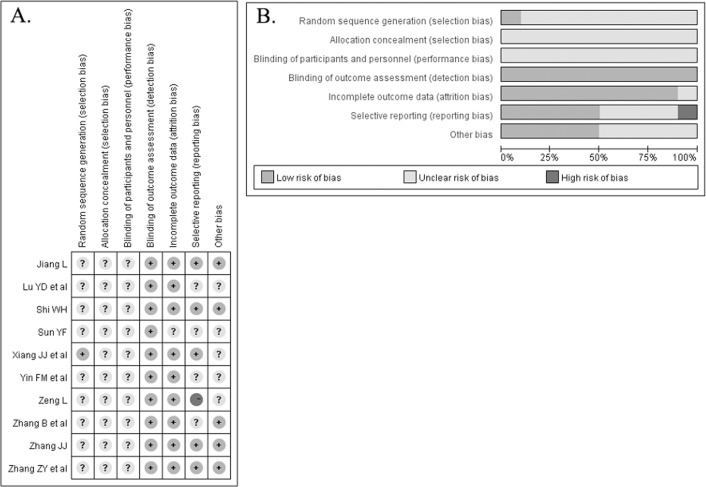
The baseline characteristics of the included trials are summarized in Table 1. A total of 787 patients were identified and analyzed. All trials were published between 2005 and 2014 and conducted in China. Monosialoganglioside was administered at a dose of 20 g per day and treatment duration ranged from 7 days to 60 days. All included trials declared a randomized control design. The detailed methodological quality of the included trials is presented in Fig 2. Overall, most of the included trials were grouped as unclear risk of bias.
Table 1. Baseline characteristics of the trials included in the meta-analysis.
| Study/Year | No. Patients | Male/female | Type of HIE | Main intervention | Treatment | Outcome | Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (GM1/Con) | (GM1/Con) | Mild | Moderate | Severe | GM1 Group | Control group | Duration | Measures | (months) | |
| Xiang JJ et al 2005 [15] | 36/30 | 20/16 17/13 |
— | 38 | 28 | GM1 20g/d, qd, IV drop + UT | UT | 7–14 days | ①+②+③+④+⑦ | 12 months |
| Lu YD et al 2008 [16] | 44/42 | 29/15 28/14 |
— | 58 | 28 | GM1 20g/d, qd, IV drop + UT | UT | 20–28 days | ⑤+⑥+⑦ | 12 months |
| Sun YF 2009 [17] | 46/43 | NP | — | NP | NP | GM1 20g/d, qd, IV drop + UT | UT | 20 days | ①+⑦ | 6 months |
| Zeng L 2009 [18] | 40/40 | 23/17 23/17 |
— | 30 | 50 | GM1 20g/d, qd, IV drop + UT | UT | 7 days | ① | 12 months |
| Zhang ZY et al 2009 [19] | 43/40 | 21/22 18/22 |
42 | 33 | 8 | GM1 20g/d, qd, IV drop + citicoline + UT | Citicoline + citicoline + UT | 20–30 days | ①+⑤+⑥+⑦ | 6 months |
| Zhang B et al 2010 [20] | 42/40 | 23/19 21/19 |
— | 58 | 24 | GM1 20g/d, qd, IV drop + UT | UT | 14 days | ⑤+⑥+⑦ | 12 months |
| Yin FM et al 2010 [21] | 31/30 | 18/13 17/13 |
— | 34 | 27 | GM1 20g/d, qd, IV drop + UT | UT | 20 days | ⑤+⑥+⑦ | 4 months |
| Shi WH 2013 [22] | 46/46 | NP | — | 42 | 50 | GM1 20g/d, qd, IV drop + UT | UT | 30–60 days | ②+③+④ | 15 months |
| Zhang JJ 2013 [23] | 36/34 | 19/17 18/16 |
37 | 28 | 5 | GM1 20g/d, qd, IV drop + UT | UT | 14 days | ①+②+③ | 12 months |
| Jiang L 2014 [24] | 39/39 | 21/18 22/17 |
17 | 33 | 28 | GM1 20g/d, qd, IV drop + citicoline + UT | Citicoline + citicoline + UT | 14 days | ①+②+③ | 12 months |
Abbreviations: Con, control; NBNA, neonatal behavioral neurological assessment; HIE, hypoxic-ischemic encephalopathy; NP, not provided; UT, usual therapy; FDP, fructose-1,6-diphosphate GM1, monosialotetrahexosylganglioside; IV, intravenous injection. ①Major neurological disability; ②Cerebral palsy; ③Mental retardation; ④Epilepsy; ⑤Mental development index; ⑥Psychomotive development index; ⑦Neonatal behavioral neurological assessment.
Fig 2.
Risk of bias summary (A) and risk of bias graph (B)
Neurodevelopmental disabilities
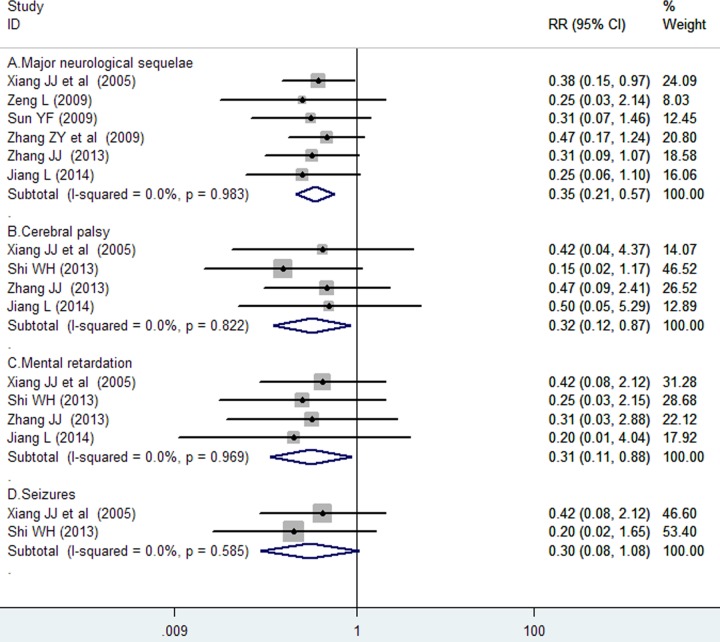
Six trials [18, 20–22, 26, 27] reported data on major neurodevelopmental disabilities during follow-up. The number of major neurological disability in the monosialoganglioside and the usual therapy groups was 18 of 240 (7.5%) and 48 of 226 (21.2%), respectively. As shown in Fig 3A, monosialoganglioside treatment was associated with a significant reduction in the incidence of major neurodevelopmental disability compared with usual therapy care (RR = 0.35; 95% CI = 0.21–0.57) in a fixed-effect model, with no evidence of significant heterogeneity (I2 = 0%, p = 0.983). Subgroup analysis indicated that the effects of monosialoganglioside in reducing major neurodevelopmental disability were observed in each predefined subgroup (Table 2). Sensitivity analysis revealed that there were minimal changes in the pooling effect sizes with the omission of anyone trials.
Fig 3. Forest plots showing risk ratio with 95% confidence interval of major neurodevelopmental disability comparing with or without monosialoganglioside treatment in a fixed-effect model.
Table 2. Subgroup analyses on major neurodevelopmental disabilities.
| Subgroup | No. of trials | Pooled RR | 95% CI | Heterogeneity between studies |
|---|---|---|---|---|
| Severity of HIE | ||||
| Moderate + severe | 3 | 0.34 | 0.16–0.72 | P = 0.931; I2 = 0% |
| All type | 3 | 0.35 | 0.18–0.69 | P = 0.762; I2 = 0% |
| Treatment duration | ||||
| >14 days | 2 | 0.41 | 0.18–0.93 | P = 0.667; I2 = 0% |
| ≤14 days | 4 | 0.31 | 0.17–0.60 | P = 0.963; I2 = 0% |
| Follow-up duration | ||||
| <12 months | 2 | 0.41 | 0.18–0.93 | P = 0.667; I2 = 0% |
| ≥12 months | 4 | 0.31 | 0.17–0.60 | P = 0.963; I2 = 0% |
Abbreviations: HIE, hypoxic-ischemic encephalopathy; RR, risk ratio; CI, confidence interval.
Meta-analysis of four trials [18, 25–27] showed a beneficial effect of monosialoganglioside therapy in reducing the incidence of cerebral palsy (RR = 0.32; 95% CI = 0.12–0.87; Fig 3B) and mental retardation (RR = 0.31; 95% CI = 0.11–0.88; Fig 3C) in a fixed-effect model, respectively. However, meta-analysis of two trials showed no significant difference in the incidence of epilepsy (RR 0.30; 95% CI 0.08–1.08; Fig 3D) between the monosialoganglioside and control groups.
Mental development index and psychomotive development index
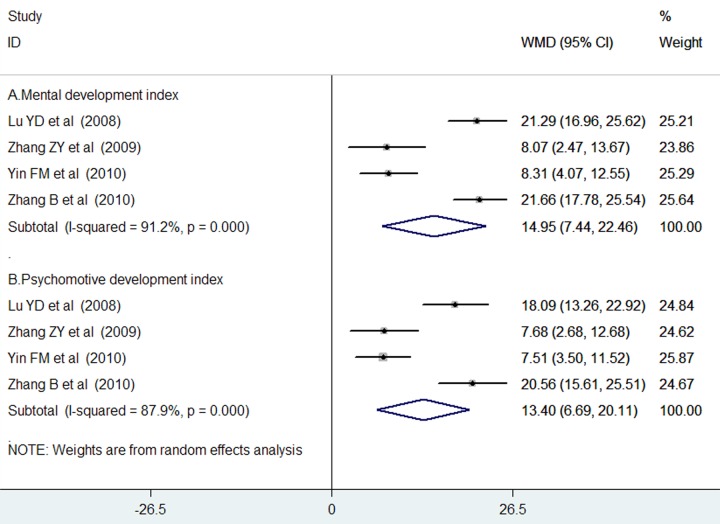
Four trials [19, 22–24] reported the data on MDI and PDI during the follow-up. As shown in Fig 4A, adjuvant treatment with monosialoganglioside resulted in a significant improvement in MDI (WMD = 14.95; 95% CI = 7.44–22.46) in a random effect model, with evidence of significant heterogeneity (I2 = 91.2%, p < 0.001). Similarly, adjuvant treatment with monosialoganglioside was associated with a significantly improvement in PDI (WMD = 13.40; 95% CI = 6.69–20.11; Fig 4B) in a random effect model, with evidence of significant heterogeneity (I2 = 87.9%, p < 0.001).
Fig 4.
Forest plots showing weighted mean difference with 95% confidence interval of the Mental Development Index (A) and Psychomotive Development Index (B) comparing with or without monosialoganglioside treatment in a random effect model.
Neonatal Behavioral Neurological Assessment
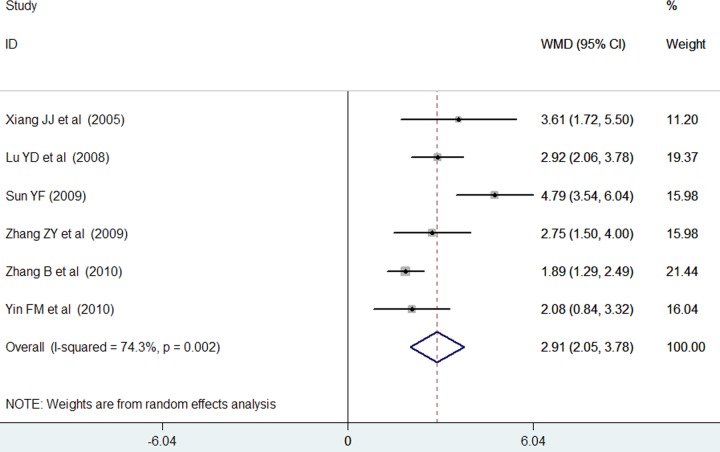
Six trials [18–20, 22–24] reported the data on NBNA at the end of monosialoganglioside treatment. As shown in Fig 5, monosialoganglioside treatment was associated with a significant increment in NBNA scores (WMD = 2.91; 95% CI = 2.05–3.78) compared with the usual treatment in a random effect model, with evidence of significant heterogeneity (I2 = 74.3%, p = 0.002). Sensitivity analysis revealed that there were no change in the direction of effect sizes when we removed anyone trials in the overall analysis.
Fig 5. Forest plots showing weighted mean difference with 95% confidence interval of Neonatal Behavioral Neurological Assessment scores comparing with or without monosialoganglioside treatment in a random effect model.
Discussion
The main findings were as follows: 1) adjuvant treatment with monosialoganglioside appeared to reduce the risk of major neurodevelopmental disabilities including cerebral palsy and mental retardation during the follow-up; 2) increment of MDI and PDI scores were more evident after monosialoganglioside treatment compared with the usual therapy during follow-up; 3) monosialoganglioside treatment was associated with a higher NANB scores at the end of treatment. However, monosialoganglioside treatment appeared to exert no obvious effect on the development of epilepsy.
Neonates with mild HIE tend to not to show increased risk of neurological disabilities, whereas those survivors with severe HIE are at a high risk to develop major neurological disabilities [28, 29]. In the current study, the incidences of major neurodevelopmental disabilities in the monosialoganglioside and the usual therapy groups were 21.2% and 7.5%, respectively. Adjuvant treatment with monosialoganglioside significantly reduced the risk of major neurodevelopmental disabilities by 65%. Major neurological disabilities include ataxia, cerebral palsy, mental retardation, seizures or epilepsy. Monosialoganglioside also significantly reduced risk of cerebral palsy and mental retardation by 68% and 69%, respectively. However, did not evidently affect the reduction of the epilepsy rate. The negative finding may be correlated to lack statistical power because only two trials were included in the analysis.
The Bayley Scale of Infant Development including MDI and PDI was used to assess the motor and psychomotor development of neonates. MDI or PDI score <70 reflect severe developmental delay. NBNA was formulated according to the method of Bra-zelton and Amiel-Tison for behavioral neurological measurement in neonates [30]. Neurobehavioral abnormalities detected using the 20-item NABA reflect early manifestations of neurological status and may represent later neurodevelopmental disabilities. This meta-analysis indicated that adjuvant treatment with monosialoganglioside significantly improved the values of MDI, PDI and NANB compared with the usual therapy. These findings suggested that monosialoganglioside achieved additional short-term and long-term benefits in improving neurodevelopmental ability. In addition, adjuvant treatment with monosialoganglioside improved the recovery time of awareness, muscle tension, and primitive reflex of HIE newborns [26].
Despite the beneficial effects of monosialoganglioside in HIE newborns, one major concern is the potential adverse effects of the monosialoganglioside. However, adverse effects were poorly reported because the included trials did not select tolerance and safety as outcomes. The reported common adverse effects of monosialoganglioside included fever, chills, cyanosis, cold sweat, skin lesion, and even cardiovascular damages [31].Therefore, the safety of monosialoganglioside use should be further evaluated in future studies.
Several potential limitations should be indicated. First, most included trials lacked sufficient information on the randomization or allocation concealment method, therefore, the methodological flaws of the included trials were an important concern. Second, potential publication bias cannot be excluded because all included trials were published in Chinese. Finally, statistical heterogeneity was present in pooling continuous data. The differences in duration of regimens, follow-up periods, and severity of HIE across the included trials may partly contribute to the observed heterogeneity.
In conclusion, this meta-analysis indicates that adjuvant treatment with monosialoganglioside appears to offer additional benefits in terms of improving short-term clinical effects and reducing long-term neurodevelopmental disabilities. Given the methodological flaws of the included trials, these findings should be interpreted with caution. Determination of the optimal duration of intervention and long-time follow-up should be considered in future trials. Future studies should explore the underlying mechanisms of the protective roles of monosialoganglioside.
Supporting information
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early human development. 2010;86(6):329–38. doi: 10.1016/j.earlhumdev.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Cai Q, Xue XD, Fu JH. Research status and progress of neonatal hypoxic ischemic encephalopathy. Chin J Pract Pediatr. 2009;24(12):968–71. [Google Scholar]
- 3.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology. 1976;33(10):696–705. [DOI] [PubMed] [Google Scholar]
- 4.de Haan M, Wyatt JS, Roth S, Vargha-Khadem F, Gadian D, Mishkin M. Brain and cognitive-behavioural development after asphyxia at term birth. Developmental science. 2006;9(4):350–8. doi: 10.1111/j.1467-7687.2006.00499.x [DOI] [PubMed] [Google Scholar]
- 5.Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. Journal of biomedicine & biotechnology. 2011;2011:609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson JO, Wassink G, van den Heuij LG, Bennet L, Gunn AJ. Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy—Where to from Here? Frontiers in neurology. 2015;6:198 doi: 10.3389/fneur.2015.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildiz EP, Ekici B, Tatli B. Neonatal hypoxic ischemic encephalopathy: an update on disease pathogenesis and treatment. Expert review of neurotherapeutics. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Lucki NC, Sewer MB. Nuclear sphingolipid metabolism. Annual review of physiology. 2012;74:131–51. doi: 10.1146/annurev-physiol-020911-153321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The role of gangliosides in neurodevelopment. Nutrients. 2015;7(5):3891–913. doi: 10.3390/nu7053891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez MR, Muraro F, Zylbersztejn DS, Abel CR, Arteni NS, Lavinsky D, et al. Neonatal hypoxia-ischemia reduces ganglioside, phospholipid and cholesterol contents in the rat hippocampus. Neuroscience research. 2003;46(3):339–47. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari G, Anderson BL, Stephens RM, Kaplan DR, Greene LA. Prevention of apoptotic neuronal death by GM1 ganglioside. Involvement of Trk neurotrophin receptors. The Journal of biological chemistry. 1995;270(7):3074–80. [DOI] [PubMed] [Google Scholar]
- 12.Gorria M, Huc L, Sergent O, Rebillard A, Gaboriau F, Dimanche-Boitrel MT, et al. Protective effect of monosialoganglioside GM1 against chemically induced apoptosis through targeting of mitochondrial function and iron transport. Biochemical pharmacology. 2006;72(10):1343–53. doi: 10.1016/j.bcp.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 13.Ballough GP, Cann FJ, Smith CD, Forster JS, Kling CE, Filbert MG. GM1 monosialoganglioside pretreatment protects against soman-induced seizure-related brain damage. Molecular and chemical neuropathology. 1998;34(1):1–23. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Wang QQ. Progress on ganglioside and early rehabilitation intervention in the treating neonatal hypoxic-ischemic encephalopathy. Clin Med Eng. 2015;22(2):251–3. [Google Scholar]
- 15.Yang X. Clinical application of the ganglioside in the management of childhood diseases. J Guangxi Univ Chin Med. 2016;19(3):66–9. [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang JJ, Wang P. Clinical observation of ganglioside in the treatment of neonatal hypoxic ischemic encephalopathy. J Clin Res. 2005;22(6):812–3. [Google Scholar]
- 19.Lu YD, Li Y, Zhou XY, Ben XM. Curative effects of monosialotetrahexosyl ganglioside on neonates with moderate and severe hypoxic-ischemic encephalopathy. J Appl Clin Pediar. 2008;23(2):150–1. [Google Scholar]
- 20.Sun YF. Efficacy of ganglioside in the treatment of neonatal hypoxic ischemic encephalopathy. Chin Prac Med. 2009;4(9):134. [Google Scholar]
- 21.Zeng L. Efficacy of ganglioside in the treatment of neonatal hypoxic ischemic encephalopathy. Chin J Nerv Dis. 2009;12(3):84–5. [Google Scholar]
- 22.Zhang ZY, Liu J, Fu YY. Clinical observation of ganglioside for the treatment of 83 cases of neonatal hypoxic ischemic encephalopathy Contemp Med. 2009;15(16):10–2. [Google Scholar]
- 23.Zhang B, Zhu J, Lu XJ, Wang J. Clinical observation of ganglioside for the treatmnt of moderate and severe neonatal hypoxic ischemic encephalopathy 40 cases. Med J Commun. 2010;24(4):697–8. [Google Scholar]
- 24.Yin FM, Shi MK, Shi LF. Clinical observation of ganglioside for the treatment of moderate and severe hypoxic ischemic encephalopathy of newborns. Mod J Integr Tradit Chin Western Med. 2010;19(14):1739–40. [Google Scholar]
- 25.Shi WH. Clinical observation of the ganglioside injection for the treatment of neonatal hypoxic-ischemic encephalopathy. Chin J Nerv Dis. 2013;16(11):79–80. [Google Scholar]
- 26.Zhang JJ. Effects of ganglioside for the treatment of 36 cases of neonatal hypoxic ischemic encephalopathy. Chin Pharmaceu 2013;22(11):146–7. [Google Scholar]
- 27.Jiang L. Clinical study on the treatment of neonatal hypoxic ischemic encephalopathy with ganglion glycosides. Chin J Mod Drug Appl. 2014;8(9):65–6. [Google Scholar]
- 28.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. European journal of pediatrics. 2007;166(7):645–54. doi: 10.1007/s00431-007-0437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta paediatrica. 1995;84(8):927–32. [DOI] [PubMed] [Google Scholar]
- 30.Bao XL, Yu RJ, Li ZS, Zhang BL. Twenty-item behavioral neurological assessment for normal newborns in 12 cities of China. Chinese medical journal. 1991;104(9):742–6. [PubMed] [Google Scholar]
- 31.Xie XY, Li QF. Literature analysis of 56 cases of adverse drug reactions induced by monosialotetrahexosylganglioside sodium injection. Chin Pharm. 2016;27(8):1067–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.