Abstract
Mammalian MRP (for mitochondrial RNA processing) RNA, also known as 7-2 RNA, is a nuclear encoded small RNA which has been reported to function in two different cellular compartments: in the mitochondria and in the nucleus. The ribonucleoprotein particle which contains the 7-2/MRP RNA, called RNase MRP, has ribonucleolytic activity and shares some structural similarity with RNase P. It has been proposed that in mitochondria, the RNase MRP is responsible for endonucleolytic cleavage of primer RNA during DNA replication. We have characterized the gene and cDNAs encoding 7-2/MRP-like RNA in Arabidopsis and tobacco, and found that in plants this RNA is enriched in nucleoli but is undetectable in purified mitochondria isolated from tobacco leaves or cells grown in suspension. In glycerol gradients tobacco 7-2/MRP RNA cosediments with large approximately 80S structures possibly representing ribosomal precursors. Fractionation of HeLa cells has also revealed that 7-2/MRP resides in the nucleolus and that most of it is associated with complexes sedimenting at approximately 80S, similar to those containing the U3 nucleolar RNA which is known to participate in pre-rRNA processing. These results indicate that the 7-2/MRP ribonucleoparticle may be involved in ribosome biogenesis, in both plant and mammalian cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Ribonuclease P. Postscript. J Biol Chem. 1990 Nov 25;265(33):20053–20056. [PubMed] [Google Scholar]
- BEERS R. F., Jr Hydrolysis of polyadenylic acid by pancreatic ribonuclease. J Biol Chem. 1960 Aug;235:2393–2398. [PubMed] [Google Scholar]
- Bennett J. L., Clayton D. A. Efficient site-specific cleavage by RNase MRP requires interaction with two evolutionarily conserved mitochondrial RNA sequences. Mol Cell Biol. 1990 May;10(5):2191–2201. doi: 10.1128/mcb.10.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland M. M., Levings C. S., 3rd, Matzinger D. F. The tobacco mitochondrial ATPase subunit 9 gene is closely linked to an open reading frame for a ribosomal protein. Mol Gen Genet. 1986 Jul;204(1):8–16. doi: 10.1007/BF00330180. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987 Mar 6;235(4793):1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Nuclear gadgets in mitochondrial DNA replication and transcription. Trends Biochem Sci. 1991 Mar;16(3):107–111. doi: 10.1016/0968-0004(91)90043-u. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Vicente O. RNA 3'-terminal phosphate cyclase from HeLa cells. Methods Enzymol. 1990;181:499–510. doi: 10.1016/0076-6879(90)81147-m. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Altman S. Similar cage-shaped structures for the RNA components of all ribonuclease P and ribonuclease MRP enzymes. Cell. 1990 Aug 10;62(3):407–409. doi: 10.1016/0092-8674(90)90003-w. [DOI] [PubMed] [Google Scholar]
- Gold H. A., Topper J. N., Clayton D. A., Craft J. The RNA processing enzyme RNase MRP is identical to the Th RNP and related to RNase P. Science. 1989 Sep 22;245(4924):1377–1380. doi: 10.1126/science.2476849. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Wiebauer K., Filipowicz W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 1990;181:148–161. doi: 10.1016/0076-6879(90)81117-d. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kung S. D., Wildman S. G. Origin of Nicotiana tabacum L. detected by polypeptide composition of Fraction I protein. Nature. 1974 Nov 15;252(5480):226–227. doi: 10.1038/252226a0. [DOI] [PubMed] [Google Scholar]
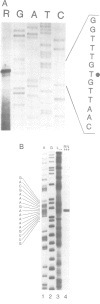
- Haas E. S., Morse D. P., Brown J. W., Schmidt F. J., Pace N. R. Long-range structure in ribonuclease P RNA. Science. 1991 Nov 8;254(5033):853–856. doi: 10.1126/science.1719634. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- James B. D., Olsen G. J., Liu J. S., Pace N. R. The secondary structure of ribonuclease P RNA, the catalytic element of a ribonucleoprotein enzyme. Cell. 1988 Jan 15;52(1):19–26. doi: 10.1016/0092-8674(88)90527-2. [DOI] [PubMed] [Google Scholar]
- Karwan R., Bennett J. L., Clayton D. A. Nuclear RNase MRP processes RNA at multiple discrete sites: interaction with an upstream G box is required for subsequent downstream cleavages. Genes Dev. 1991 Jul;5(7):1264–1276. doi: 10.1101/gad.5.7.1264. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Kiss T., Antal M., Solymosy F. Plant small nuclear RNAs. III. The complete primary and secondary structure of broad bean U2 RNA: phylogenetic and functional implications. Nucleic Acids Res. 1987 Feb 11;15(3):1332–1332. doi: 10.1093/nar/15.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Evidence against a mitochondrial location of the 7-2/MRP RNA in mammalian cells. Cell. 1992 Jul 10;70(1):11–16. doi: 10.1016/0092-8674(92)90528-k. [DOI] [PubMed] [Google Scholar]
- Kiss T., Marshallsay C., Filipowicz W. Alteration of the RNA polymerase specificity of U3 snRNA genes during evolution and in vitro. Cell. 1991 May 3;65(3):517–526. doi: 10.1016/0092-8674(91)90469-f. [DOI] [PubMed] [Google Scholar]
- Kiss T., Tóth M., Solymosy F. Plant small nuclear RNAs. Nucleolar U3 snRNA is present in plants: partial characterization. Eur J Biochem. 1985 Oct 15;152(2):259–266. doi: 10.1111/j.1432-1033.1985.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Baer M., Craft J., Altman S. An immunological determinant of RNase P protein is conserved between Escherichia coli and humans. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8717–8721. doi: 10.1073/pnas.86.22.8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshallsay C., Kiss T., Filipowicz W. Amplification of plant U3 and U6 snRNA gene sequences using primers specific for an upstream promoter element and conserved intragenic regions. Nucleic Acids Res. 1990 Jun 25;18(12):3459–3466. doi: 10.1093/nar/18.12.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshallsay C., Kiss T., Filipowicz W. Amplification of plant U3 and U6 snRNA gene sequences using primers specific for an upstream promoter element and conserved intragenic regions. Nucleic Acids Res. 1990 Jun 25;18(12):3459–3466. doi: 10.1093/nar/18.12.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Smith D. Ribonuclease P: function and variation. J Biol Chem. 1990 Mar 5;265(7):3587–3590. [PubMed] [Google Scholar]
- Pica-Mattoccia L., Attardi G. Expression of the mitochondrial genome in HeLa cells. IX. Replication of mitochondrial DNA in relationship to cell cycle in HeLa cells. J Mol Biol. 1972 Mar 14;64(2):465–484. doi: 10.1016/0022-2836(72)90511-6. [DOI] [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Reimer G., Raska I., Scheer U., Tan E. M. Immunolocalization of 7-2-ribonucleoprotein in the granular component of the nucleolus. Exp Cell Res. 1988 May;176(1):117–128. doi: 10.1016/0014-4827(88)90126-7. [DOI] [PubMed] [Google Scholar]
- STEELE W. J., OKAMURA N., BUSCH H. EFFECTS OF THIOACETAMIDE ON THE COMPOSITION AND BIOSYNTHESIS OF NUCLEOLAR AND NUCLEAR RIBONUCLEIC ACID IN RAT LIVER. J Biol Chem. 1965 Apr;240:1742–1749. [PubMed] [Google Scholar]
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Newton K. J. Isolation of plant mitochondrial RNA. Methods Enzymol. 1986;118:488–496. doi: 10.1016/0076-6879(86)18095-5. [DOI] [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Characterization of human MRP/Th RNA and its nuclear gene: full length MRP/Th RNA is an active endoribonuclease when assembled as an RNP. Nucleic Acids Res. 1990 Feb 25;18(4):793–799. doi: 10.1093/nar/18.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Secondary structure of the RNA component of a nuclear/mitochondrial ribonucleoprotein. J Biol Chem. 1990 Aug 5;265(22):13254–13262. [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. Structure of U2 snRNA genes of Arabidopsis thaliana and their expression in electroporated plant protoplasts. EMBO J. 1988 Mar;7(3):791–799. doi: 10.1002/j.1460-2075.1988.tb02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibel F., Filipowicz W. RNA-polymerase specificity of transcription of Arabidopsis U snRNA genes determined by promoter element spacing. Nature. 1990 Jul 12;346(6280):199–202. doi: 10.1038/346199a0. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Singh R., Reddy R. Rat nucleolar 7-2 RNA is homologous to mouse mitochondrial RNase mitochondrial RNA-processing RNA. J Biol Chem. 1989 Sep 5;264(25):14835–14839. [PubMed] [Google Scholar]
- Yuan Y., Tan E., Reddy R. The 40-kilodalton to autoantigen associates with nucleotides 21 to 64 of human mitochondrial RNA processing/7-2 RNA in vitro. Mol Cell Biol. 1991 Oct;11(10):5266–5274. doi: 10.1128/mcb.11.10.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]