Abstract
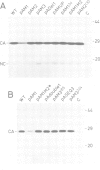
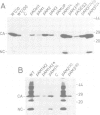
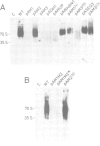
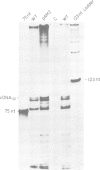
The Rous sarcoma virus (RSV) RNA leader sequence carries three open reading frames (uORFs) upstream of the AUG initiator of the gag gene. We studied, in vivo, the role of these uORFs by changing two or three nucleotides of the three AUGs or by deleting the first uORF. Our results show that (i) unlike most previously characterized uORFs, which decrease translation, the first uORF (AUG1) of RSV acts as an enhancer of translation, since absence of the first AUG decreased translation; AUG3 also modulates translation, probably by interfering with scanning ribosomes as described for other upstream ORFs, and mutation of AUG2 had no effect on translation. (ii) Mutation of each of the upstream AUGs lowered the infectivity of progeny virions. (iii) Unexpectedly, mutation of AUG1 and/or AUG3 dramatically reduced RNA packaging by 50-to 100-fold, unlike mutation of AUG2 which did not alter RNA packaging efficiency. Additional mutants in the vicinity of uORF1 and uORF3 were constructed in order to elucidate the mechanism by which uORFs affect RNA packaging: a translation model requiring uORFs 1 and 3, and involving ribosome pausing at AUG 3 is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Miller P. F., Jackson B. M., Hinnebusch A. G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991 Jan;11(1):486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyar A., Cobrinik D., Ge Z., Kung H. J., Leis J. Interaction between retroviral U5 RNA and the T psi C loop of the tRNA(Trp) primer is required for efficient initiation of reverse transcription. J Virol. 1992 Apr;66(4):2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick B. A., Lee A. L., Grendell R. L., Derynck R. Inhibition of translation of transforming growth factor-beta 3 mRNA by its 5' untranslated region. Mol Cell Biol. 1991 Sep;11(9):4306–4313. doi: 10.1128/mcb.11.9.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Aiyar A., Ge Z., Katzman M., Huang H., Leis J. Overlapping retrovirus U5 sequence elements are required for efficient integration and initiation of reverse transcription. J Virol. 1991 Jul;65(7):3864–3872. doi: 10.1128/jvi.65.7.3864-3872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Soskey L., Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988 Oct;62(10):3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A., Jaton J. C. In vitro, the major ribosome binding site on Rous sarcoma virus RNA does not contain the nucleotide sequence coding for the N-terminal amino acids of the gag gene product. J Virol. 1979 Feb;29(2):597–611. doi: 10.1128/jvi.29.2.597-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz P., Oertle S., Meric C., Damay P., Spahr P. F. Point mutations in the proximal Cys-His box of Rous sarcoma virus nucleocapsid protein. J Virol. 1990 Oct;64(10):4978–4987. doi: 10.1128/jvi.64.10.4978-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Gordon K., Sanfaçon H., Bonneville J. M., Hohn T. Positive and negative control of translation by the leader sequence of cauliflower mosaic virus pregenomic 35S RNA. EMBO J. 1990 Jun;9(6):1697–1707. doi: 10.1002/j.1460-2075.1990.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Translation of a polycistronic mRNA in the presence of the cauliflower mosaic virus transactivator protein. EMBO J. 1991 Dec;10(12):3887–3896. doi: 10.1002/j.1460-2075.1991.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Mocarski E. S. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J Virol. 1988 Sep;62(9):3334–3340. doi: 10.1128/jvi.62.9.3334-3340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Dalton M. W., Johnson D. P., Petersen R. B. Phylogenetic and physical analysis of the 5' leader RNA sequences of avian retroviruses. Nucleic Acids Res. 1991 Dec 25;19(24):6929–6934. doi: 10.1093/nar/19.24.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Petersen R. B., Hensel C. H., Albericio F., Gunderson S. I., Palmenberg A. C., Barany G. Synthesis in vitro of a seven amino acid peptide encoded in the leader RNA of Rous sarcoma virus. J Mol Biol. 1986 Jul 5;190(1):45–57. doi: 10.1016/0022-2836(86)90074-4. [DOI] [PubMed] [Google Scholar]
- Hensel C. H., Petersen R. B., Hackett P. B. Effects of alterations in the leader sequence of Rous sarcoma virus RNA on initiation of translation. J Virol. 1989 Nov;63(11):4986–4990. doi: 10.1128/jvi.63.11.4986-4990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Involvement of an initiation factor and protein phosphorylation in translational control of GCN4 mRNA. Trends Biochem Sci. 1990 Apr;15(4):148–152. doi: 10.1016/0968-0004(90)90215-w. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Cullen B. R., Malavarca R., Skalka A. M. Role of the avian retrovirus mRNA leader in expression: evidence for novel translational control. Mol Cell Biol. 1986 Feb;6(2):372–379. doi: 10.1128/mcb.6.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Brady J., Khoury G. Translational regulation of SV40 early mRNA defines a new viral protein. Cell. 1987 Feb 27;48(4):639–645. doi: 10.1016/0092-8674(87)90242-x. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Méric C. A procedure for Northern blot analysis of native RNA. Anal Biochem. 1986 Nov 15;159(1):227–232. doi: 10.1016/0003-2697(86)90332-5. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Koyama T., Harada F., Kawai S. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biochemical properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):154–162. doi: 10.1128/jvi.51.1.154-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. L., Miller A. D. Retroviral RNA packaging: sequence requirements and implications. Curr Top Microbiol Immunol. 1990;157:125–152. doi: 10.1007/978-3-642-75218-6_5. [DOI] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Miller P. F., Hinnebusch A. G. Sequences that surround the stop codons of upstream open reading frames in GCN4 mRNA determine their distinct functions in translational control. Genes Dev. 1989 Aug;3(8):1217–1225. doi: 10.1101/gad.3.8.1217. [DOI] [PubMed] [Google Scholar]
- Méric C., Gouilloud E., Spahr P. F. Mutations in Rous sarcoma virus nucleocapsid protein p12 (NC): deletions of Cys-His boxes. J Virol. 1988 Sep;62(9):3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M., Junker-Niepmann M., Schaller H. Translational inactivation of RNA function: discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell. 1990 Dec 21;63(6):1357–1363. doi: 10.1016/0092-8674(90)90431-d. [DOI] [PubMed] [Google Scholar]
- Oertle S., Bowles N., Spahr P. F. Complementation studies with Rous sarcoma virus gag and gag-pol polyprotein mutants. J Virol. 1992 Jun;66(6):3873–3878. doi: 10.1128/jvi.66.6.3873-3878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N. T., Cohen E. A., Darveau A., Rosen C., Haseltine W., Sonenberg N. Mutational analysis of the 5' non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J. 1988 Sep;7(9):2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem. 1989 Mar 25;264(9):5031–5035. [PubMed] [Google Scholar]
- Pelletier J., Flynn M. E., Kaplan G., Racaniello V., Sonenberg N. Mutational analysis of upstream AUG codons of poliovirus RNA. J Virol. 1988 Dec;62(12):4486–4492. doi: 10.1128/jvi.62.12.4486-4492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Borchelt D., Resnick R. J. Participation of 5'-terminal leader sequences in in vitro translation of Rous sarcoma virus RNA. J Biol Chem. 1982 Jun 10;257(11):6551–6555. [PubMed] [Google Scholar]
- Petersen R. B., Hackett P. B. Characterization of ribosome binding on Rous sarcoma virus RNA in vitro. J Virol. 1985 Dec;56(3):683–690. doi: 10.1128/jvi.56.3.683-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Hensel C. H., Hackett P. B. Identification of a ribosome-binding site for a leader peptide encoded by Rous sarcoma virus RNA. J Virol. 1984 Sep;51(3):722–729. doi: 10.1128/jvi.51.3.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Moustakas A., Hackett P. B. A mutation in the short 5'-proximal open reading frame on Rous sarcoma virus RNA alters virus production. J Virol. 1989 Nov;63(11):4787–4796. doi: 10.1128/jvi.63.11.4787-4796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss M. R., Degnin C. R., Geballe A. P. Translational control of human cytomegalovirus gp48 expression. J Virol. 1991 Dec;65(12):6782–6789. doi: 10.1128/jvi.65.12.6782-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman S. A., Good P. J., Mertz J. E. Leader-encoded open reading frames modulate both the absolute and relative rates of synthesis of the virion proteins of simian virus 40. J Virol. 1989 Sep;63(9):3884–3893. doi: 10.1128/jvi.63.9.3884-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Khokha R., Denhardt D. T. Modulation of translation by the 5' leader sequence of the mRNA encoding murine tissue inhibitor of metalloproteinases. J Biol Chem. 1990 Apr 5;265(10):5585–5589. [PubMed] [Google Scholar]
- Werner M., Feller A., Messenguy F., Piérard A. The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell. 1987 Jun 19;49(6):805–813. doi: 10.1016/0092-8674(87)90618-0. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]