Abstract
Background
Coagulase-negative staphylococci (CoNS) are recognized as a large reservoir of staphylococcal cassette chromosome mec (SCCmec) harboured by Staphylococcus aureus. However, data of SCCmec in CoNS are relatively absent particularly in China.
Methods
Seventy-eight CoNS clinical and 47 community isolates were collected in Beijing. PCR was performed to classify SCCmec types. Under oxacillin treatment, quantitative real-time reverse transcription PCR (qRT-PCR) was performed to compare mecA mRNA levels and mRNA half-life between isolates with single SCCmec element and those with multiple one. Their growth curves were analysed. Their bacterial cell wall integrity was also compared by performing a Gram stain. All ccr complex segments were sequenced and obtained ccr segments were analysed by phylogenetic analyses.
Results
All 78 clinical isolates had mecA segments compared with 38% in community isolates (total 47). Only 29% clinical isolates and 33% community isolates (among mecA positive isolates) harboured a single previously identified SCCmec type; notably, 17% clinical isolates and 28% community isolates had multiple SCCmec types. Further studies indicated that isolates with multiple SCCmec elements had more stable mecA mRNA expression compared with isolates with single SCCmec elements. CoNS with multiple SCCmec elements demonstrated superior cell wall integrity. Interestingly, phylogenetic analyses of obtained 70 ccr segments indicated that horizontal gene transfer of the ccr complex might exist among various species of clinical CoNS, community CoNS and S. aureus.
Conclusions
CoNS recovered from patients carried extremely diverse but distinctive SCCmec elements compared with isolates from the community. More attention should be given to CoNS with multiple SCCmec not only because they had superior cell wall integrity, but also because CoNS and S. aureus might acquire multiple SCCmec through the ccr complex.
Electronic supplementary material
The online version of this article (doi:10.1186/s12941-017-0231-z) contains supplementary material, which is available to authorized users.
Keywords: Coagulase-negative staphylococci, Multiple SCCmec, Bacterial cell wall integrity
Background
Coagulase-negative staphylococcus (CoNS) is a part of the commensal bacterial microflora of healthy people. However, with the development of interventional therapy and the increasing number of immunocompromised patients, these bacteria are becoming the most important causes of nosocomial infections [1, 2]. CoNS bloodstream infections have been estimated in as many as 250,000 cases annually in the US. The mortality rate of these infections is 1–25%, representing a great burden to the public health system [3]. The most common CoNS in nosocomial infection are Staphylococcus epidermidis, followed by Staphylococcus haemolyticus, Staphylococcus hominis and Staphylococcus capitis [4]. Another important reason for the increasing concern for CoNS is the fact that they also harbour SCCmec elements, which are found in methicillin-resistant S. aureus (MRSA). SCCmec elements harbour mec genes (mecA/mecC), providing resistance to methicillin and nearly all other beta-lactam antibiotics [5].
In general, SCCmec has two essential components, i.e., the mec gene complex and the cassette chromosome recombinase (ccr) gene complex. The mec gene complex consists of mecA/mecC, regulatory genes and associated insertion sequences and has been classified into five main classes, i.e., class A, class B, class C1, class C2, class D, which has been observed only in Staphylococcus caprae, and newly found class E [6, 7]. Encoding recombinases mediating integration and excision of SCCmec into and from the chromosome, ccr genes (ccrC or the pair of ccrA and ccrB) play an important role in the transfer of SCCmec elements [8]. The ccr gene(s) and surrounding genes form the ccr gene complex. At present, two distinct ccr gene complexes have been reported based on the composition of ccr genes, one carrying two adjacent ccr genes, ccrA and ccrB, and the second carrying ccrC. The ccrA and ccrB genes identified in S. aureus strains are categorized into four and five allotypes respectively, resulting in six ccr gene complex types, designated as type 1 (ccrA1B1), type 2 (ccrA2B2), type 3 (ccrA3B3), type 4 (ccrA4B4), type 7 (ccrA1B6) and type 8 (ccrA1B3). In contrast, all identified ccrC variants to date show high nucleotide similarity and are designed to only one allotype, ccrC1, constituting type 5 of ccr gene complex [7, 9]. Because of the high diversity of ccr gene complex and mec gene complex, an extensive genetic diversity of SCCmec elements has been revealed in S. aureus and a total of twelve types of SCCmec have been assigned for S. aureus based on the classes of the mec gene complex and ccr gene complex [9].
Previous studies have found that specific SCCmec elements, or components, exist in particular CoNS. For example, type IV was preferentially associated with S. epidermidis and type V was prevalently found in S. haemolyticus in the hospital [10, 11]. However, in recent years, more diverse SCCmec elements including non-mecA-encoding cassettes had been revealed from CoNS, and many SCCmec elements in methicillin-resistant CoNS (MR-CoNS) could not be typed using currently available schemes applied to MRSA. Moreover mecA gene has been found more widely distributed among CoNS than among S. aureus indicating a potential reservoir for the transfer of SCCmec elements to S. aureus [10, 12, 13]. However, only a small number of SCCmec elements of CoNS have been characterized in China [14]. In addition, the precise role of SCCmec elements of CoNS in the emergence and evolution of MRSA remains obscure, which requires characterization of additional SCCmec elements. Furthermore, multiple SCCmec have been found in CoNS recovered from patients in several studies [14, 15]. To obtain information on the SCCmec of local CoNS in Beijing and to reveal the function of multiple SCCmec in CoNS, clinical and community isolates were investigated and the features (including mecA mRNA quantity, mRNA half life, growth curve, bacterial cell wall integrity) of CoNS with multiple SCCmec elements were compared with those harbouring a single SCCmec element.
Methods
Sample collection and bacterial isolation
Only one isolate from each subject was collected and further analysed in the study. The demographic, hospital, and microbiological data were anonymously collected. Clinical CoNS isolates were collected from two hospitals in Beijing from July 2013 to December 2015. These CoNS strains were isolated from the blood of inpatients. The ages ranged from 34 to 98 years (mean ± SD, 50 ± 16). These patients were hospitalized for more than 48 h and were suspected of having a blood bacterial infection. Collected blood samples were inoculated into aerobic BacT/Alert FAN blood culture bottles and incubated in the BACT/Alert machine (bioMérieux, Marcy l’Etoile, France) for up to 5 days. Positive culture samples were directly inoculated onto Mueller–Hinton Broth (MH broth, Oxoid LID, Basingstoke, Hampshire, England) supplemented with 2% NaCl and incubated aerobically at 37 °C for 72 h. Species identification were determined using the Vitek II (bioMerieux, Durham, NC, USA) automated microbiology system and further confirmed by partially sequencing 16S rRNA genes amplified with primers 5F and 1194R and rpoB genes with primers 2491F and 3241R [13].
Community CoNS isolates were collected from healthy subjects (aged from 20 to 48) in two communities in Beijing in June 2016. Three groups of subjects, including office workers, construction workers and soldiers, were recruited. Samples were collected from the forehead and elbow with cotton swabs wetted with sterilized PBS. The swabs were placed into Mueller–Hinton Broth (MH broth, Oxoid LID, England) supplemented with 2% NaCl and incubated aerobically at 37 °C for 72 h. Ten microliters of culture suspected of bacteria growth were inoculated onto Brain Heart Infusion (BHI, Oxoid LID, England) agar and suggestive colonies with white color and smooth edge were subjected to screening tests with partial sequencing of 16S rRNA and rpoB genes as described above.
Detection of mec gene and SCCmec typing
The existence of the mecA gene was identified using primers met1/met2 and mecC gene with primers mecCF/mecCR [5, 16]. For mecA positive isolates, SCCmec typing was defined by the combination of ccr type and mec class, which were obtained using PCR [6, 9]. The mec class was assigned with five primers to identify the gene lineages of mecA–mecI (class A mec with primer mA7/mI6), mecA-IS1272 (class B mec with primer mA7/IS7), mecA-IS431 (class C mec with primer mA7/IS2) and mecA-IS431L (class C1 mec with primer mA7/IS2L). To further discriminate class C1 or C2 mec complexes, sequences between IS431 and mecA were examined using PCR with primer (IS431-F2 or IS431-R1) located in either direction of IS431 paired with primer mecA-R2 in mecA [14]. Five ccr gene complexes were identified with eight primers: four primers consisting of a common reverse primer (common to ccrB1-3, i.e., primer BC) and three forward primers specific for ccrA1, ccrA2, and ccrA3 to confirm ccr1–3 based on differences in ccrA genes; two primers to identify ccr4; and two primers to identify ccrC [6, 11].
For mecA negative isolates, ccr complexes were also analysed with primers as described above. The primers and lengths of amplicons used to identify the mec gene, mecA classes and ccr complexes are listed in Additional file 1: Table S1. PCR products of ccr complexes from all isolates were sent to Sangon Biotechnology Company (Sangon Biotech, Shanghai, China) for sequencing. The results were blasted with sequences in GenBank, and ccr genes with nucleotide identities more than 85% were designed to the same allotype [9].
Those with two mecA classes were designated CoNS with multiple SCCmec elements; those with one class of mecA complex and one ccr complex detected were classified into CoNS with a single SCCmec element. All strains with multiple SCCmec elements and those with single elements were further characterized.
Determination of minimal inhibitory concentrations (MIC) to oxacillin
For MIC determination, a broth microdilution broth susceptibility assay was performed according to CLSI guidelines [17]. The oxacillin (oxacillin sodium monohydrate, Sigma-Aldrich, St. Louis, MO, USA) concentration ranged from 256 to 0.125 μg/ml. The plates were incubated under normal atmospheric conditions for 24 h at 37 °C. The presence of a white pellet on the bottom of the tube indicated bacterial growth. The MIC value was identified by the lowest concentration of oxacillin at which no visible growth could be observed.
Growth curves of CoNS under oxacillin treatment
Samples of bacterial culture were prepared as follows: a single colony of the strain was cultured with MH broth overnight at 37 °C. Bacterial suspensions were diluted with MH broth to 0.5 McFarland standards and added to oxacillin (final concentration 2 μg/ml) and cultured at 37 °C. Two millilitres of culture was removed at the indicated time point. The growth curves were measured by plate counts on MHI agar (Oxoid LID, Basingstoke, Hampshire, England). The experiments were repeated three times, and the results were reported as an average of the replicate samples.
Quantification of mecA mRNA in CoNS under oxacillin treatment
Samples of bacterial culture were prepared as described above. Total RNA was extracted using the OMEGA bacterial RNA kit (OMEGA Biotech, Doraville, GA, USA) and eluted into 50 μl ddH2O. Three microliters of RNA was reverse transcribed into cDNA using the TransScript TM Two-Step RT-PCR Super Mix (TransGene Biotech, Beijing, China) in a total volume of 20 μl. Using standard PCR and real-time PCR, 2 μl of cDNA was used to evaluate and represent the quantity of total mecA mRNA in each 2 ml sample because it held the same proportion of total RNA in each sample.
For standard PCR, 2 μl of cDNA was amplified via PCR with TransScript 2× PCR Super Mix (Transgene Biotech, Beijing, China) in a total volume of 30 μl. The primer pairs (mecAF/mecAR) are shown in Additional file 1: Table S1 [18].
For qRT-PCR analyses, real-time PCR amplification was performed in a total volume of 25 μl containing 12.5 μl of 2× SYBR Fast qPCR Mix (TAKARA, Dalian, China), 1.0 μl primer and 2 μl template cDNA. The specific primers (mecA-1501F, mecA-1598R) used for the detection of mecA gene are listed in Additional file 1: Table S1 [19]. Data were presented as the relative copies of mecA mRNA levels compared with that of untreated CoNS with a single SCCmec element.
mecA mRNA half-life identification
Samples of bacterial culture were prepared as described above except with oxacillin cultured at 37 °C for 3 h. Transcriptional arrest was induced with actinomycin D as references except that the dosage of actinomycin D was modified to 2 μg/ml according to a preliminary experiment [20]. Two millilitres of culture was removed at the indicated time point. Total RNA was extracted and subjected to mecA mRNA analyses as described above.
Bacterial cell wall integrity assays
Samples of bacterial culture were prepared as described above except with 8 μg/ml oxacillin treatment. Samples were collected for Gram stain at 0, 1, and 3 h to visualize the bacterial cell wall integrity under a microscope.
Phylogenetic analyses of ccr
The reference sequences of the ccr complex in GenBank (Additional file 1: Table S2) and those derived here (Additional file 1: Tables S3, S4) were used to construct a phylogenetic tree. Using MEGA version 5.0, neighbour-joining trees were constructed with the maximum composite likelihood model assuming rate uniformity and pattern homogeneity.
Statistical analysis
Statistical analysis and graphic presentations were performed with Microsoft Excel XP software. The results are expressed as the average of three assays. A P value of 0.05 (Student’s t test) was considered significant.
Results
Sample collection
In this study, a total of 78 clinical CoNS isolates and 47 community CoNS isolates were recovered and identified to species.
Identification of CoNS
CoNS obtained from the clinic were classified into 4 different Staphylococcal species. These included S. epidermidis (n = 30), S. hominis (n = 20), S. capitis (n = 15) and S. haemolyticus (n = 13). However, most of the CoNS recovered from community were S. epidermidis (n = 40), and the other CoNS were few, including S. hominis (n = 5) and S. haemolyticus (n = 2).
Extremely diverse SCCmec types and multiple SCCmec elements
No mecC gene was detected in all these isolates. Not surprisingly, mecA was detected in 100% (78/78) clinic isolates compared to 38% (18/47) of the community isolates. The SCCmec typing results of these isolates are summarized in Table 1. Interestingly, only a small portion of CoNS were assigned as harbouring a single previously identified SCCmec type in both the clinical and community strains (23/78, 29% and 6/18, 33%, respectively). Those identified in clinical isolates included SCCmec type III (n = 7), type V (n = 7), type IV (n = 3), type VIII (n = 3), type II (n = 2), and type IX (n = 1). For community strains, only six strains of S. epidermidis were confirmed to harbour single SCCmec type II. Moreover, 11% (9/78) of clinical CoNS and 5% (1/18) of community isolates recovered carried a new single SCCmec type. Strains with an previously identified single SCCmec type or new single SCCmec type were assigned as CoNS with a single SCCmec element.
Table 1.
Detection of mecA segments and SCCmec typing results of CoNS isolates
| Character of SCCmec | Source | Species | mecA PCR | mecA class | ccr type | SCCmec type | No. of isolate | Example isolate |
|---|---|---|---|---|---|---|---|---|
| Previously identified single SCCmec type | Clinic | S. homi | + | A | 4 | VIII | 3 | H80, H86 |
| + | A | 2 | II | 1 | H59 | |||
| S. epid | + | A | 3 | III | 4 | H8 | ||
| + | B | 2 | IV | 3 | H67, H11 | |||
| + | C2 | 5 | V | 2 | H81 | |||
| + | A | 2 | II | 1 | ||||
| S. haem | + | C2 | 1 | IX | 1 | |||
| S. capi | + | A | 3 | III | 3 | H4 | ||
| + | C2 | 5 | V | 5 | H7, H26, H54, H60, H85 | |||
| Total number | 23 | |||||||
| Community | S. epid | + | A | 2 | II | 6 | ||
| Total number | 6 | |||||||
| New identified single SCCmec type | Clinic | S. homi | + | A | 1 | New | 3 | H29, H34 |
| S. haem | + | C1 | 4 | New | 5 | H2 | ||
| + | C1 | 2 | New | 1 | ||||
| Total number | 9 | |||||||
| Community | S. haem | + | C1 | 2 | New | 1 | CJ31-1 | |
| Total number | 1 | |||||||
| New type with a single mecA class and multi ccr complexes | Clinic | S. homi | + | A | 1, 4 | New | 2 | H1 |
| + | A | 1, 2 | New | 1 | H73 | |||
| + | A | 2, 4 | New | 2 | ||||
| + | A | 3, 4 | New | 1 | HA1 | |||
| + | A | 1, 4, 5 | New | 2 | H6 | |||
| + | A | 1, 3, 4, 5 | New | 1 | ||||
| S. epid | + | A | 1, 3 | New | 5 | H22 | ||
| + | A | 1, 3, 4 | New | 2 | H21 | |||
| + | A | 3, 4, 5 | New | 1 | H92 | |||
| + | C2 | 4, 5 | New | 1 | H76 | |||
| + | C2 | 1, 2, 5 | New | 1 | ||||
| + | B | 2, 4 | New | 3 | H24, H77 | |||
| S. haem | + | A | 1, 2, 4 | New | 1 | |||
| + | C2 | 2, 4 | New | 1 | H28 | |||
| + | C2 | 4, 5 | New | 1 | H68 | |||
| + | C2 | 1, 4 | New | 2 | H15, H36 | |||
| S. capit | + | A | 3, 4 | New | 1 | H23 | ||
| + | A | 1, 2, 4, 5 | New | 1 | H37 | |||
| + | A | 1, 2, 3, 5 | New | 1 | H83 | |||
| + | A | 1, 2, 3, 4, 5 | New | 1 | H72 | |||
| + | C2 | 2, 4, 5 | New | 1 | H78 | |||
| + | C2 | 1, 2, 4, 5 | New | 1 | ||||
| Total number | 33 | |||||||
| New type with a single mecA class and multi ccr complexes | Community | S. epid | + | A | 2, 4 | New | 1 | |
| + | A | 2, 4, 5 | New | 1 | ||||
| + | B | 1, 2 | New | 3 | ||||
| + | B | 2, 4 | New | 1 | ||||
| Total number | 6 | |||||||
| New type with multi SCCmec | Clinic | S. homi | + | A, C2 | 1, 4, 5 | New | 1 | |
| + | A, C2 | 1, 2, 4, 5 | New | 2 | H33-2 | |||
| + | A, C2 | 1, 2, 3, 4, 5 | New | 1 | H62 | |||
| S.epid | + | A, B | 2, 4, 5 | New | 1 | |||
| + | A, B | 2, 3, 5 | New | 1 | ||||
| + | A, B | 2, 3, 4, 5 | New | 1 | H30 | |||
| + | A, B | 1, 2, 3, 4, 5 | New | 1 | ||||
| + | A, C2 | 1, 5 | New | 1 | ||||
| + | A, C2 | 2, 4, 5 | New | 1 | H87 | |||
| + | A, C2 | 1, 2, 3, 4, 5 | New | 1 | H57 | |||
| S. haem | + | A, B | 1, 2, 3, 4 | New | 1 | HA11 | ||
| S.capit | + | A, C2 | 1, 2, 4, 5 | New | 1 | H14 | ||
| Total number | 13 | |||||||
| Community | S. homi | + | A, B | 2 | New | 1 | ||
| + | A, B | 1 | New | 1 | CV34 | |||
| + | A, B | 2, 4 | New | 1 | C3-1 | |||
| S. epid | + | A, B | 1, 2 | New | 1 | |||
| + | A, C2 | 2, 5 | New | 1 | ||||
| Total number | 5 | |||||||
| Isolates without mecA detected | Community | S. homi | − | − | 2 | N | 2 | C13-2 |
| S. epid | − | − | 2 | N | 15 | C5-1, CJ29 | ||
| − | − | 1, 2 | N | 1 | ||||
| − | − | 2, 5 | N | 2 | ||||
| − | − | 5 | N | 2 | CJ28-3 | |||
| − | − | 2, 4 | N | 1 | CV33-1 | |||
| − | − | – | N | 5 | ||||
| S. haem | − | − | 2, 4 | N | 1 | |||
| Total number | 29 | |||||||
S. homi, S. hominis; S. epid, S. epidermidis; S. haem, S. haemolyticus; S.capit, S. capitis, −, mecA negative; N, not available
Interestingly, 54% (42/78) of clinical isolates and 39% (7/18) of community isolates (including new single SCCmec types, new types with a single mecA class and multiple ccr complexes) could not be classified into any SCCmec type, most of them carried more than one ccr complex. Surprisingly, 17% (13/78) of the clinical isolates and 28% (5/18) of the community isolates had two classes of SCCmec types (assigned as CoNS with multiple SCCmec elements), particularly some clinical isolates that harboured five ccr complexes (i.e., ccrA1B1, ccrA2B2, ccrA3B3, ccrA4B4 and ccrC).
Strains (both clinical and community isolates) that did not fit into the SCCmec typing criteria, including those harbouring multiple SCCmec elements, were designated new SCCmec types (total n = 67), which accounted for approximately 70% of the total CoNS with mecA segments determined. It was unexpected that among the 29 community strains without a mecA or mecC segment detected, 24 (83%) contained a ccr complex. Furthermore, multiple ccr complexes were detected in 5 (21%) strains.
More stable mecA mRNA transcription in CoNS with multiple SCCmec elements compared to single elements
As shown in Fig. 1b, total mecA mRNA in CoNS with multiple SCCmec elements exhibited constant and stable expression during the 10 h experiment. However, total mecA mRNA in CoNS with single SCCmec element was transcribed unsteadily during this experimental course (Fig. 1a). To further assay mecA mRNA stability, mecA mRNA half-life was analysed in randomly selected S. epidermidis H67 and H30. As demonstrated in Fig. 1c, the mecA mRNA half-life was approximately 40–50 min in S. epidermidis H30. In contrast, mecA mRNA was very unstable in S. epidermidis H67, in which the mecA mRNA half-time probably was no more than 10 min.
Fig. 1.
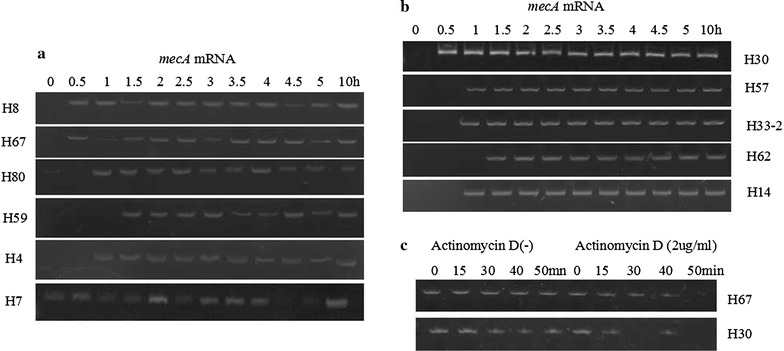
mRNA expression analyses of the amplified fragments of mecA in CoNS with single SCCmec elements (a) and those with multiple SCCmec elements (b). Two-millilitres culture of CoNS treated with oxacillin (2 μg/ml) for 10 h were used for mecA mRNA transcription analyses by reverse transcription PCR (RT-PCR); after treatment with oxacillin (2 μg/ml) and actinomycin D (2 μg/ml), mecA mRNA half-lives were determined by RT-PCR (c). mRNA expression levels were described in terms of intensity using Quantity One Imager. Data are shown as the image of three independent experiments with similar results
Quantitative RT-PCR analyses of randomly selected isolates further confirmed that the total mecA mRNA in S. epidermidis H30 demonstrated continuously sustainable expression during the 10 h experiment. In contrast, the total mecA mRNA in S. epidermidis H8 showed very unstable transcription during this experimental course. Furthermore, the number of mecA mRNA relative copies of S. epidermidis H8 at many time points was significantly less than that of S. epidermidis H30 (lower right panel in Fig. 2). However, no significant differences in growth curves were observed between these two isolates (upper left panel in Fig. 2). Moreover, qRT-PCR of mecA mRNA transcription and growth curve assays in other S. epidermidis isolates and CoNS species also demonstrated the same phenomenon (Additional file 2: Figure S1).
Fig. 2.
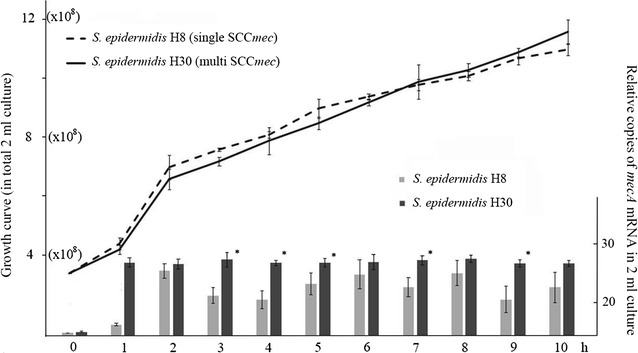
Growth curve indicated total cells in a 2-ml culture of S. epidermidis H8, H30 treated with oxacillin at each time point for 10 h (upper left). The corresponding relative total mecA mRNA in 2-ml sample treated with oxacillin at each time point was measured by quantitative RT real-time PCR (lower right). Data were presented as the relative copies of mecA mRNA levels compared with that of S. epidermidis H8 (0 h). Each bar represents the mean ± SD of at least three independent experiments. *P < 0.05 between two strains at each time point
CoNS with multiple SCCmec elements demonstrated better bacterial cell wall integrity than those carrying a single element
Randomly selected S. epidermidis H30, H57 (with multiple SCCmec elements) and S. epidermidis H8, H67 (with single SCCmec) were recruited for analyses of bacterial cell wall integrity. As shown in Fig. 3, most cells of S. epidermidis H57, H30 still demonstrated Gram-positivity after 3 h treatment of oxacillin (91.6 ± 1.3, 82.6 ± 2.8%, respectively). In contrast, a smaller number of cells of S. epidermidis H8, H67 demonstrated Gram-positivity at 3 h under oxacillin treatment (12.3 ± 1.7, 14.2 ± 2.6%, respectively). The differences between S. epidermidis H30, H57 and S. epidermidis H8, H67 were significant (P < 0.001).
Fig. 3.
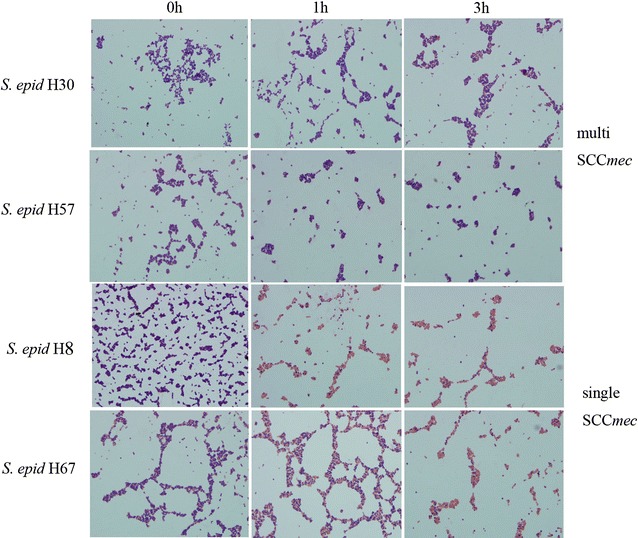
Bacterial cell wall integrity under oxacillin treatment (8 μg/ml). Samples were collected for Gram staining at 0, 1, and 3 h to visualize the bacterial cell wall under a microscope. The proportion of bacteria cells demonstrated as Gram-negative among every 100 total cells, was manually calculated according to three microscope fields for each sample. The results are shown as the mean value of three microscope fields. Data are shown as the images of three independent experiments with similar results
Phylogenetic association of ccr among CoNS and S. aureus
A total of 70 ccr complex segments were sequenced successfully in this study, including 60 clinical isolates and 10 community isolates (Additional file 1: Tables S3, S4). No new ccr allotypes or alleles were identified. The ccrAB alleles were assigned based on a BLAST search with sequences in GenBank, resulting in 13 ccrA1B1, 13 ccrA2B2, 9 ccrA3B3, 10 ccrA4B4 and 15 ccrC from clinical CoNS and 1 ccrA1B1, 5 ccrA2B2, 3 ccrA4B4, and 1 ccrC from community CoNS. As shown in Fig. 4, all species of CoNS recovered from patients contained various types of ccr complexes except ccrA3B3, which had not been discovered from S. haemolyticus in this study. For community isolates, ccrA3B3 had not been recovered in this study. In each phylogenetic tree, ccr segments recovered from clinical CoNS were grouped with those from community isolates in this study and those from around the world. No specific cluster formed for either the clinical isolates or community isolates.
Fig. 4.
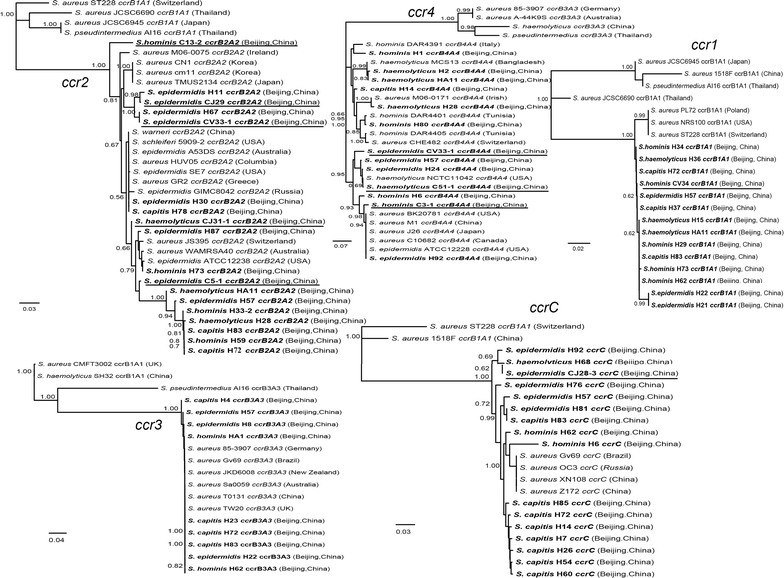
Neighbour-joining tree based on ccr sequences determined in this study and those downloaded from GenBank. Those with bold italic font represent ccr sequences recovered in this study and those underlined represent isolates from the community
Importantly, ccrA3B3 segments in clinical CoNS demonstrated high similarity (nearly 100%) with those in S. aureus worldwide. In contrast, ccrA1B1 segments from both the clinic and community were grouped together but separated from those found in S. aureus. Interestingly, ccrA2B2, ccrA4B4 and ccrC showed an intermediate state, i.e., some clustered with those in S. aureus and others had separated from them.
Discussion
In general, the existence of different types of SCCmec in MR-CoNS is dependent on the host species and geographical locations [11]. In this study, SCCmec types, II, III, and V were prevalent in MR-CoNS in Beijing. Another study performed from southwest China demonstrated that SCCmec types III, IV and V were dominant in MR-CoNS [14]. Moreover, specific SCCmec types (in some instances, specific ccr or mec complex genes) were found particularly in specific CoNS species. Consistent with previous reports, type IV SCCmec was preferentially associated with S. epidermidis in our study. However, most type V SCCmec had been recovered in S. capitis in this study, unlike previous study which demonstrated type V dominates in S. haemolyticus [10–12]. Although in this study class C mec was dominantly associated with S. haemolyticus as previous reports [21, 22], most S. haemolyticus isolates (clinic or community) carried new SCCmec type. We also did not find type VII SCCmec in CoNS in this study as other researchers; however, one isolate of type IX was disclosed in S. haemolyticus in clinic, which had not been previously confirmed in CoNS [12, 23]. A recent study also reported SCCmec type IX in CoNS in the community [24]. Significantly, most CoNS strains recovered in this study (clinic and community) consisted of untypable SCCmec elements as many other reports [14–16, 23, 25]. Importantly, the differences between clinical CoNS and community isolates might result from sampling bias as most CoNS recovered from the community were S. epidermidis.
Intriguingly, various combinations of ccr types were revealed in a single CoNS strain, including clinical and community isolates in this study. Multiple copies of ccr complex have been reported in S. aureus, S. epidermidis and other CoNS. However, most are combinations of ccrAB and ccrC [6, 25]. To the best of our knowledge, this is the first report of a heterogeneous combination of ccr complex in a single CoNS strain, particularly all five types of ccr complexes existing in a single clinical isolate. Thus, it was not surprising that these CoNS strains contained multiple SCCmec elements. Multiple SCCmec elements have been reported in clinical MR-CoNS and the incidences were as high as that observed in our finding [6, 14]. Although it was likely that the two SCCmec elements actually constituted a composite rather than two independent units, multiple copies of mecA existed in one single CoNS strain both in the clinic and community as revealed in our study. Intriguingly, whether the existence of multiple SCCmec in community isolates was attributed to spill over from the hospital or to antibiotic abuse in the community requires further investigation.
It is well-known that acquisition of antibiotic resistance in bacterial cells is often accompanied by fitness cost in the absence of antibiotics, most of which demonstrated slower growth rates and finally resulted in the dilution of antibiotic resistant genes [26]. However, we did not identify any significant differences in the growth rates between CoNS strains with multiple SCCmec and those with a single isolate with or without oxacillin, and no correlation of multiple SCCmec with MIC in response to oxacillin was disclosed (data not shown). We speculated that MIC in response to oxacillin might also be correlated with other antibiotic genes [27]. Interestingly, we demonstrated that multiple SCCmec elements in CoNS strains ensure stable and continuous transcription of antibiotic-resistant genes, i.e., mecA gene, whose transcript, PBP2a, was capable of maintaining cell wall integrity [28]. Further analyses by Gram staining demonstrated that the cell wall in CoNS with multiple SCCmec demonstrated much stronger resistance and better integrity under oxacillin treatment than those CoNS with single SCCmec element. Interestingly, although the bacteria cell number dramatically increased, total mecA mRNA levels sustained constantly from 1 h to 10 h incubation with oxacillin. We suspected that the amount of mecA mRNA at the 1 h time point was sufficient to resist the antibiotics added into the culture.
Finally, phylogenetic analyses of ccr indicated potential horizontal gene transfer among different CoNS species of clinic and community isolates, even among CoNS and S. aureus. In particular, nearly 100% similarity of all ccrA3B3 might result from a recent gene transfer among different CoNS species and S. aureus. As observed in previous studies [16], we also detected the ccr complex in mec negative strains. Specifically, some community CoNS strains had multiple ccr but lacked the mec gene. Their potential to acquire mec genes with these ccr complexes requires further attention. However, the limitation of our work is that we could not determine which ccr complex was linked to the specific SCCmec, particularly for those that had two types of mecA classes (multiple SCCmec). The presence of untypable and multiple SCCmec elements represent great challenges for SCCmec typing in MR-CoNS [11, 14, 15, 23]. Whole genome sequencing of more MR-CoNS would be helpful to construct a new typing method through understanding the relative position and precise composition of multiple SCCmec and to further elucidate the role of ccr complexes in spreading SCCmec elements among CoNS and S. aureus.
Conclusions
Overall, CoNS recovered in Beijing carried extremely diverse SCCmec elements including multiple SCCmec elements, which demonstrated superior cell wall integrity. Our data revealed potential horizontal gene transfer among different CoNS species of clinic and community isolates, even among CoNS and S. aureus.
Additional files
Additional file 1: Table S1. Primers used for mec gene detection and SCCmec Typing. Table S2. The ccr gene sequences obtained from GenBank for phylogenetic analyses. Table S3. The ccr gene sequences obtained in this study for phylogenetic analyses. Table S4. Origins of the CoNS strains whose ccr segments were applied to phylogenetic analyses.
Additional file 2: Figure S1. Growth curve indicating the total cells in a 2-ml culture of CoNS treated with oxacillin at each time point for 10 h (upper left in panel A, B, and C). The corresponding relative total mecA mRNA in the 2-ml sample treated with oxacillin at each time point was measured by quantitative RT real-time PCR (lower right in each panel). Data are presented as the relative copies of mecA mRNA levels compared with that of S. epidermidis H8 (0 h). Each bar represents the mean±SD of at least three independent experiments. *P<0.05 between two strains at each time point.
Authors’ contributions
XPC, SML and JXL wrote the manuscript. XPC, WGL and HZ implemented PCR and sequence analyses; HYD, LZ, and LZ performed bacterial culture experiments; JC and YW did raw data analyses. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files 1 and 2.
Ethics approval and consent to participate
The ethics committee of National Institute of Communicable Disease Control and Prevention approved the protocol before the beginning of this research.
Funding
This work was supported by the National Key Technology Support Program (2012BAI11B05) and National Natural Science Foundation of China (Grants 81201250).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CoNS
coagulase-negative staphylococci
- SCCmec
staphylococcal cassette chromosome mec
- ccr
cassette chromosome recombinase
- MIC
minimal inhibitory concentrations
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12941-017-0231-z) contains supplementary material, which is available to authorized users.
Xiao-Ping Chen and Wen-Ge Li contributed equally to this work
Contributor Information
Xiao-Ping Chen, Email: chenxiaoping@icdc.cn.
Wen-Ge Li, Email: liwenge@icdc.cn.
Hao Zheng, Email: zhenghao@icdc.cn.
Hai-Yan Du, Email: duhh099123@sina.com.
Li Zhang, Email: zhangll8820@sina.com.
Lei Zhang, Email: zhang088463@sina.com.
Jie Che, Email: chejie@icdc.cn.
Yuan Wu, Email: wuyuan@icdc.cn.
Shu-Mei Liu, Phone: 86-10-88062158, Email: lsmlucky@126.com.
Jin-Xing Lu, Phone: 86-10-58900763, Email: lujinxing6688@yahoo.com.
References
- 1.Ghazal SS, Stevens MP, Bearman GM, Edmond MB. Utility of surveillance blood cultures in patients undergoing hematopoietic stem cell transplantation. Antimicrob Resist Infect Control. 2014;3:20. doi: 10.1186/2047-2994-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widerström M. Significance of Staphylococcus epidermidis in health care-associated infections, from contaminant to hospital relevant pathogen: this is a wake-up call! J Clin Microbiol. 2016;54:1679–1681. doi: 10.1128/JCM.00743-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis. 2007;7:645–657. doi: 10.1016/S1473-3099(07)70235-9. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Speer CP. The role of Staphylococcus epidermidis in neonatal sepsis: guarding angel or pathogenic devil? Int J Med Microbiol. 2014;304:513–520. doi: 10.1016/j.ijmm.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 5.García-Álvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011;8:595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turlej A, Hryniewicz W, Empel J. Staphylococcal cassette chromosome mec (SCCmec) classification and typing methods: an overview. Pol J Microbiol. 2011;60:95–103. [PubMed] [Google Scholar]
- 8.Misiura A, Pigli YZ, Boyle-Vavra S, Daum RS, Boocock MR, Rice PA. Roles of two large serine recombinases in mobilizing the methicillin-resistance cassette SCCmec. Mol Microbiol. 2013;88:1218–1229. doi: 10.1111/mmi.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Working Group on the Classification of Staphylococcal Cassette chromosome elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fessler AT, Billerbeck C, Kadlec K, Schwarz S. Identification and characterization of methicillin-resistant coagulase-negative staphylococci from bovine mastitis. J Antimicrob Chemother. 2010;65:1576–1582. doi: 10.1093/jac/dkq172. [DOI] [PubMed] [Google Scholar]
- 11.Ruppé E, Barbier F, Mesli Y, Maiga A, Cojocaru R, Benkhalfat M, et al. Diversity of staphylococcal cassette chromosome mec structures in methicillin-resistant Staphylococcus epidermidis and Staphylococcus haemolyticus strains among outpatients from four countries. Antimicrob Agents Chemother. 2009;53:442–449. doi: 10.1128/AAC.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahem S, Salmenlinna S, Virolainen A, Kerttula AM, Lyytikäinen O, Jägerroos H, et al. Carriage of methicillin-resistant Staphylococci and their SCCmec types in a long-term-care facility. J Clin Microbiol. 2009;47:32–37. doi: 10.1128/JCM.01085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petti CA, Simmon KE, Miro JM, Hoen B, Marco F, Chu VH, International Collaboration on Endocarditis-Microbiology Investigators et al. Genotypic diversity of coagulase-negative staphylococci causing endocarditis: a global perspective. J Clin Microbiol. 2008;46:1780–1784. doi: 10.1128/JCM.02405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong Z, Peng C, Lü X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci hospital isolates. PLoS ONE. 2011;6:e20191. doi: 10.1371/journal.pone.0020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tulinski P, Fluit AC, Wagenaar JA, Mevius D, van de Vijver L, Duim B. Methicillin-resistant coagulase-negative staphylococci on pig farms as a reservoir of heterogeneous staphylococcal cassette chromosome mec elements. Appl Environ Microbiol. 2012;78:299–304. doi: 10.1128/AEM.05594-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanssen AM, Kjeldsen G, Sollid JU. Local variants of staphylococcal cassette chromosome mec in sporadic methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci: evidence of horizontal gene transfer? Antimicrob Agents Chemother. 2004;48:285–296. doi: 10.1128/AAC.48.1.285-296.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M07-A8. 8. CLSI: Wayne; 2009. [Google Scholar]
- 18.Lee JW, Ji YJ, Lee SO, Lee IS. Effect of Saliva miltiorrhiza bunge on antimicrobial activity and resistant gene regulation against methicillin-resistant Staphylococcus aureus (MRSA) J Microbiol. 2007;45:350–357. [PubMed] [Google Scholar]
- 19.Wang HY, Kim S, Kim J, Park SD, Uh Y, Lee H. Multiplex real-time PCR assay for rapid detection of methicillin-resistant staphylococci directly from positive blood cultures. J Clin Microbiol. 2014;52:1911–1920. doi: 10.1128/JCM.00389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Yoza BK, El Gazzar M, Hu JY, Cousart SL, McCall CE. RelB sustains IkappaBalpha expression during endotoxin tolerance. Clin Vaccine Immunol. 2009;16:104–110. doi: 10.1128/CVI.00320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berglund C, Söderquist B. The origin of a methicillin-resistant Staphylococcus aureus isolate at a neonatal ward in Sweden-possible horizontal transfer of a staphylococcal cassette chromosome mec between methicillin-resistant Staphylococcus haemolyticus and Staphylococcus aureus. Clin Microbiol Infect. 2008;14:1048–1056. doi: 10.1111/j.1469-0691.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 22.Bouchami O, Ben Hassen A, de Lencastre H, Miragaia M. High prevalence of mec complex C and ccrC is independent of SCCmec type V in Staphylococcus haemolyticus. Eur J Clin Microbiol Infect Dis. 2012;31:605–614. doi: 10.1007/s10096-011-1354-3. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Wang X, Gao Q, Lu Y. Molecular characterization of Staphylococcus epidermidis strains isolated from a teaching hospital in Shanghai, China. J Med Microbiol. 2009;58:456–461. doi: 10.1099/jmm.0.007567-0. [DOI] [PubMed] [Google Scholar]
- 24.Sinlapasorn S, Lulitanond A, Angkititrakul S, Chanawong A, Wilailuckana C, Tavichakorntrakool R, et al. SCCmec IX in meticillin-resistant Staphylococcus aureus and meticillin-resistant coagulase-negative staphylococci from pigs and workers at pig farms in Khon Kaen, Thailand. J Med Microbiol. 2015;64:1087–1093. doi: 10.1099/jmm.0.000119. [DOI] [PubMed] [Google Scholar]
- 25.Hanssen AM, Sollid JU. Multiple staphylococcal cassette chromosomes and allelic variants of cassette chromosome recombinases in Staphylococcus aureus and coagulase-negative staphylococci from Norway. Antimicrob Agents Chemother. 2007;51:1671–1677. doi: 10.1128/AAC.00978-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki S, Horinouchi T, Furusawa C. Phenotypic changes associated with the fitness cost in antibiotic resistant Escherichia coli strains. Mol BioSyst. 2016;12:414–420. doi: 10.1039/C5MB00590F. [DOI] [PubMed] [Google Scholar]
- 27.Wassenaar TM, Ussery D, Nielsen LN, Ingmer H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol (Bp). 2015;5:44–61. doi: 10.1556/EuJMI-D-14-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemeyer DM, Pucci MJ, Thanassi JA, Sharma VK, Archer GL. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1996;178:5464–5471. doi: 10.1128/jb.178.18.5464-5471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primers used for mec gene detection and SCCmec Typing. Table S2. The ccr gene sequences obtained from GenBank for phylogenetic analyses. Table S3. The ccr gene sequences obtained in this study for phylogenetic analyses. Table S4. Origins of the CoNS strains whose ccr segments were applied to phylogenetic analyses.
Additional file 2: Figure S1. Growth curve indicating the total cells in a 2-ml culture of CoNS treated with oxacillin at each time point for 10 h (upper left in panel A, B, and C). The corresponding relative total mecA mRNA in the 2-ml sample treated with oxacillin at each time point was measured by quantitative RT real-time PCR (lower right in each panel). Data are presented as the relative copies of mecA mRNA levels compared with that of S. epidermidis H8 (0 h). Each bar represents the mean±SD of at least three independent experiments. *P<0.05 between two strains at each time point.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional files 1 and 2.


