Several years ago, the polyketide natural product frenolicin B, produced by the actinomycete Streptomyces roseofulvus, underwent preliminary testing for use as an anticoccidial agent in poultry, owing to its promising activity against Eimeria tenella.1 To probe its pharmacophore, a limited structure activity relationship study was undertaken using a series of ~20 semisynthetic derivatives of frenolicin B modified at the 1, 2, 4, 8, 10 and 11 positions.2–4 All variants resulted in a loss of potency except for esterification of the phenol at position 11. This esterified derivative likely acted as a pro-drug, increasing the in vivo efficacy of frenolicin by a factor of 2.3 Aside from its oral bioavailability in poultry, frenolicin B is tolerated with no acute toxicity in rats fed diets containing 100 mg kg−1 per day, though some chronic effects potentially stemming from chelation of dietary copper were noted after 4 weeks.5 To further explore the scope of frenolicin B’s antiparasitic activity, we investigated its efficacy against the human malarial pathogen Plasmodium falciparum. This Apicomplexan parasite infects ~225 million people and is responsible for ~780 000 deaths annually. We also explored the antimalarial efficacy of frenolicin B in vivo using the murine malaria model P. berghei.
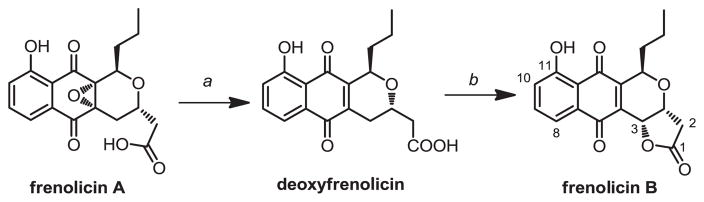
Frenolicins are produced by S. roseofulvus in three isolable forms (Figure 1).6 Based on earlier work by Whaley et al.7 we developed a simple method to convert frenolicin A to deoxyfrenolicin, followed by lactonization to convert deoxyfrenolicin to frenolicin B.8 This method gives high yields and conversion efficiencies when performed on pure frenolicin A (>80% yield over two steps). Importantly, it can be performed on crude isolate flushed through a silica column. Additionally the method requires no purification of the deoxyfrenolicin intermediate between the de-epoxidation and lactonization steps. Details are provided in the Experimental procedure.
Figure 1.
Conversion of S. roseofulvus frenolicins to frenolicin B. a: H2, PtO2 in methanol at 0 °C for 30 min; b: bubbled O2, pyridine, in methanol at 60 °C overnight.
With purified frenolicin B in hand, we tested its effectiveness in vitro against three laboratory strains of P. falciparum: HB3 (chloroquine and amodiaquine susceptible); Dd2 (highly chloroquine resistant and moderately amodiaquine resistant) and 7G8 (moderately chloroquine resistant and highly amodiaquine resistant).9 Parasite growth inhibition by frenolicin B was tested in vitro and the ICs of 50 (IC50) and 90 (IC90) percent of parasite population were determined using a standard 72 h antimalarial response assay, which uses SYBR green to detect DNA and estimates parasite multiplication10 (see Experimental procedure for details). To evaluate whether common liver enzymes would metabolize frenolicin B and affect its antiparasitic activity, the drug was exposed to a panel of human cytochrome P450 enzymes (CYPs) and then retested against each parasite strain by standard growth inhibition assay (see Experimental procedure for details). Parasite in vitro growth inhibition by frenolicin B was similar against each of the different strains, with IC50 values in the range of 600–800 nM (Table 1), implying that the natural product’s mode of action is distinct from chloroquine and amodiaquine. Additionally, similar or lower IC50 values obtained for frenolicin B after incubation with cytochrome P450 enzymes suggests the drug is not metabolized or if so, its potency is improved by the liver enzymes tested, prompting us to investigate its activity in vivo.
Table 1.
Growth inhibition of three strains of Plasmodium falciparum by frenolicin B
| Strain | Frenolicin B | P450 | 1A2 | 2C8 | 2C9 | 2C19 | 2D6 | 3A4 |
|---|---|---|---|---|---|---|---|---|
| IC50 values (nM)a | ||||||||
| HB3 | 600±100 (8) | 1000±190 (7) | 800±260 (4) | 600±200 (4) | 600±150 (4) | 600±160 (4) | 700±210 (4) | 700±190 (4) |
| Dd2 | 800±200 (10) | 800±150 (7) | 600±130 (4) | 490±70 (4) | 600±140 (4) | 500±100 (4) | 600±120 (4) | 600±140 (4) |
| 7G8 | 800±150 (8) | 1400±260 (7) | 490±30 (2) | 400±20 (2) | 470±5 (2) | 430±30 (2) | 520±20 (2) | 480±2 (2) |
| IC90 values (nM)b | ||||||||
| HB3 | 1500±240 | 3100±750 | 2000±720 | 1600±580 | 1700±590 | 1500±440 | 2000±740 | 1800±630 |
| Dd2 | 2100±490 | 2800±440 | 1800±460 | 2100±660 | 1600±530 | 1500±460 | 2100±670 | 1800±590 |
| 7G8 | 2000±440 | 7400±2917 | 1100±60 | 850±1 | 990±7 | 1000±20 | 1270±60 | 960±50 |
Values are reported as IC50±s.e. (number of replicates).
Values are reported as IC90±s.e.
The number of replicates is the same as in the IC50 values above. The column P450 refers to frenolicin B incubated with a variety of P450 s, whereas the latter columns refer to frenolicin B incubated with a specific P450 isoform.
We chose to test the compound in a 4-day P. berghei suppression model in 8-week-old CD1 mice at doses of 10, 20, 40 and 63 mg kg−1 per day. For each dosing level, a cohort of five mice inoculated with 1×107 P. berghei parasites was given a daily oral gavage of frenolicin B for 4 days beginning 1 h after inoculation. On the fifth day the mice were killed, their blood was drawn and parasitemia levels were determined (that is the percent of infected red blood cells; see Experimental procedure for details). Table 2 shows the parasitemia levels of the mice on day 5 versus an uninfected control, as well as the number of mice cured in each group. Mice were designated cured if their parasitemia level was <1.0%. A dose-dependent trend in both the parasitemia levels and number of cured mice could be observed. At the dose of 63 mg kg−1, parasitemias were reduced by >50% and two of five mice were cured. This activity was still considerably lower than piperaquine, a drug used in combination therapy with the artemisinin derivative dihydroartemisinin (Table 2). These data illustrate that frenolicin B has a dose-dependent, orally bioavailable activity against P. berghei in mice.
Table 2.
In vivo efficacy of frenolicin B in mice
| Dose (mg kg−1 per day) | Percentage of parasitemia±s.d. | No. of mice cured/totala |
|---|---|---|
| Vehicle | 31±6.4 | 0/5 |
| 10 | 39±13 | 0/5 |
| 20 | 37±6.3 | 0/5 |
| 40 | 33±19 | 1/5 |
| 63 | 15±16 | 2/5 |
| 2.5 (piperaquine) | 1.4±0.7 | 2/5 |
| 5.0 (piperaquine) | 0 | 5/5 |
Each cohort contained five mice whose parasitemia level was determined after 4 days of daily dosing. Each mouse that had a parasitemia <1.0 % was deemed cured. As a control for the efficacy of the in vivo assay, mice were also treated with piperaquine at a dose of 2.5 or 5.0 mg kg−1 per day.
In summary we have shown that frenolicin B possesses good antiparasitic activity against P. falciparum in vitro and orally against P. berghei in mice. Additionally, frenolicin B appears to exert its bioactivity via a different mode of action than chloroquine and amodiaquine, making it a potentially useful starting point in the development of new agents to treat drug-resistant strains of malaria.
EXPERIMENTAL PROCEDURE
Isolation of frenolicins from S. roseofulvus and conversion to frenolicin B
S. roseofulvus was cultured on semi-solid R5 plates as previously described.11 Optimization of antibiotic production showed a maximum yield of frenolicins after 4 days of growth at 30 °C, with frenolicin B, deoxyfrenolicin and frenolicin A produced in a ratio of ~1:20:5. After growth, the agar was mashed and extracted 1:1 with 1% acetic acid in 99% ethyl acetate overnight, followed by a second overnight extraction. Purification was performed by flushing the crude extract through a silica gel column using 1% acetic acid in 99% ethyl acetate as the mobile phase. The eluate was dried in vacuo and subjected to the procedure outlined in Figure 1 to convert all frenolicins present to frenolicin B. It was found that both the de-epoxidation and lactonization reactions described below could be run on this silica gel eluate instead of pure frenolicin A with a minimal loss of yield.
As an example of the reaction run on pure compound, 160 mg frenolicin A (0.46 mmol) was dissolved in 10 ml anhydrous methanol (Acros Organics, Fair Lawn, NJ, USA) and added to a round bottom flask containing 21 mg (20% mol cat.) platinum (IV) oxide (Sigma-Aldrich, St Louis, MO, USA) under nitrogen. Hydrogen gas was bubbled through the mixture for one min before the flask was placed under hydrogen atmosphere and the mixture stirred for 30 min at O °C. The mixture was then filtered through celite to remove any accumulated Pt black, the filtrate was dried and redissolved in 50 ml methanol, and then added to a 250 ml round-bottom flask. To this mixture 365 mg pyridine was added (4.6 mmol) before heating at 60 °C overnight while bubbling air through the solution. The solvent was then evaporated yielding 150 mg of material, which showed near complete conversion (>95%) of the frenolicin A to frenolicin B by NMR.
After conversion of the frenolicins in this crude material to frenolicin B through the method outline in Figure 1, frenolicin B was isolated by preparative HPLC on a Beckman 126 solvent module equipped with at Beckman 166 UV detector (Beckman Coulter, Brea, CA, USA). The material was dissolved in 3 ml methanol and injected on a reverse-phase column (250 mm ×22 mm, C18, Vydac, Deerfield, IL, USA). A gradient starting at 15% acetonitrile in water was increased to 40% over 20 min, then increased to 46% over 50 min, yielding 10 mg l−1 of isolated product (see Fitzgerald et al.12 for characterization).
Testing of frenolicin B against P. falciparum in vitro
P. falciparum parasites were cultured in vitro and their dose-response profiles were measured as described elsewhere.9,10 Briefly, parasites were maintained in complete RPMI 1640 media (KD Medical, Columbia, MD, USA) containing L-glutamine, 25 mM Hepes, 50 μg ml−1 hypoxanthine, 10 μg ml−1 gentamicin, 0.225% NaHCO3 and 0.5% Albumax I (Invitrogen, Carlsbad, CA, USA) at 37°C under an atmosphere of 5% CO2/5% O2/90% N2. Frenolicin B was dissolved in dimethyl sulfoxide and serially diluted from 80 to 0.002 μM in 96-well plates. Synchronized ring-stage parasite cultures at 1% parasitemia were incubated with the various drug concentrations for 72 h when cells were harvested and exposed to SYBR Green for parasite growth quantification. Percentage of growth inhibition was calculated by the ratio of SYBR Green signal from each well with the drug over the signal obtained from wells with no drug (control wells with maximum parasite growth). Growth inhibition curves, IC50 and IC90 values, and statistical analyses were determined using GraphPad Prism software (GraphPad Software, San Diego, CA, USA) and Excel. An in vitro method to metabolize frenolicin B and other antimalarials was developed by pre-incubating the drugs with various recombinant cytochrome P450 enzymes and using the supernatant to perform the serial dilutions for the in vitro growth inhibition assay described above (O Twu, M Krause and JM Sá, unpublished data to be submitted to Malaria Journal in 2011).
Testing of frenolicin B against P. berghei in vivo
Frenolicin B was dissolved in 100 μl of 0.5% aqueous hydroxyethylcellulose, with 0.1% Tween 80 for dosing by oral gavage in 8-week-old CD1 mice (Charles River Laboratories, Wilmington, MA, USA). Mice were inoculated with 107 parasites by i.p. injection 1 h before first dosing. Following 4 days of daily dosing, parasitemia was measured on day 5. For this, tail vein blood was collected in citrate phosphate dextrose solution/phosphate-buffered saline supplemented with 5% fetal bovine serum. Infected erythrocytes were fluorescently stained using SYBR Green I (Invitrogen) combined with MitoTracker Deep Red (Invitrogen) in 1×phosphate-buffered saline, pH 7.3, containing 5% fetal bovine serum.13 Cells were labeled for 40 min at 37 °C and dual-channel fluorescence was detected with an Accuri C6 (Becton-Dickinson, Franklin Lakes, NJ, USA) attached to a Hypercyt sampler (Intellicyt, Albuquerque, NM, USA). SYBR Green was excited with a 488-nm diode laser and emission was detected with a 530 nm filter, whereas a 640-nm laser was used for MitoTracker Deep Red (Invitrogen) with a 675 nm filter. Parasitemias were determined following sample analysis using FlowJo software (Tree Star, OR, USA).
Acknowledgments
This research was partially supported by a grant from the National Institutes of Health to CK (R01 CA 77248) and to DF (R01 AI079709), and by the Intramural Research Program of the NIH, NIAID.
References
- 1.Omura S, Tsuzuki K, Iwai Y. Anticoccidal activity of frenolicin B and its derivatives. J Antibiot. 1985;38:1447–1448. doi: 10.7164/antibiotics.38.1447. [DOI] [PubMed] [Google Scholar]
- 2.Armer RE, et al. Anticoccidial activity of novel semi-synthetic analogues of deoxyfrenolicin and frenolicin B (part II) Heterocycl Commun. 1998;4:345–350. [Google Scholar]
- 3.Armer RE, et al. Anticoccidial activity of novel semi-synthetic analogues of deoxyfrenolicin and frenolicin B (part I) Heterocycl Commun. 1998;4:309–315. [Google Scholar]
- 4.Armer RE, et al. Carbocyclic frenolicin analogues: novel anticoccidial agents. Bioorg Med Chem Lett. 1998;8:139–142. doi: 10.1016/s0960-894x(97)10200-1. [DOI] [PubMed] [Google Scholar]
- 5.Kossor DC, et al. Administration of the oral antibiotic frenolicin-B selectively alters copper nutriture in male rats. J Nutr. 2001;131:3247–3250. doi: 10.1093/jn/131.12.3247. [DOI] [PubMed] [Google Scholar]
- 6.Iwai Y, et al. Production of deoxyfrenolicin and a new antibiotic, frenolicin B by Streptomyces roseofulvus strain AM-3867. J Antibiot. 1978;31:959–965. doi: 10.7164/antibiotics.31.959. [DOI] [PubMed] [Google Scholar]
- 7.Whaley HA, Ellestad GA. US patent #3452051. Deoxyfrenolicins. 1969 [Google Scholar]
- 8.Brimble MA, Narin MR, Prabaharan H. Synthetic strategies towards pyranonaphthoquinone antibiotics. Tetrahedron. 2000;56:1937–1992. [Google Scholar]
- 9.SJM, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci USA. 2009;106:18883–9. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smilkstein M, et al. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. The John Innes Foundation; Norwich, UK: 2000. pp. 472–481. [Google Scholar]
- 12.Fitzgerald JT, Ridley CP, Khosla C. Engineered biosynthesis of the antiparasitic agent frenolicin B and rationally designed analogs in a heterologous host. J Antibiot. doi: 10.1038/ja.2011.86. e-pub ahead of print 21 September 2011. doi:10:1038/ja.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekland EH, Schneider J, Fidock DA. Identifying apicoplast targeting antimalarials using high-throughput compatible approaches. FASEB J. 2011 doi: 10.1096/fj.11-187401. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]