Abstract
Background
Early prognosis in comatose survivors after cardiac arrest due to ventricular fibrillation (VF) is unreliable, especially in patients undergoing mild hypothermia. We aimed at developing a reliable risk-score to enable early prediction of cerebral performance and survival.
Methods
Sixty-one out of 239 consecutive patients undergoing mild hypothermia after cardiac arrest, with eventual return of spontaneous circulation (ROSC), and comatose status on admission fulfilled the inclusion criteria. Background clinical variables, VF time and frequency domain fundamental variables were considered. The primary and secondary outcomes were a favorable neurological performance (FNP) during hospitalization and survival to hospital discharge, respectively. The predictive model was developed in a retrospective cohort (n=32; September 2006–September 2011, 48.5 ± 10.5 months of follow-up) and further validated in a prospective cohort (n = 29; October 2011–July 2013, 5 ± 1.8 months of follow-up).
Results
FNP was present in 16 (50.0%) and 21 patients (72.4%) in the retrospective and prospective cohorts, respectively. Seventeen (53.1%) and 21 patients (72.4%), respectively, survived to hospital discharge. Both outcomes were significantly associated (p < 0.001). Retrospective multivariate analysis provided a prediction model (sensitivity= 0.94, specificity = 1) that included spectral dominant frequency, derived power density and peak ratios between high and low frequency bands, and the number of shocks delivered before ROSC. Validation on the prospective cohort showed sensitivity = 0.88 and specificity = 0.91. A model-derived risk-score properly predicted 93% of FNP. Testing the model on follow-up showed a c-statistic ≥ 0.89.
Conclusions
A spectral analysis-based model reliably correlates time-dependent VF spectral changes with acute cerebral injury in comatose survivors undergoing mild hypothermia after cardiac arrest.
Keywords: Cardiac arrest, Cerebral injury, Early prognosis, Ventricular fibrillation, Dominant frequency
1. Introduction
Both in-hospital and out-of-hospital cardiac arrests due to ventricular fibrillation (VF) are associated with high mortality rates and significant cerebral disability [1,2]. VF-derived cerebral injury is a very sensitive time-dependent condition with dramatic social and personal consequences. The absence of cerebral blood flow during VF leads to ischemic damage within a few minutes, which increases after reperfusion due to generation of oxygen free radicals and activation of degradation enzymes, together with other mediators [3]. To date, mild hypothermia is the only therapy that has shown to increase both survival rates and functional outcomes in comatose survivors of cardiac arrest due to VF [4–6], even though cardiopulmonary resuscitation (CPR) technique and some specific drugs are considered influential to improve return of spontaneous circulation (RSOC) and survival outcomes during resuscitation [7–9]. However, the use of sedative and neuromuscular blocking drugs in cooled patients may mask neurological damage and delay examination. Furthermore, early prognosis within the first 72 h after cardiac arrest remains unreliable, which is especially relevant in those patients undergoing highly specialized intensive care who might not have any hopes for recovery.
Reducing the time to DC shock after VF onset is vital to restore spontaneous circulation and minimize cerebral injury [10]. However, the exact time in VF is difficult to determine even after witnessed cardiac arrest. Many VF episodes may initiate as ventricular tachycardia and cerebral blood flow might still persist until VF develops [11]. Reliable experimental data from waveform analysis during VF indicate that both spectral dominant frequency (DF) and median frequency decrease after onset of VF [12,13]. In the clinical setting, such a decrease in spectral values correlates with poor defibrillation success and no ROSC [14]. Moreover, retrospective data in patients with out-of-hospital cardiac arrest and VF have shown that a 5.61 Hz DF threshold can serve as a good predictor for 1-year survival after discharge [15]. Therefore, we hypothesize that spectral analysis of VF before a DC shock will accurately reflect the degree of acute cerebral injury as a consequence of time in VF and concomitant myocardial ischemia.
Here, we analyzed VF waveform properties before the first DC shock in patients undergoing therapeutic hypothermia due to comatose status after advanced life support (ALS) and ROSC. We aimed to identify spectral parameters that in combination with clinical variables may serve to develop a reliable model and risk score to enable early prediction of cerebral performance and survival to hospital discharge. We also studied the capacity of the model to predict both outcomes at follow-up.
2. Methods
2.1. Study design
The study was performed in a referral center for out-of-hospital cardiac arrest (Hospital Universitario La Paz, Madrid, Spain), in which mild therapeutic hypothermia is routinely used in comatose survivors after the event. The emergency service and ambulances in the region have trained medical staff and nurses, all coordinated by a central station to minimize both time to ALS and transportation to a referral center. The study included consecutive patients who underwent mild hypothermia after cardiac arrest due to VF, eventually with ROSC, and comatose status (Glasgow Coma Scale ≤ 8) on admission. Patients with witnessed or un-witnessed documented VF were eligible for the study, as long as VF traces before the first DC shock had enough quality and duration (≥3 s) for digitization and analysis of spectral parameters, respectively. We excluded patients with early mortality or hemodynamic instability leading to incomplete 24 h of mild hypothermia, and absence of subsequent withdrawal of sedation to assess cerebral performance. Other exclusion criteria were age < 18 years, Glasgow Coma Scale score after ROSC > 8, non-shockable or shockable rhythms other than VF, a terminal illness or cognitive deterioration present before the cardiac arrest, and possible causes of coma other than cardiac arrest. The study was divided into two groups, as follows: group 1 with eligible patients from September 2006 to September 2011 and retrospective data analysis, and group 2 with eligible patients from October 2011 to July 2013, in whom we prospectively studied the utility of the predictive algorithm developed in group 1. All data were collected from a prospective registry. The institutional ethics review committee approved prospective analysis of the patients, in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and European guidelines for good clinical practice.
2.2. Hypothermia protocol
Patients admitted to the acute cardiac care unit (ACCU) underwent routine neurological evaluation before sedation, drug-induced paralysis and initiation of hypothermia protocol as described elsewhere [6]. Briefly, cooling with intravenous cold saline (<8 °C) was initiated on admission. This was followed by direct cooling of the blood using the Icy catheter (ZOLL Medical Corporation, Chelmsford, MA) positioned at the level of the inferior vena cava through the femoral vein. Cooling was set at a maximum rate with a target temperature either at 32, 33 or 34 °C, which was maintained during 24 h. Rewarming was controlled at a set rate of 0.1 to 0.3 °C/h to reach 37 °C in 12 to 24 h. Mechanical ventilation was adjusted to ensure normoxemia and normocapnia. Mean blood pressure was maintained between 85 and 100 mm Hg. Blood glucose level was ensured at <10 mmol/l. Limitation of active ALS was considered in patients who remained deeply comatose after 5 days of evolution, as long as it was possible to reach an agreement with their representatives.
2.3. Spectral analysis
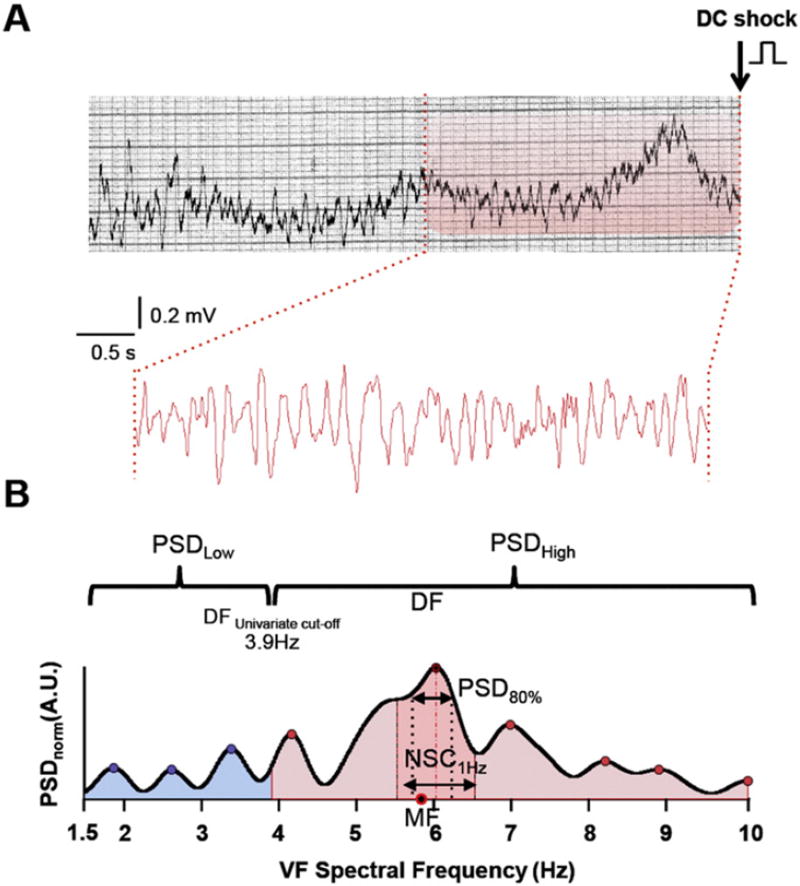
For each patient, we analyzed VF epochs prior to the first DC shock. Digitization was performed using a supervised semi-automatic approach based on region of interest selection, histogram thresholding and intensity transformations. Up to 5-s long segments were extracted after segmentation and signal codification from artifact-free VF tracings. Signals were band-pass filtered between 1.5 and 40 Hz. Quality of extraction was visually inspected by two independent investigators. Averaged power spectral density was obtained at each frequency using the non-parametric Welch method for using fast Fourier transform and normalized to the peak power in the 1.5–10 Hz band for each patient. Both time and frequency domain variables were quantified across patients. Those included VF amplitude over time, amplitude spectral area (AMSA), DF, median frequency, approximate entropy regularity index, spectral regularity index, 1-Hz DF spectral concentration and normalized 80% power spectral density (see Fig. 1 and Supplemental Methods for details). Investigators blinded to clinical outcome performed all data analysis, extraction and quantification using custom-made scripts of MATLAB software (V. 2010b, The Mathworks, Inc., Natick, MA).
Fig. 1.
Digitization and signal processing of a representative VF trace. A. Upper panel, single lead VF trace from paper ECG prior to the first DC shock. Lower panel, 5-s VF epoch after digitization, segmentation and codification. B. Representative spectra of the VF trace shown in A. DF, MF, 1-Hz DF spectral concentration and PSD80%, are shown. The univariate cut-off at 3.9 Hz was used to define low and high PSD bands. DF = dominant frequency. MF =median frequency. NSC = normalized spectral concentration. PSD = power spectral density.
2.4. Outcome
The primary outcome was a favorable neurological performance (FNP) during hospitalization. All patients were classified using the Pittsburgh outcome categorization of brain injury as follows: cerebral performance categories (CPCs) 1 and 2 (good and moderate disability, respectively) were considered as FNP, and CPCs 3, 4 and 5 (severe disability, vegetative state and brain death, respectively) were considered as a non-FNP (Supplemental Table 1) [16]. Neurological outcome was established after in-hospital stabilization or before hospital discharge. In patients from group 1, retrospective data were obtained from clinical records during hospitalization.
The secondary outcome measure was survival to hospital discharge.
2.5. Follow-up
Neurological outcome was prospectively assessed by in-person interview in all survivors after October 2011, either from group 1 or group 2. Specifically, patients from group 2 were evaluated between 3 and 6 months after hospitalization. Neurological outcome was also determined in both groups using the mini-mental state examination as follows: any score ≥24 points (out of 30) indicated a good cognition. Scores <24 indicated cognitive impairment [17]. Only patients with both CPC ≤2 and mini-mental state examination score ≥24 were considered to have FNP at follow-up.
Survival after hospitalization was assessed in group 1 after October 2011. In patients from group 2, survival was assessed at 6 months after hospital discharge.
2.6. Statistical analysis
All values are presented as median ± SEM (25th, 75th percentiles) except where noted. The retrospective cohort was used to develop a model for predicting the primary outcome. Each of the clinical, spectral and time domain VF variables underwent univariate analysis to evaluate its association with in-hospital FNP. Normal distribution of variables was assessed with the Shapiro–Wilk test. Statistical significance was assessed by the T-test or the Mann–Whitney–Wilcoxon test, as appropriate. If necessary, we used Bonferroni correction for multiple comparisons. Categorical variables and percentile comparisons were compared using a Chi-squared test or the Fisher exact test, as appropriate. P < 0.05 was considered statistically significant. Non-correlated variables, among statistically significant ones (Supplemental Fig. 1), and clinical relevant variables were regressed out against the primary outcome by using a stepwise backward multivariate logistic regression approach.
We aimed at predicting in-hospital FNP with the highest sensitivity and specificity achievable using the minimum number of variables. We validated the predictive accuracy of the model in the prospective cohort and tested the model during follow-up. We also studied in both groups the accuracy of the model in predicting survival. Patients from both groups were categorized according to their risk scores obtained in the multivariate analysis. Goodness of fit was assessed through Pearson residuals and Chi-squared deviance. To correct for bias, we obtained bootstrapped standard errors for weights. To guarantee robustness we used the Jackknife fitted regression weights to confirm the minimum mean squared error (see Supplemental Methods for details). All analyses were done using SSPS v21 and custom Matlab scripts for mathematical assistance.
3. Results
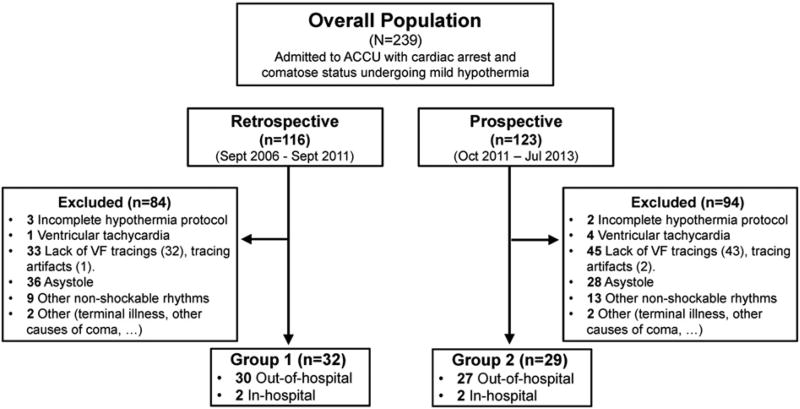
The work flow of the study is depicted in Fig. 2. A total of 239 patients undergoing mild hypothermia (n= 116, retrospective cohort and n = 123, prospective cohort) were assessed for eligibility during the study period. Sixty-one patients (n = 31, group 1 and n = 29, group 2) fulfilled the inclusion criteria. The vast majority of patients were included after out-of-hospital cardiac arrest (n= 57). However, two patients in each group were included after in-hospital cardiac arrest due to VF, since comatose status was present after DC shock and ROSC. Baseline clinical characteristics and background treatment of both groups are shown in Table 1. Female sex, family history of sudden cardiac death and younger age were more frequent in group 2. Overall, the main cause of VF was coronary heart disease, either acute coronary syndromes (n = 27, 45%) or chronic coronary disease (n = 14, 23%), followed by idiopathic VF (n = 6, 10%) and dilated cardiomyopathy (n= 6, 10%) (Supplemental Fig. 2).
Fig. 2.
Workflow of patients included in group 1 and group 2.
Table 1.
Baseline clinical characteristics.
| Clinical characteristics | Retrospective (n = 32) |
Prospective (n = 29) |
Overall (n = 61) |
P |
|---|---|---|---|---|
| Age (years) | 63.5 ± 4.5 | 55 ± 5.7 | 55 ± 3.72 | 0.0409 |
| Male, n (%) | 31 (96.9) | 23 (79.4) | 54 (88.5) | 0.031 |
| Family history of SCD, n (%) | 1 (3.57) | 8 (40.0) | 9 (15.8) | 0.009 |
| Hypertension, n (%) | 13 (40.6) | 15 (51.7) | 28 (45.9) | 0.385 |
| Dyslipedemia, n (%) | 9 (28.1) | 13 (44.8) | 22 (36.1) | 0.174 |
| Diabetes, n (%) | 7 (21.9) | 3 (10.3) | 10 (16.4) | 0.224 |
| Smoking habit, n (%) | 12 (37.5) | 13 (44.8) | 22 (36.1) | 0.561 |
| Atrial fibrillation, n (%) | 5 (15.6) | 6 (20.7) | 11 (18) | 0.849 |
| Heart failure, n (%) | 10 (31.3) | 6 (20.7) | 12 (26.2) | 0.234 |
| Previous myocardial infarction, n (%) | 6 (18.8) | 8 (27.6) | 14 (22.9) | 0.654 |
| Previous revascularization, n (%) | 4 (12.5) | 4 (13.8) | 6 (13.1) | 0.402 |
| Previous stroke, n (%) | 1 (3.1) | 1 (3.4) | 2 (3.3) | 0.943 |
| Chronic renal failure, n (%) | 2 (6.3) | 0 (0) | 2 (3.3) | 0.271 |
| DCM, n (%) | 2 (6.3) | 0 (0) | 2 (3.3) | 0.17 |
| COPD, n (%) | 3 (9.4) | 5 (17.2) | 8 (13.1) | 0.363 |
| HCM, n (%) | 0 (0) | 3 (10.3) | 3 (4.9) | 0.101 |
| Severe valvulopathy, n (%) | 2 (6.3) | 1 (3.4) | 3 (4.9) | 0.613 |
| Preexcitation, n (%) | 0 (0) | 1 (3.4) | 1 (1.6) | 0.289 |
| Time to ALS, (min) | 8 ± 2.4 | 8 ± 2.0 | 8 ± 1.5 | 0.887 |
| Time performing ALS, (min) | 15 ± 5.9 | 10 ± 5.3 | 15 ± 3.9 | 0.282 |
| Number of shocks delivered before ROSC | 3.5 ± 1.6 | 3 ± 0.87 | 3 ± 0.9 | 0.185 |
| Background treatment | ||||
| Aspirin, n (%) | 4 (12.5) | 5 (17.2) | 9 (14.7) | 0.602 |
| Thienopyridines, n (%) | 1 (3.1) | 0 (0) | 1 (1.6) | 0.524 |
| Betablockers, n (%) | 7 (21.9) | 9 (31) | 16 (26.2) | 0.416 |
| Amiodarone, n (%) | 2 (6.3) | 0 (0) | 2 (3.3) | 0.271 |
| ACE inhibitors, n (%) | 6 (18.8) | 7 (24.1) | 13 (21.3) | 0.557 |
| ARBs, n (%) | 1 (3.1) | 3 (10.3) | 4 (6.6) | 0.239 |
| Statins, n (%) | 8 (25) | 10 (34.5) | 18 (29.5) | 0.417 |
| Calcium antagonists, n (%) | 1 (3.1) | 2 (6.9) | 3 (4.9) | 0.496 |
| Diuretics, n (%) | 8 (25) | 6 (20.7) | 14 (22.9) | 0.689 |
| Aldosterone inhibitors, n (%) | 1 (3.1) | 2 (6.9) | 3 (4.9) | 0.476 |
| Anticoagulants, n (%) | 5 (15.6) | 6 (20.7) | 12 (19.7) | 0.849 |
ROSC: return of spontaneous circulation. DCM: dilated cardiomyopathy. COPD: chronic obstructive pulmonary disease. HCM: hypertrophic cardiomyopathy. ALS: advanced life support. ACE: angiotensin-converting enzyme. ARBs: Angiotensin II receptor blockers.
3.1. Outcomes
Primary and secondary outcomes are shown in Table 2. Sixteen patients in group 1 (50.0%) and 21 patients in group 2 (72.4%) achieved FNP during hospitalization. Seventeen patients in group 1 (53.1%) and 21 patients in group 2 (72.4%) survived to hospital discharge. After a median follow-up of 48.5±10.5 months (27.0, 68.7) in group 1, 16 patients (50.0%) were still alive, albeit 15 (46.8%) showed FNP. In group 2, 20 patients (68.9%) were alive at 6 months after hospital discharge and 19 (65.5%) showed FNP after 5 ± 1.8 months (3.5, 7.5). Hospitalization outcomes were not statistically different between groups (Table 2). No patients were missed during follow-up. Outcomes and follow-up of each individual patient are depicted in Supplemental Fig. 3. Four patients died (6.5%) despite FNP. There was a statistically significant association between FNP and survival (Supplemental Table 2) in both groups (p < 0.001).
Table 2.
Primary and secondary outcomes.
| Outcome | Retrospective (n = 32) |
Prospective (n = 29) |
Overall (n = 61) |
P (χ2test) |
|---|---|---|---|---|
| Favorable neurological performance | ||||
| In-hospital | 16 (50.00%) | 21 (72.41%) | 37 (60.66%) | 0.074 |
| Follow-upa | 15 (46.88%) | 19 (65.52%) | 34 (55.74%) | |
| Survival | ||||
| Hospital discharge | 17 (53.12%) | 21 (72.41%) | 38 (62.30%) | 0.121 |
| Follow-upa | 16 (50.00%) | 20 (68.97%) | 36 (59.02%) | |
Median ± SEM (25th, 75th percentiles). Retrospective: 48.5 ± 10.5 (27.0, 68.7) months. Prospective: 5 ± 1.8 (3.5, 7.5) months.
3.2. Prediction model
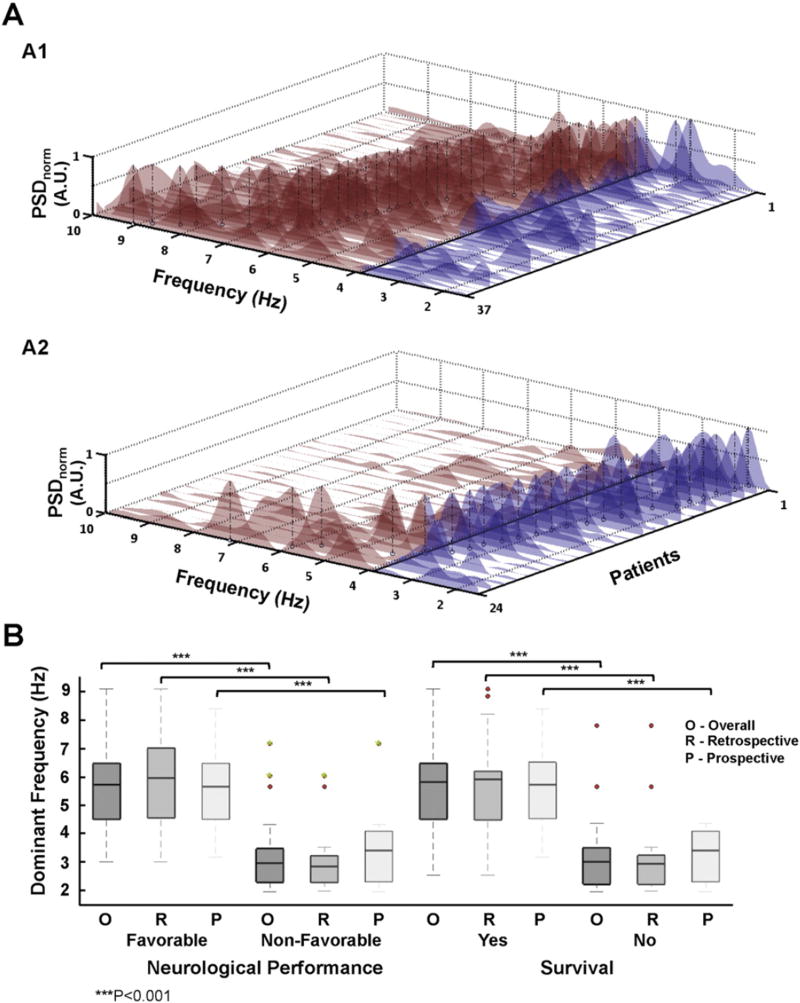
In creating the model we only considered the primary endpoint since mortality may occur in patients with FNP due to other causes non-directly related with cardiac arrest injury. The interval to ALS and total time of ALS reached statistical significance among clinical variables. All fundamental spectral and time domain VF variables, except VF signal amplitude, were significantly associated to the primary endpoint (p < 0.05) (Table 3). Interestingly, DF was strongly associated with FNP, which was reflected by the best univariate independent predictive accuracy in the retrospective and prospective cohorts (average 0.884) (Table 3). A cut-off at 3.9 Hz showed the highest sensitivity (0.88) and specificity (0.94) in predicting the primary endpoint in the retrospective cohort. Therefore, we used such a cut-off value to obtain two derived, also significant (p < 0.001. Table 3), spectral variables as follows: high-to-low power spectral density ratio (HL-PSDR), as the relative power between high (3.9–10 Hz) and low (1.5–3.9 Hz) bands, and high-to-low peak ratio (HL-pKR), as the relative number of spectral peaks above and below 3.9 Hz with power above 40% of the DF (Fig. 1). Graphic representation of individual spectra and DF peaks of the entire population are shown in Fig. 3A. The vast majority of patients with FNP during hospitalization showed DF values above 3.9 Hz (Fig. 3A1), unlike those individuals with non-FNP, who had DF values below 3.9 Hz (Fig. 3A2). Such differences were statistically significant both in the retrospective and prospective cohorts, as well as in the entire population (p < 0.001. Fig. 3B). Moreover, DF values also showed significant differences between patients who survived and those who did not survive to hospital discharge (p < 0.001. Fig. 3B).
Table 3.
Univariate analysis and independent predictive accuracy.
| Neurological performance | Univariate unadjusted odds ratio | |||
|---|---|---|---|---|
|
|
|
|||
| Categories | P value | OR (CI 95%) | Univariate P | ACC (CI 95%)a |
| Clinical variables | ||||
| Age | 0.11 | |||
| Gender | 0.309 | |||
| Hypertension | 0.281 | |||
| Dyslipedemia | 0.694 | |||
| Diabetes | 0.199 | |||
| Smoking habit | 0.465 | |||
| Atrial fibrillation | 0.07 | 2.158 (0.864–5.391) | 0.099 | 0.672 (0.506–0.838) |
| Heart failure | 0.063 | 1.940 (0.945–3.986) | 0.071 | 0.655 (0.486–0.824) |
| Previous myocardial infarction | 0.233 | |||
| Previous revascularization | 0.219 | |||
| Previous stroke | 0.309 | |||
| Chronic renal failure | 0.516 | |||
| DCM | 0.144 | |||
| COPD | 0.544 | |||
| HCM | 0.565 | |||
| Severe valvulopathy | 0.516 | |||
| Number of shocks delivered before ROSC | 0.023 | 3.025 (0.991–9.229) | 0.052 | 0.671 (0.503–0.837) |
| Time to ALS | <0.001 | 10.312 (2.044–52.032) | 0.005 | 0.689 (0.524–0.851) |
| Time performing ALS | 0.007 | 2.770 (1.046–7.336) | 0.04 | 0.676 (0.509–0.84) |
| Background treatment | ||||
| Aspirin | 0.285 | |||
| Thienopyridines | 0.309 | |||
| Betablockers | 0.669 | |||
| Amiodarones | 0.144 | |||
| ACE inhibitors | 0.365 | |||
| ARBs | 0.309 | |||
| Statins | 0.414 | |||
| Calcium antagonists | 0.31 | |||
| Diuretics | 0.314 | |||
| Anticoagulants | 0.133 | |||
| Aldosterone inhibitors | 0.309 | |||
| VF variables | ||||
| Spectral domain | ||||
| Fundamental spectral variables | ||||
| Dominant frequency | <0.001 | 0.089 (0.020–0.403) | 0.002 | 0.884 (0.77–0.988) |
| Median frequency | <0.001 | 0.073 (0.015–0.355) | 0.001 | 0.86 (0.741–0.98) |
| Normalized 80% PSD | 0.005 | 3.121 (1.270–7.669) | 0.013 | 0.579 (0.4–0.75) |
| 1 Hz DF spectral concentration | 0.023 | 2.198 (1.027–4.704) | 0.042 | 0.634 (0.463–0.805) |
| Amplitude spectrum area (AMSA) | <0.001 | 0.053 (0.008–0.362) | 0.003 | 0.85 (0.723–0.977) |
| Spectral Regularity Index | 0.07 | |||
| Derived spectral variables | ||||
| High-to-low peak ratio | <0.001 | 0.073 (0.014–0.372) | 0.002 | 0.817 (0.678–0.954) |
| High-to-low PSD ratio | <0.001 | 0.034 (0.003–0.396) | 0.007 | 0.847 (0.721–0.969) |
| Time domain | ||||
| Mean amplitude | 0.11 | |||
| Approximate Entropy Regularity Index | <0.001 | 0.032 (0.003–0.355) | 0.005 | 0.796 (0.652–0.937) |
Univariate predictive accuracy averaged for training (group 1, retrospective) and validation (group 2, prospective). OR represent univariate odds ratios for non-FNP.
AMSA: amplitude spectrum area. PSD: power spectral density. ROSC: return of spontaneous circulation. Other abbreviations as in Table 1.
Fig. 3.
A. Power spectral density (PSD) of all patients with in-hospital favorable (A1) and non-favorable (A2) neurological performance. The patients are sorted based on their DF values. DF peaks are pointed out for each individual (black vertical dashed-line). The cut-off threshold of 3.9 Hz was chosen for color-coding above (red) and below (blue) the PSD. B. Boxplot representation of DF comparing patients from both groups for primary and secondary endpoints. Boxes depict median and interquartile range (25–75%). Red dots are outliers at least twice the interquartile range from the median. Green dots represent outliers to hospital discharge who improved neurological performance during follow-up.
Multivariate analysis identified DF, HL-pKR, HL-PSDR and the number of shocks delivered before ROSC as the best performance model to predict in-hospital FNP. Multivariate adjusted odds ratios are shown in Table 4. For the primary endpoint, the model achieved sensitivity = 0.94 and specificity=1 (c-statistic=0.98). Validation on the prospective cohort also showed high sensitivity (0.88) and specificity (0.91) (c-statistic = 0.89). The multivariate model achieved sensitivity = 0.94 and specificity = 0.94 to predict in-hospital survival in the retrospective cohort (c-statistic=0.95). For the secondary endpoint, predictive performance was also high in the prospective group (sensitivity= 0.88, specificity=0.91, c-statistic=0.92). ROC curves of the multivariate model are shown in Supplemental Fig. 4. In-hospital performance of the model is shown in Table 5. Performance of the model for both outcomes at follow-up also reached high sensitivity, specificity and c-statistic values (Supplemental Table 3).
Table 4.
Multivariate best performance model.
| Variable | Multivariate adjusted odds ratio for non-FNP (OR) |
CI 95% |
|---|---|---|
| Dominant frequency (Hz) | 0.252 | 0.227–0.281 |
| High-to-low peak ratio (A.U.) | 0.164 | 0.145–0.185 |
| High-to-low PSD ratio (A.U.) | 0.264 | 0.237–0.295 |
| Number of shocks delivered before ROSC | 5.14 | 4.79–5.51 |
Abbreviations as in Table 3.
Table 5.
In-hospital performance of the best multivariate predictive model.
| Se | Sp | C-Stat | ACC | |
|---|---|---|---|---|
| Favorable neurological performance | ||||
| Retrospective group (Training) | 0.94 | 1 | 0.98 | 0.97 |
| Prospective group (Validation) | 0.88 | 0.91 | 0.89 | 0.90 |
| Survival outcome (test) | ||||
| Retrospective group | 0.94 | 0.94 | 0.95 | 0.93 |
| Prospective group | 0.88 | 0.91 | 0.92 | 0.89 |
3.3. Risk score based on the predictive performance of the model
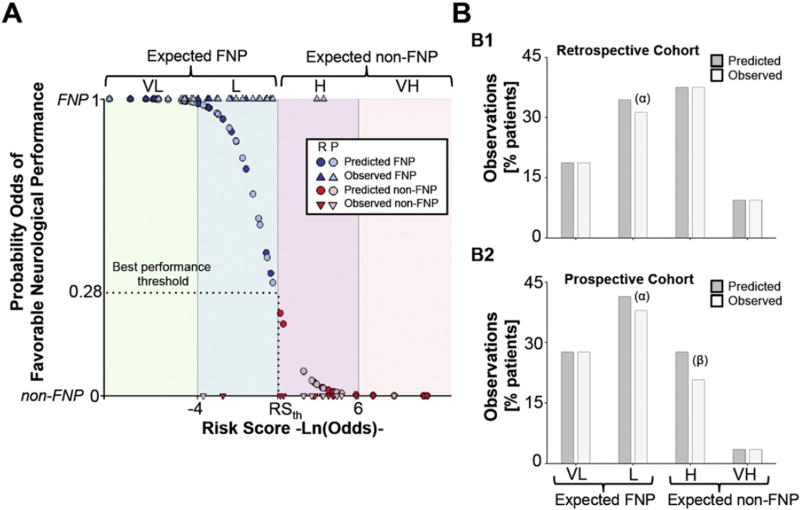
We used the best performance threshold obtained by the prediction model to define four risk subsets of the population for non-FNP, as follows: very low and low risk of non-FNP (expected FNP), high and very high risk of non-FNP (expected non-FNP). Interquartile ranges of individual variables within each of the risk score groups are shown in Supplemental Table 4. Fig. 4A shows the observed and predicted probability of in-hospital FNP for the entire population. The risk score correctly classifies more than 93% of observations. Only 1 patient in the retrospective cohort was classified as FNP, although the patient was non-FNP during hospitalization (false negative, Fig. 4B1). Three patients in the prospective cohort were identified as false negative and false positives (1 and 2 patients, respectively). The risk score was very reliable in predicting neurological performance in the subgroups of very low and very high risk of non-FNP (Fig. 4B1 and B2). Multivariate adjusted logistic regression weights and statistics for each of the four variables included in the risk score are shown in Supplemental Table 5.
Fig. 4.
Risk score based on the predictive performance of the model. A. Observed (triangles) and predicted (circles) probability of FNP for the entire population. Blue and red represent FNP and non-FNP, respectively (dark fill, retrospective; light fill, prospective).We defined four risk groups of non-FNP performance according to their risk scores as follows: expected FNP; very low (VL) and low risk (L) and expected non-FNP; high (H) and very high risk (VH). B. Percentage of patients (predicted, dark gray and observed, light gray) who belong to each of the risk score groups in both the retrospective (B1) and prospective cohorts (B2). (α) and (β) represent false negative and false positive individuals, respectively. FNP= favorable neurological performance.
To test the reproducibility of the spectral variables present in the risk score, we quantified changes in the spectral components of VF prior to the first reported DC shock using a 3-s sliding window shifted 0.2-s from the DC shock. Interestingly, spontaneous variability of the spectral components a few seconds (maximum available=12 s) before the DC shock did not reflect significant changes in neurological performance prediction and risk score classification for the entire population (Supplemental Fig. 5).
Further risk score validation in patients without comatose status after DC shock who did not undergo hypothermia (N = 11), showed that risk score stratification properly provided FNP values in all cases, as expected (Supplemental Fig. 6).
3.4. Contribution of spectral parameters to the predictive performance of the model
The predictive performance of our model was highly dependent on incorporating the spectral variables. Thus, only considering the spectral parameters, the prediction model achieved high sensitivity (0.88) and specificity (0.86) in validation (c-statistic = 0.88), compared with sensitivity = 0.62 and specificity = 0.66 (c-statistic = 0.67) using the number of shocks delivered before ROSC alone. Moreover, the best clinical prediction model (5-variable model) using the most influential clinical factors in the univariate analysis (Table 3) achieved sensitivity = 0.50 and specificity = 0.71 (c-statistic= 0.69) in the prospective cohort. The inclusion of additional clinically-relevant variables to achieve better prediction in training resulted in a further decrease in the predictive performance of the model in the prospective cohort (c-statistic=0.63). Overall, clinical models did not achieve c-statistic values greater than 0.69 in the prospective cohort (Supplemental Table 6 and Supplemental Methods), which highlights the objective and reliable significance of spectral parameters.
4. Discussion
We have introduced a novel practical approach aimed at predicting neurological performance and survival in patients undergoing therapeutic hypothermia after cardiac arrest due to VF and comatose status on admission. In brief, DF before the first DC shock is a strong independent predictor for both FNP and survival using a cut-off value of 3.9 Hz. To increase the predictive accuracy, multivariate analysis identified DF, HL-pKR, HL-PSDR and the number of shocks delivered before ROSC as the best performance model to predict both primary and secondary outcomes. The model showed sensitivity and specificity values above 0.88 and 0.91 in the validation prospective cohort. We also developed a risk score that properly predicted 93% of the in-hospital neurological outcome observed in the entire cohort.
Currently, the reliability of early prognosis in comatose survivors after cardiac arrest due to VF is very limited, which severely impairs the ability of physicians to provide accurate information to patients' relatives and to optimize the use of intensive-resource care. Standardization of mild hypothermia delays neurological evaluation and prognostication due to sedation as well as higher rates of misleading biomarker values within the first 24–48 h [18]. Moreover, the large variability of threshold biomarker values used to predict poor outcome and different measurement techniques make it necessary to exert caution and question the prognostic accuracy provided by biochemical markers.
Clinical variables are also inconsistent in their ability to predict both survival and neurological performance [19], as we also showed after developing and validating the best clinical prediction models. Advanced age seems to be associated with decreased survival after cardiac arrest and resuscitation [20]. Interestingly, old age is not associated with non-FNP [10], which supports the role of early and appropriate resuscitation to prevent cerebral injury [21]. However, FNP does not prevent later complications that may lead to in-hospital mortality, especially in old patients. Univariate analysis of our retrospective cohort also showed younger age as significantly associated with survival (data not shown). Conversely, age was not significantly associated with FNP. Likewise, inclusion of clinically-relevant variables in the multivariate analysis did not result in age as a variable present in the predictive model.
Time to CPR after collapse has been shown to correlate with functional outcome [10]. Moreover, when performed properly, CPR improves functional outcome [21]. However, the quality of CPR administered by a bystander might be extremely variable even if performed by trained personnel [21,22], which might not add a significant improvement in outcome [4]. The strong predictive value of DF and derived spectral variables may be explained by their ability to provide reliable information of both the time from VF onset and the quality of CPR. Thus, as shown in both humans and animal models, as the VF episode evolves, progressive myocardial ischemia leads to a gradual decline in DF values [12,13,23,24]. Stewart et al. have shown that DF values fall with an increased duration of collapse from 5.5 Hz at 3 min to a mean of 2.1 Hz at 20 min [13]. Patients with a mean DF of 3.89 ± 0.25 Hz did not survive longer than 6 h after resuscitation, unlike patients with a mean DF of 5.60 ± 0.25 Hz who did survive [13]. Similar DF values (5.61 Hz) have been reported by Goto et al. to predict 1-year survival in a retrospective cohort of patients after out-of-hospital cardiac arrest, although data about neurological performance were missed [15]. Our risk score identifies DF values of 5.6 ± 0.53 Hz and 6.35 ± 0.75 Hz at low and very low risk of non-FNP, respectively. The latter does not necessarily correlate with survival, which is reflected by the fact that 2 patients during hospitalization and another 2 patients during follow-up died despite FNP. Previous series have shown that the longer the time between CPR and ROSC the lower the survival rate [1,10], which is similar to what we observed in both cases with FNP and in-hospital mortality after the hypothermia protocol, in whom long CPR (20 and 42 min) and a high number of shocks (4 and 5) before ROSC were present. A significantly higher number of shocks delivered before ROSC was also present in patients with DF < 3.9 Hz (Supplemental Fig. 7), which highlights the difficulties of the heart for acute recovery. Mortality may also occur during the hypothermia protocol before withdrawing sedation. Thus, data from 4 out of 5 patients, who were excluded due to early mortality and impossibility to assess the primary outcome, showed that risk score stratification would have predicted FNP in 3 out of 4 (Supplemental Table 7). However, early mortality due to other medical circumstances did not allow recovery and neurological assessment.
Interestingly, CPR may increase DF values while coronary blood flow rises [24,25], which is also associated with increased probability of successful rescue shocks [26]. Increase in DF during resuscitation may explain false positive cases to predict FNP when CPR is delayed after collapse and DF is already low. The latter is supported by recent data by Freese et al. in patients with out-of-hospital cardiac arrest due to VF [27]. The authors showed that a waveform analysis algorithm to decide whether to apply an immediate defibrillatory shock or a CPR interval before the shock, did not improve overall survival to hospital discharge compared with a standard shock-first protocol. In the study, prognosis may have been determined by the spectral values at the beginning of CPR. Yet, an increase in favorable VF waveform parameters during CPR increased the probability of ROSC and survival to admission [27].
DF alone showed the best univariate independent predictive accuracy among spectral variables. However, relying on DF alone may not be accurate in some cases; for instance when the DF peak is close to the cut-off value. Our multivariate model includes 2 additional derived measures (HL-PSDR and HL-pKR) that aided in clarifying such cases. The probability of a favorable outcome increases as the relative power of high spectral bands (3.9–10 Hz) and the number of significant spectral peaks above 3.9 Hz also increase (Supplemental Fig. 8). Recent data by Schoene et al. also highlights the role of AMSA over the course of the first 3 shocks during resuscitation in predicting survival and FNP in a large retrospective cohort of patients with out-of-hospital cardiac arrest due to VF [28]. Calculation of AMSA will typically provide higher AMSA values in traces with more high-frequency content, which agrees with our results based on DF. However, Schoene et al. did not distinguish between patients with or without comatose status on admission. Moreover, the authors did not provide information about post-cardiac arrest care using mild hypothermia. Here, we focused on a population with baseline comatose status on admission and mild hypothermia as uniform therapy to minimize post-cardiac arrest syndrome.
Finally, recent data prompted us to consider the role of hypothermia after cardiac arrest and whether a target temperature rather than controlling the body temperature at 36 °C must be pursued [29]. We speculate that temperature may have a minimum role in patients with very low risk (i.e., favorable risk score) or very high risk of cerebral injury at baseline (i.e., asystole). However, target temperature may matter in patients with borderline values to minimize post-cardiac arrest syndrome. Altogether, the results support the clinical relevance of the predictive model and risk score to assist physicians and patients' relatives who deal with difficult decisions after out-of-hospital cardiac arrest due to VF.
5. Limitations
This is a single center study with a limited number of patients, in which survival rates may be higher than expected. However, our data is consistent with similar series in dedicated units that included patients after admission to the ACCU, with an initial rhythm reported as VF and using mild hypothermia [29,30]. Therefore, the study population may be skewed towards patients with FNP, since patients who died in transit or survived to hospital admission but died in the emergency department or during the hypothermia protocol, before withdrawing sedation, were excluded.
Differences between groups regarding age, gender and family history of sudden cardiac death may have occurred due to the study design. However, the highly reliable predictive performance of the spectral-based model compared with clinical models in the validation cohort, suggests that the model is suitable for clinical practice upon developing appropriate clinical tools. The proposed risk score will nevertheless benefit from further validation in a multicenter study with more patients. Additionally, a significant number of traces were not available on admission, which may raise the concern of selection bias. However, our results are consistent with previous studies, which reduce likelihood for such bias [13,15,27,28]. In the future, either incorporating the algorithm into external defibrillators or automated signal transferring from defibrillators to portable medical devices, for signal processing and risk score calculation, may avoid such limitation. The model will also benefit from future studies aiming at direct comparisons of current biochemical and neurological markers to establish the net benefit on early prognosis.
6. Conclusions
A spectral analysis-based model demonstrates high reliability in predicting in-hospital FNP and survival to discharge in patients with comatose status on admission after cardiac arrest due to VF.
Supplementary Material
Acknowledgments
We thank Dr. Stuart Pocock and Dr. Martín Laclaustra for their assistance with the statistical analysis.
Grant support: the CNIC is supported by the Spanish Ministry of Economy and Competitiveness and the Pro-CNIC Foundation.
Abbreviations
- FNP
Favorable neurological performance
- HL-PSDR
High-to-low power spectral density ratio
- HL-pKR
High-to-low peak ratio
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijcard.2015.03.074.
References
- 1.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 2.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid. Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-de-Sa E, Rey JR, Armada E, Salinas P, Viana-Tejedor A, Espinosa-Garcia S, et al. Hypothermia in comatose survivors from out-of-hospital cardiac arrest: pilot trial comparing 2 levels of target temperature. Circulation. 2012;126:2826–2833. doi: 10.1161/CIRCULATIONAHA.112.136408. [DOI] [PubMed] [Google Scholar]
- 7.Idris AH, Guffey D, Aufderheide TP, Brown S, Morrison LJ, Nichols P, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125:3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stiell IG, Brown SP, Christenson J, Cheskes S, Nichol G, Powell J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit. Care Med. 2012;40:1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 10.Rogove HJ, Safar P, Sutton-Tyrrell K, Abramson NS. Old age does not negate good cerebral outcome after cardiopulmonary resuscitation: analyses from the brain resuscitation clinical trials. The Brain Resuscitation Clinical Trial I and II Study Groups. Crit. Care Med. 1995;23:18–25. doi: 10.1097/00003246-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am. Heart J. 1989;117:151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 12.Brown CG, Dzwonczyk R, Werman HA, Hamlin RL. Estimating the duration of ventricular fibrillation. Ann. Emerg. Med. 1989;18:1181–1185. doi: 10.1016/s0196-0644(89)80056-3. [DOI] [PubMed] [Google Scholar]
- 13.Stewart AJ, Allen JD, Adgey AA. Frequency analysis of ventricular fibrillation and resuscitation success. Q. J. Med. 1992;85:761–769. [PubMed] [Google Scholar]
- 14.Strohmenger HU, Lindner KH, Lurie KG, Welz A, Georgieff M. Frequency of ventricular fibrillation as a predictor of defibrillation success during cardiac surgery. Anesth. Analg. 1994;79:434–438. doi: 10.1213/00000539-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Goto Y, Suzuki I, Inaba H. Frequency of ventricular fibrillation as predictor of one-year survival from out-of-hospital cardiac arrests. Am. J. Cardiol. 2003;92:457–459. doi: 10.1016/s0002-9149(03)00667-2. [DOI] [PubMed] [Google Scholar]
- 16.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 17.Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46:54–58. (63, 6) [PubMed] [Google Scholar]
- 18.Pfeifer R, Borner A, Krack A, Sigusch HH, Surber R, Figulla HR. Outcome after cardiac arrest: predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation. 2005;65:49–55. doi: 10.1016/j.resuscitation.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.A randomized clinical study of cardiopulmonary–cerebral resuscitation: design, methods, and patient characteristics. Brain Resuscitation Clinical Trial I Study Group. Am. J. Emerg. Med. 1986;4:72–86. [PubMed] [Google Scholar]
- 20.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33:237–245. doi: 10.1007/s00134-006-0326-z. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher EJ, Lombardi G, Gennis P. Effectiveness of bystander cardiopulmonary resuscitation and survival following out-of-hospital cardiac arrest. JAMA. 1995;274:1922–1925. [PubMed] [Google Scholar]
- 22.Wik L, Kramer-Johansen J, Myklebust H, Sorebo H, Svensson L, Fellows B, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 23.Martin DR, Brown CG, Dzwonczyk R. Frequency analysis of the human and swine electrocardiogram during ventricular fibrillation. Resuscitation. 1991;22:85–91. doi: 10.1016/0300-9572(91)90067-9. [DOI] [PubMed] [Google Scholar]
- 24.Umapathy K, Foomany FH, Dorian P, Farid T, Sivagangabalan G, Nair K, et al. Real-time electrogram analysis for monitoring coronary blood flow during human ventricular fibrillation: implications for CPR. Heart Rhythm. 2011;8:740–749. doi: 10.1016/j.hrthm.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Achleitner U, Wenzel V, Strohmenger HU, Lindner KH, Baubin MA, Krismer AC, et al. The beneficial effect of basic life support on ventricular fibrillation mean frequency and coronary perfusion pressure. Resuscitation. 2001;51:151–158. doi: 10.1016/s0300-9572(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 26.Strohmenger HU, Lindner KH, Keller A, Lindner IM, Pfenninger EG. Spectral analysis of ventricular fibrillation and closed-chest cardiopulmonary resuscitation. Resuscitation. 1996;33:155–161. doi: 10.1016/s0300-9572(96)01003-9. [DOI] [PubMed] [Google Scholar]
- 27.Freese JP, Jorgenson DB, Liu PY, Innes J, Matallana L, Nammi K, et al. Waveform analysis-guided treatment versus a standard shock-first protocol for the treatment of out-of-hospital cardiac arrest presenting in ventricular fibrillation: results of an international randomized, controlled trial. Circulation. 2013;128:995–1002. doi: 10.1161/CIRCULATIONAHA.113.003273. [DOI] [PubMed] [Google Scholar]
- 28.Schoene P, Coult J, Murphy L, Fahrenbruch C, Blackwood J, Kudenchuk P, et al. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Heart Rhythm. 2014;11:230–236. doi: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 30.Boyce LW, Vliet Vlieland TP, Bosch J, Wolterbeek R, Volker G, van Exel HJ, et al. High survival rate of 43% in out-of-hospital cardiac arrest patients in an optimised chain of survival. Neth. Heart J. 2015;23:20–25. doi: 10.1007/s12471-014-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.