Abstract
In the surgical management of epilepsy, the resection of cortex exhibiting interictal fast ripples (250–500 Hz) on electrocorticography has been linked to postoperative seizure-freedom. Although fast ripples appear to accurately identify the epileptogenic zone—the minimum tissue that must be removed at surgery to achieve seizure-freedom—it has not been established that fast ripples are a superior biomarker in comparison with multimodal presurgical neuroimaging and other electrocorticography abnormalities. Hence, in the prediction of postoperative seizure-freedom, we compared the value of fast ripples with other intraoperative electocorticography abnormalities including focal slowing, paroxysmal fast activity, intermittent spike discharges, continuous epileptiform discharges, focal attenuation, and intraoperative seizures, as well as complete resection of the lesion defined by MRI and other neuroimaging. In a cohort of 60 children with lesional epilepsy and median postsurgical follow-up exceeding 4 years, who underwent resective epilepsy surgery with intraoperative electrocorticography, we evaluated the extent to which removal of each intraoperative electrocorticography abnormality impacts time to first postoperative seizure using the Kaplan-Meier method and Cox proportional hazards regression. Secondly, we contrasted the predictive value of resection of each competing electrocorticography abnormality using standard test metrics (sensitivity, specificity, positive predictive value, and negative predictive value). In contrast with all other intraoperative electrocorticography abnormalities, fast ripples demonstrated the most favorable combination of positive predictive value (100%) and negative predictive value (76%) in the prediction of postoperative seizures. Among all candidate electrocorticography features, time to first postoperative seizure was most strongly associated with incomplete resection of fast ripples (hazard ratio = 19.8, p <0.001). In multivariate survival analyses, postoperative seizures were independently predicted by incomplete resection of cortex generating fast ripples (hazard ratio = 25.4, 95%CI 6.71 – 96.0, p < 0.001) and focal slowing (hazard ratio = 5.79, 95%CI 1.76 – 19.0, p = 0.004), even after adjustment for the impact of an otherwise complete resection. All children with incomplete resection of interictal FR-generating cortex exhibited postoperative seizures within six months. Notably, this cohort included many patients with large resections and thus limited opportunity to exhibit unresected fast ripples. Future study in a cohort with small resection volume, or a clinical trial in which resection margins are guided by fast ripple distribution, would likely yield a more precise estimate of the risk posed by unresected fast ripples. With a high detection rate during brief intraoperative electrocorticography and favorable positive and negative predictive value, interictal fast ripple characterization during surgery is a feasible and useful adjunct to standard methods for epilepsy surgery planning, and represents a valuable spatially-localizing biomarker of the epileptogenic zone, without the need for prolonged extraoperative electrocorticography.
Keywords: High Frequency Oscillation, Epilepsy Surgery, Seizure Outcome, Intraoperative Electrocorticography, EEG, Biomarker
1. Introduction
High frequency cortical oscillations termed fast ripples (FR, 250–500 Hz) are thought to identify epileptogenic neocortex (Bragin et al., 1999) and have been proposed as a biomarker of the epileptogenic zone (EZ), defined as the area of cortex indispensable for the generation of seizures (Rosenow and Lüders, 2001). In the surgical management of epilepsy, multiple retrospective studies using intraoperative (van Klink et al., 2014; van ’t Klooster et al., 2015b; J. Wu et al., 2010a) or extraoperative (Akiyama et al., 2011; Haegelen et al., 2013; Jacobs et al., 2010; Okanishi et al., 2014) electrocorticography (ECoG), as well as a single prospective report (Hussain et al., 2016), have linked resection of FR-generating cortex to postoperative seizure freedom. Still, like many surgical interventions, the use of FR data to guide resection has not been validated in a clinical trial, though a randomized non-inferiority study (“The HFO Trial”) is now underway (van ’t Klooster et al., 2015a). Furthermore, there has been only limited comparison of FR to other abnormalities routinely observed during conventional intraoperative ECoG, such as focal background slowing, intermittent spikes or sharp-waves, continuous epileptiform discharges (Gambardella et al., 1996), paroxysmal fast activity (PFA, 30–100 Hz, also known as gamma) (Wu et al., 2008), focal attenuation, and ictal events. Utilizing a large cohort with extended postoperative follow-up, we set out to (1) better define the value of FR as a biomarker of the EZ by comparing intraoperatively-identified FR with other ECoG abnormalities in the prediction of postoperative epilepsy outcomes, and (2) quantify the independent hazard posed by incomplete FR resection, even when surgical resection is otherwise complete on the basis of neuroimaging.
2. Material and Methods
2.1 Standard protocol approvals
The institutional review board at the University of California Los Angeles (UCLA) approved the use of human subjects (protocol #11-000771) and waived the requirement for written informed consent, as all testing (i.e. intraoperative ECoG) was performed on a routine clinical basis.
2.2 Patients
All children with medically refractory epilepsy who underwent epilepsy surgery with the UCLA Pediatric Epilepsy Surgery Program between August 2007 and August 2009 were included in this study and have been previously reported (Hussain et al., 2016; J. Wu et al., 2010a). Refractory epilepsy was defined as at least monthly seizures despite failure of at least three appropriate antiepileptic drugs (Kwan et al., 2010). There were no exclusion criteria.
2.3 Patient evaluation and surgical planning
Our detailed approach to identify and treat pediatric epilepsy surgery candidates has been described previously (Hemb et al., 2010; Hussain et al., 2016; Salamon et al., 2008; Wu et al., 2010a; Wu et al., 2010b). Briefly, we emphasize complete resection of an EZ chiefly defined by non-invasive testing, and especially MRI. We have found that co-registration of MRI and 18-fluorodeoxyglucose positron emission tomography (PET) is valuable in identifying the full extent of MRI abnormalities (Salamon et al., 2008), and thus the EZ. We occasionally utilize magnetic source imaging when other studies are inconclusive. In the operating room, we routinely utilize neuronavigation of the MRI-PET co-registration in combination with brief intraoperative ECoG to refine the ultimate cortical margins of surgical resection (Olson et al., 1990). Extended extraoperative ECoG is reserved for exceptional cases requiring functional mapping of eloquent cortex. This approach has resulted in favorable rates of postoperative seizure-freedom, infrequent use of extraoperative ECoG, and a presumptive bias toward larger resections of well-defined lesions (Hemb et al., 2010; Hussain et al., 2016; Wu et al., 2010a; Wu et al., 2010b). We acknowledge that the ideal approach to epilepsy surgery in children is the subject of ongoing debate (Cross et al., 2006; Harvey et al., 2008; Jayakar et al., 2016).
2.4 Data abstraction
Clinical characteristics of the study population were abstracted from the electronic medical record. With respect to the lesion defined by neuroimaging, all cases underwent MRI six months following surgery and the area resected was compared with pre-surgical imaging to classify each resection as complete or incomplete. For our analyses, resections were not classified as complete/incomplete on the basis of any intraoperative ECoG findings. In evaluating the impact of etiology/histopathology, each patient was classified as developmental (cortical dysplasia, hememegalencephy, and tuberous sclerosis complex) or acquired (all other etiologies) (Devlin et al., 2003).
2.5 ECoG recording and FR identification
Our procedures for FR characterization, including anesthetic considerations (Constant et al., 2005), have been previously described in detail (Hussain et al., 2016; Wu et al., 2010a). Briefly, intraoperative ECoG was performed during all surgeries and recorded with grid and strip macroelectrodes (AdTech, Racine, WI). Electrodes were sequentially repositioned over the putative EZ as needed (typically 4 to 5 total positions), with recording lasting several minutes at each position. Simultaneously, for all non-hemispherectomy cases, at least one “control” strip of electrodes was placed over cortex which was believed to be distant from the EZ. ECoG was recorded with a 2000 Hz sampling rate with an anti-aliasing 500 Hz high frequency (low-pass) filter, no low frequency (high-pass) filter, and no notch filter, on the Harmonie long-term monitoring system (Alpine Biomed; Montreal, Canada). All ECoG took place on a pre-resection intraoperative basis before resection and no child in this cohort underwent extraoperative or post-resection ECoG. Electrocorticography tracings were reviewed with a referential montage, referenced to a contralateral scalp electrode.
As previously reported, FR identification was retrospective between August 2007 and September 2008 (n = 30) (Wu et al., 2010a) and either “live” in the operating room (n = 11) or “prospective” within one week of surgery (n = 19) between October 2008 and August 2009 (Hussain et al., 2016). For each electrode, we tabulated whether or not it was included in the resection and whether or not FR were detected. Accordingly, we determined whether surgical resection encompassed all, some, or none of the cortex generating FR events. As in our prior publications (Hussain et al., 2016; Wu et al., 2010a), and as advocated for statistical considerations (van ’t Klooster et al., 2015b), we have focused on the presence or absence of FR and other ECoG markers rather than normalized or otherwise adjusted event rates which take into consideration the total number of channels in which any particular ECoG abnormality is observed.
2.6 Conventional ECoG Interpretation
For all ECoG studies, conventional intraoperative ECoG was performed to guide surgical resection, and official clinical interpretation was carried out within one week of surgery, and without knowledge of FR data. The same ECoG studies were retrospectively re-reviewed by a second board-certified pediatric electroencephalographer (JYW), blinded to FR localization and postoperative outcome, to confirm the presence and location of each of the abnormal findings on ECoG: focal slowing, focal voltage attenuation, isolated spikes/sharp waves, continuous epileptiform discharges, PFA, and ictal events. Seizures, focal slowing, focal voltage attenuation, and focal spikes were identified according to the definitions of Sperling (Sperling, 2003), PFA was identified according to the description of Wu and colleagues (Wu et al., 2008), and continuous epileptiform discharges were identified according to the description of Gambardella and colleagues (Gambardella et al., 1996). Each of the six findings were reviewed independently of FR characterization. In parallel to our approach for FR, we tabulated whether or not each abnormality was within the final resection.
2.7 Postoperative outcome assessment
Follow-up occurred on a routine clinical basis and began no later than six months after surgery, and at least annually thereafter following a standard protocol (Hemb et al., 2010). Seizure outcome was noted at each of these visits, and anti-epileptic drugs were adjusted when clinically appropriate.
2.8 Statistical methods
Continuous summary data were presented as median and interquartile range (IQR) based on non-parametric distributions. Comparisons of proportions and medians were accomplished using the Fisher exact test and Wilcoxon rank-sum test, respectively. Survival analyses were conducted using the Kaplan-Meier procedure and Cox proportional hazards regression. Sensitivity was defined as the proportion of patients with postoperative seizures who had incomplete resection of cortex exhibiting the ECoG finding of interest, and similarly, specificity was defined as the proportion of patients with postoperative seizure-freedom who exhibited complete resection of each ECoG finding. Positive predictive value (PPV) was defined as the proportion of patients with retained cortex generating the ECoG finding of interest who developed postoperative seizures. Likewise, negative predictive value (NPV) was defined as the proportion of patients without retained cortex with the ECoG abnormality of interest who remained seizure-free. 95% confidence intervals for proportions were calculated using the Agresti-Coull method (Agresti and Coull, 1998). Patients without a given ECoG finding detected were excluded from univariate analyses specific to that ECoG finding, but retained in multivariate analyses so as to avoid biasing the estimated hazard of competing ECoG predictors for postoperative outcome. All comparisons were two-sided and significant results were considered at p < 0.05. STATA software (version 11, College Station, Texas, USA) was used for all calculations.
3. Results
3.1 Cohort Characteristics
Clinical attributes for the study population are summarized in Table 1. Sixty consecutive children with intractable lesional epilepsy underwent surgery with intraoperative ECoG assessment between August 2007 and August 2009. Of note, the majority of children (78%) exhibited extratemporal lobe epilepsy. Latency to surgery was substantial: Median age at onset of epilepsy was 9.9 months and median age at surgery was 9.1 years, similar to previous cohorts reported by our group (Hemb et al., 2010; Hussain et al., 2016; Wu et al., 2010a; Wu et al., 2010b). Twenty-one (35%) patients had a history of infantile spasms, including 10 (17%) with active spasms at the time of surgery. Half of resections were limited to a single lobe, and 43% of patients underwent hemispherectomy. Resections were classified as “complete” based on postoperative neuroimaging in all but one case in which the child underwent the first resection in a two-stage hemispherectomy for Rasmussen encephalitis. This patient’s second surgery was not included in our statistical analyses. Median follow-up duration was 4.1 years (2.6 – 6.6).
Table 1.
Clinical characteristics of the study population
| Demographics | |
| Female sex, n (%) | 30 (50.0%) |
| Age of onset of epilepsy, months, median (IQR) | 9.9 (3.0 – 78.0) |
| Epilepsy | |
|
| |
| Duration of epilepsy, y, median (IQR) | 3.6 (1.8 – 7.3) |
| AEDs at surgery, median (IQR) | 2 (1 – 3) |
| History of infantile spasms (%) | 21 (35.0%) |
| Ongoing infantile spasms at surgery, n (%) | 10 (16.6%) |
|
| |
| Surgery | |
| Age at surgery, years, median (IQR) | 9.1 (2.7 – 13.4) |
| Post-surgical follow-up, years, median (IQR) | 4.1 (2.6 – 6.6) |
|
| |
| Scope of resection | |
| Unilobar, n (%) | 30 (50.0%) |
| Multilobar, n (%) | 4 (6.7%) |
| Hemispherectomy, n (%) | 26 (43.3%) |
Abbreviations: IQR, interquartile range
In comparison to the previously reported retrospective subgroup (August 2007 to September 2008, n=30) (Wu et al., 2010a), the subsequent prospective subgroup (October 2008 to August 2009, n=30) (Hussain et al., 2016), did not differ with respect to age at epilepsy onset (p = 0.43), age at surgery (p = 0.98), duration of epilepsy (p = 0.59), sex (p = 1.00), number of AEDs at the time of surgery (p = 0.61), and type of surgical resection (p = 0.78). Accordingly, all subsequent analyses were pooled for the retrospective and prospective groups (n = 60).
3.2 ECoG Sampling and FR Findings
Of the 60 ECoG recordings, one lobe was sampled in three (completion of frontal lobectomy or hemispherectomy), two lobes were sampled in 18, three lobes were sampled in 34, and all four lobes were sampled in five patients. The median ECoG duration was 10.0 minutes (IQR, 7.0 – 13.7 minutes). FR were identified 48 studies (80%). Among the 12 patients that did not exhibit FR on ECoG, sampling was quite limited, as described previously (Hussain et al., 2016; Wu et al., 2010a). Although the median duration of ECoG tended to be longer among patients with observed FR (10.0 minutes, IQR 6.0 – 11.0) in comparison with patients without observed FR (8.5 minutes, IQR 7.0 – 15.5), the difference was not statistically significant.
3.3 Findings of other ECoG abnormalities
Conventional ECoG abnormalities were tabulated in the same fashion as FR. PFA was found in 29 recordings (48.3%), focal slowing in 52 (86.7%), continuous epileptiform discharges in nine (15.0%), intermittent spikes and sharp waves in 45 (75.0%), focal voltage attenuation in 10 (16.7%), and ictal events in five (8.3%). Although the vast majority of studies exhibited multiple coexistent abnormalities, none of these conventional ECoG abnormalities co-occurred with a frequency greater than would be expected by chance alone (all p > 0.05). However, the detection of FR was highly correlated with the identification of PFA (p = 0.014), such that patients with observed PFA were more likely to exhibit FR, and vice versa. Of interest, among patients with coexistent FR and PFA, these phenomena often occurred simultaneously.
Seizures occurred during five ECoG studies. FR and ictal onset were (1) localized to the same single electrode in one patient, with FR observed two minutes before the seizure, (2) localized to neighboring electrodes (1 cm apart, measured center-to-center) in two patients, or (3) localized to disparate electrodes (2 cm apart) in two patients. Cortex corresponding to these ictal onsets was completely resected in all five patients, and FR were completely resected in four. The single patient with incomplete FR resection is the same aforementioned patient with intentionally incomplete resection in the setting of staged hemispherectomy. He suffered postoperative seizures and underwent successful hemispherectomy completion 12 months later. Importantly, there were no cases with complete FR resection and incomplete resection of either the MRI lesion and/or cortex with ictal onset.
3.4 Association of ECoG abnormalities and clinical characteristics
In a series of exploratory analyses, we evaluated the association of individual ECoG abnormalities with the following clinical variables: sex, age of onset of epilepsy, duration of epilepsy, age at surgery, number of AEDs at surgery, etiologic/histopathologic classification. In pairwise comparisons, the presence of FR and PFA were individually associated with female sex (p = 0.021 and p = 0.038, respectively) and younger age at surgery (p = 0.044 and p = 0.0010, respectively). In other pairwise analyses, the detection of intermittent spikes and sharp waves was associated with a higher burden of AEDs at the time of surgery (p = 0.011), and the presence of slowing was associated with fewer AEDs at surgery (p = 0.046) and a longer duration of epilepsy (p = 0.045). Neither historical nor active infantile spasms at surgery were associated with detection of any ECoG abnormality. There were no other significant pairwise associations.
In considering etiology/histopathology, the most common cause of epilepsy was cortical dysplasia (CD, n = 30), followed by TSC (n = 7), stroke (n = 7), Rasmussen encephalitis (n = 6), tumor (n = 5), Sturge-Weber Syndrome (n = 2), hemimegalencephaly (HME, n = 1), post-infectious injury (n = 1), and post-traumatic injury (n=1). Etiologic/histopathologic classification, categorized as developmental (CD, HME, and TSC) or acquired (all other etiologies), was not associated with the presence of FR. Although there was a trend toward more frequent discovery of FR among developmental etiologies (p = 0.083), this comparison may be confounded by age; those patients with developmental etiologies were considerably younger at the time of surgery than patients with acquired etiologies (p = 0.017). Among other ECoG findings, only continuous epileptiform discharges exhibited an association with etiologic/histopathologic classification, such that all nine instances of continuous epileptiform discharges were observed among children with developmental etiologies (p = 0.023).
3.5 Postoperative outcome
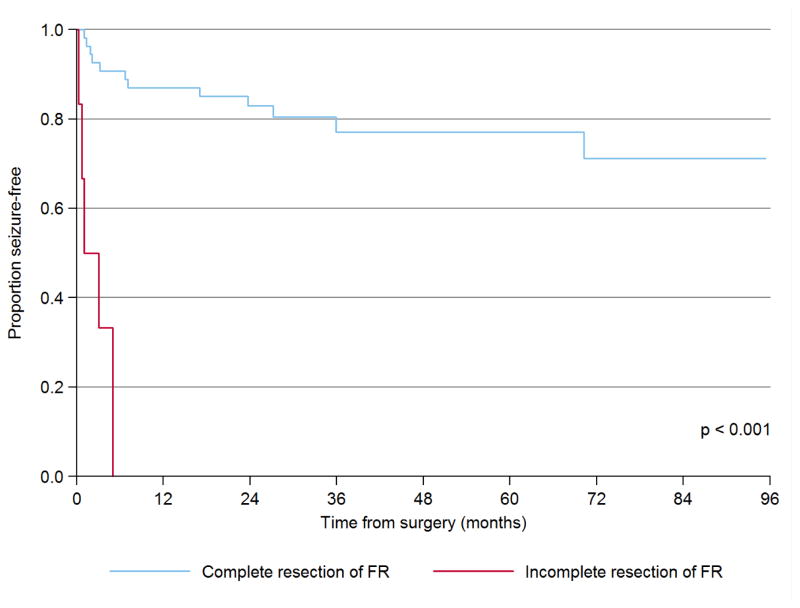
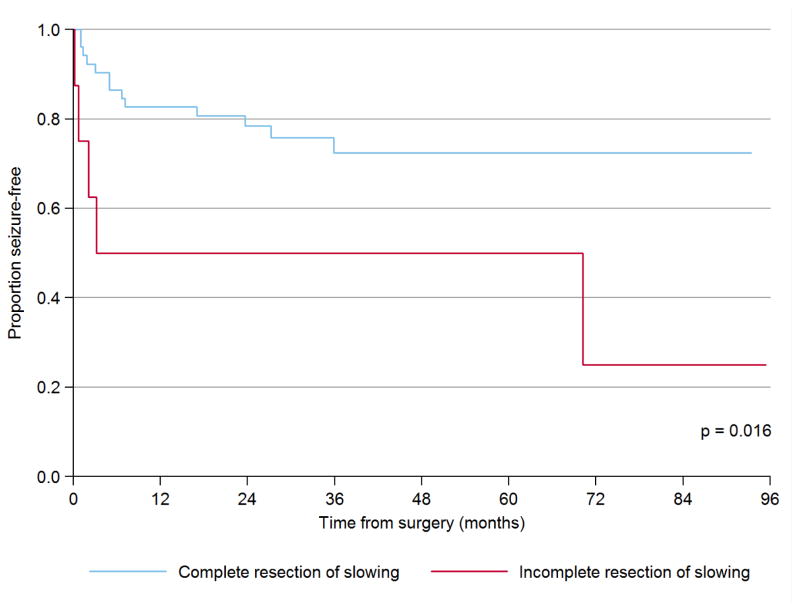
Median postoperative follow-up for this cohort was 4.1 years (2.6 – 6.6) and seizure freedom was achieved in 42 (70.0%) children, which is similar to previous reports from our institution (Hemb et al., 2010; Hussain et al., 2016; Wu et al., 2010a; Wu et al., 2010b). As expected from the literature (Fallah et al., 2015; Jayakar et al., 2016), and as summarized in Table 2, univariate Cox proportional hazards regression revealed that time to first postoperative seizure is linked to incomplete resection of the EZ (classified on the basis of preoperative evaluation—especially MRI—and specifically not considering ECoG findings), with a nearly twenty-fold hazard (HR = 19.8, p < 0.001). Similarly, time to postoperative first seizure was linked to incomplete resection of FR (HR = 25.4, p <0.001, Figure 1) and focal slowing on ECoG (HR 3.6, p = 0.016, Figure 2). However, in multivariate analysis, an incomplete resection on the basis of presurgical neuroimaging was no longer associated with elevated hazard (HR 3.0, p = 0.36) and only FR (HR 25.4, p < 0.001) and slowing (HR 5.8, p = 0.004) remained independent predictors of time to first postoperative seizure. Thus, the complete resection of FR and slowing each confer independent benefit, above and beyond the impact of an otherwise complete resection by neuroimaging. Foremost, all six children with incomplete resection of FR exhibited early surgical failure with seizures resuming within six months of surgery. None of these associations were modified by other clinical and demographic factors on multivariate analysis, and the impact of incomplete resection of FR and slowing remained highly significant when limiting the analyses to patients who did not undergo hemispherectomy, i.e. those patients among whom ECoG abnormalities could be both detected and incompletely resected (Table 2).
Table 2.
Time to first postoperative seizure
| All Patients (n = 60) | Excluding hemispherectomy (n = 34) | |||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Predictor | HR | 95%CI | Sig. | HR | 95%CI | Sig. |
|
|
|
|
||||
| Univariate analysis | ||||||
| Demographics and etiology | ||||||
| Female sex | 0.93 | 0.37 – 2.34 | 0.87 | 1.28 | 0.43 – 3.84 | 0.65 |
| Age at onset, years | 1.00 | 0.91 – 1.10 | 0.98 | 0.97 | 0.87 – 1.07 | 0.52 |
| Age at surgery, years | 1.02 | 0.94 – 1.10 | 0.62 | 0.97 | 0.89 – 1.06 | 0.57 |
| Etiology and resection | ||||||
| Etiologya | 0.59 | 0.19 – 1.79 | 0.59 | 0.90 | 0.25 – 3.23 | 0.87 |
| Incomplete Resectionb | 19.2 | 2.00 – 185.0 | 0.010 | 10.6 | 1.10 – 101.7 | 0.041 |
| ECoG Findingsc | ||||||
| FR | 19.8 | 5.95 – 65.8 | <0.001 | 12.5 | 3.49 – 45.1 | <0.001 |
| Focal slowing | 3.56 | 1.26 – 10.0 | 0.016 | 2.34 | 0.79 –7.14 | 0.126 |
| Spikesd | 2.05 | 0.27 – 15.6 | 0.489 | 1.31 | 0.17 – 10.3 | 0.80 |
| PFA | 2.85 | 0.64 – 12.6 | 0.169 | 1.83 | 0.40 – 8.38 | 0.43 |
|
|
|
|
||||
| Multivariate analysis | ||||||
| FR | 25.4 | 6.71 – 96.0 | <0.001 | 21.1 | 4.62 – 95.9 | <0.001 |
| Focal slowing | 5.79 | 1.76 – 19.0 | 0.004 | 5.60 | 1.39 – 22.5 | 0.015 |
| Spikes | 4.48 | 0.54 – 37.0 | 0.16 | 4.38 | 0.45 – 42.4 | 0.20 |
| PFA | 0.82 | 0.17 – 3.93 | 0.80 | 0.87 | 0.18 – 4.20 | 0.86 |
| Incomplete Resectionb | 3.03 | 0.28 – 33.2 | 0.36 | 2.81 | 0.25 – 31.1 | 0.40 |
Etiology was classified as developmental (e.g. focal cortical dysplasia) versus acquired (e.g. stroke)
Resections were classified as complete if the resection included the entire epileptogenic zone as defined by postoperative neuroimaging.
Hazard ratios were not calculated for continuous epileptiform discharges, focal attenuation, and ictal events, based on the paucity of cases observed with incomplete resection of these abnormalities
Spikes refer to intermittent spike or sharp-wave discharges
Abbreviations: HR, hazard ratio; ECoG, electrocorticography; FR, fast ripples; PFA, paroxysmal fast activity
Figure 1.
Impact of incomplete resection of fast ripples on postoperative seizure freedom
This is a Kaplan-Meier plot of time to first postoperative seizure as a function of complete or incomplete resection of fast ripples. The p-value is derived by multivariate Cox proportional hazards regression.
Abbreviations: FR, fast ripples
Figure 2.
Impact of incomplete resection of focal slowing on postoperative seizure freedom
This is a Kaplan-Meier plot of time to first postoperative seizure as a function of complete or incomplete resection of focal slowing. The p-value is derived by multivariate Cox proportional hazards regression.
3.6 Characteristics of individual ECoG findings
The resection of cortex corresponding to each ECoG finding was assessed using test metrics and results are summarized in Table 3. For FR, we found sensitivity of 38% (95%CI, 18 – 61), specificity of 100% (87 – 100), PPV of 100% (56 – 100), NPV of 76% (61 – 87), and accuracy of 79% (66 – 88). Of particular note, FR was the only ECoG finding with a high detection rate (prevalence), high overall accuracy, and favorable positive and negative predictive values.
Table 3.
Diagnostic/prognostic test characteristics of each ECoG abnormality
| ECoG Abnormality | Complete Resection | Incomplete Resection | Prevalence(%) | Sense (%) | Specf (%) | PPVg (%) | NPVh (%) | Accuracyi (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sz-Free | Sz | Sz-Free | Sz | |||||||
| FRa (n=48) | 32 | 10 | 0 | 6 | 80 (68 – 88) | 38 (18 – 61) | 100 (87 – 100) | 100 (56 – 100) | 76 (61 – 87) | 79 (66 – 88) |
| Slowingb (n=52) | 34 | 10 | 3 | 5 | 87 (76 – 93) | 33 (15 – 58) | 92 (78 – 98) | 63 (30 – 87) | 77 (63–87) | 75 (62 – 85) |
| Spikesc (n=45) | 30 | 13 | 1 | 1 | 75 (63 – 84) | 7 (0 – 34) | 97 (82 – 100) | 50 (9 – 91) | 70 (55 – 81) | 69 (54 – 81) |
| PFA (n=29) | 22 | 3 | 2 | 2 | 48 (36 – 61) | 40 (12 – 77) | 92 (73 – 99) | 50 (15 – 85) | 88 (69 – 97) | 83 (65 – 93) |
| CEDs (n=9) | 3 | 5 | 1 | 0 | 15 (8 – 26) | 0 (0 – 49) | 75 (29 – 97) | 0 (0 – 83) | 38 (13 – 70) | 33 (12 – 65) |
| Attend (n=10) | 4 | 5 | 1 | 0 | 17 (9 – 28) | 0 (0 – 49) | 80 (36 – 98) | 0 (0 – 83) | 44 (19 – 73) | 40 (17 – 69) |
| Seizures (n=5) | 3 | 2 | 0 | 0 | 8 (3 – 18) | 0 (0 – 71) | 100 (38 – 100) | N/Al | 60 (23 – 88) | 60 (23 – 88) |
All proportions are accompanied by a 95% confidence interval.
Fast ripples;
Focal slowing;
Intermittent spike and/or sharp-wave discharges (rhythmic discharges without evolution);
Focal attenuation;
Sensitivity was defined as the proportion of patients with postoperative seizures who exhibited incomplete resection of the ECoG abnormality;
Specificity is the proportion of patients with postoperative seizure freedom who exhibited complete resection of the ECoG abnormality;
PPV was defined as the proportion of patients with incomplete resection of an ECoG abnormality who suffered postoperative seizures;
NPV was defined as the proportion of patients with complete resection of the ECoG abnormality who became seizure-free.
Accuracy was defined by the sum of patients with (1) incomplete resection of the ECoG abnormality and seizures, and (2) complete resection of the ECoG abnormality and seizure-freedom, divided by the total number of patients exhibiting the ECoG abnormality.
No PPV could be calculated as there were no patients with incomplete resection.
Abbreviations: HR, hazard ratio; ECoG, electrocorticography; FR, fast ripples; PFA, paroxysmal fast activity; PPV. positive predictive value; NPV, negative predictive value; Sens, sensitivity; Spec, specificity; Sz, seizure; CEDs, continuous epileptiform discharges
4. Discussion
Having combined our retrospective (Wu et al., 2010a) and prospective (Hussain et al., 2016) series to yield a much larger cohort with median postoperative follow-up exceeding 4 years, this is the largest and longest study to evaluate the impact of FR on epilepsy surgery outcomes. In this study, we found evidence demonstrating that FR appear to be a superior biomarker of the EZ in comparison with other intraoperative ECoG abnormalities. Echoing our previous reports, our findings indicate that complete removal of cortex generating FR—and to a lesser extent focal slowing—greatly increases the likelihood of postoperative seizure freedom. The impact of FR appears to be especially robust as the association with seizure freedom was not confounded by demographic or etiologic factors, and that incomplete removal of FR-generating cortex independently confers more than 20-fold increased risk of surgical failure, above and beyond the impact of an otherwise complete resection by neuroimaging. In addition to adding value in surgical planning and prognosis, the feasibility of FR characterization at surgery is apparent with the high prevalence of FR (80%) on brief intraoperative ECoG and our previous demonstration that FR can be identified “live” in the operating room (Hussain et al., 2016).
Our findings are congruent with results from multiple independent investigators and previous studies. The resection of FR has been statistically linked to favorable postoperative epilepsy outcomes in multiple studies using diverse cohorts and varied methodology in the characterization of FR (Akiyama et al., 2011; Cho et al., 2014; Hussain et al., 2016; Jacobs et al., 2010; Okanishi et al., 2014; van Klink et al., 2014; van ’t Klooster et al., 2015b; J. Wu et al., 2010a). In contrast to previous reports, the large size of our cohort and the long duration of follow-up have enabled us to not only identify a cross-sectional association, but to implement longitudinal multivariate survival analyses. This allowed identification of surgical failure risk that is specifically attributable to FR, while adjusting for the effect of competing ECoG abnormalities as well as the impact of an otherwise complete resection.
Despite the risk of postoperative seizures that we have attributed to incomplete FR resection, FR characterization is not perfect, and is by no means a standalone biomarker of the EZ. In particular, eight patients in this series, and similar proportions in other reports (Jacobs et al., 2010; van Klink et al., 2014), with apparently complete FR resection went on to have postoperative seizures. Although some epileptogenic cortices may not produce FR, we believe it is more likely that we simply failed to sample EZs that were inaccessible by intraoperative ECoG. As we cannot ensure complete resection of FR-containing cortex if such cortex is never sampled, it is important to note that the use of FR data is critically dependent upon the topographic extent of ECoG sampling. Moreover, as surgical approaches (and thus exposure of cortex that can be sampled) are largely guided by preoperative neuroimaging, we may often be unable to adequately sample cortex that is distant from MRI abnormalities. In this study, our sampling may have been more extensive than would be expected at other centers because a high percentage of surgeries were hemispherectomies. Conversely, had we employed extended extraoperative ECoG, we might have had a greater opportunity to identify FR with lower event rates. This is clearly problematic as the distribution of neuroimaging abnormalities is often discordant with the topography of FR abnormalities (Hussain et al., 2016; Wu et al., 2010a). More expansive ECoG would mitigate such bias, though the potential benefit of additional sampling must be weighed against the risks of invasive monitoring, especially extended extraoperative monitoring (Schmidt et al., 2016).
It is also possible that our identification of FR was limited on a technical basis such that FR may be inadequately distinguished from ECoG background activity—especially when dependent on human visual identification. The recent demonstration that a custom low-noise amplifier increased FR-detection rates—and importantly—improved prediction of postoperative outcome is an encouraging development (Fedele et al., 2017). Further technological innovation is clearly needed, especially as it pertains to ongoing efforts to identify FR on scalp EEG (Pizzo et al., 2016).
Inasmuch as sampling limitations and bias may lead to the false impression that FR resection is complete, the opposite phenomenon is more concerning. Whereas we have reported that all six patients with incomplete FR resection suffered postoperative seizures (PPV = 100%), others have reported somewhat lower risk (PPV = 75%) attributed to residual FR (van ’t Klooster et al., 2015b). One potential explanation is that some residual FR may be physiologic rather than pathologic. Indeed, the presence of physiologic and pathologic high frequency oscillations with similar morphology certainly complicates FR characterization. However, physiologic high frequency oscillations most often occur in the ripple range (80 – 250 Hz) (Axmacher et al., 2008; Kerber et al., 2014; Melani et al., 2013; Wang et al., 2013), and although physiologic FR have been reported, they tend to be stimulus-evoked rather than spontaneous (Matsumoto et al., 2013; Nagasawa et al., 2012). A key exception may be FR arising from occipital cortex, where spontaneously occurring physiologic FR can masquerade as pathologic FR. To avoid misclassification, we emphasize FR (>250 Hz) rather than ripples (<250 Hz), and interpret occipital FR with caution.
With regard to internal validity, there is no consensus as to how FR are best identified and quantified. Inter-rater reliability in the manual identification FR has not been established, and future study is warranted to contrast the merits and limitations of manual versus automated FR detection. An initial validation of a contemporary automated FR detector was successful in that channels with automatically-detected FR matched channels with expert-identified FR well, and automatically-detected FR predicted postoperative outcomes with high PPV (Fedele et al., 2016). With regard to the identification of many established EEG phenomena (e.g. epileptiform discharges, slowing, attenuation) we have previously demonstrated that inter-rater reliability is poor (Hussain et al., 2015). Broadly speaking, we must seek consensus as to how FR are quantified (presence/absence versus event rates), determine whether ECoG sampling is best accomplished on an extraoperative or intraoperative basis, and decide whether the search for FR should be conducted before and/or after resection.
Several methodologic limitations of this study must be acknowledged. Foremost, this study is not a controlled clinical trial, and the resection of any abnormality was simply observed, rather than assigned in a randomized and blinded fashion. Although we are confident that specific ECoG abnormalities (especially FR) were, or were not, completely removed based on observed resection margins, we did not conduct post-resection ECoG in any of these patients and therefore cannot verify with certainty that specific ECoG abnormalities were completely resected. In particular, it is possible that surgical resection may lead to the production of FR not observed preoperatively, with the hypothesis that tissue at the margin of resection may become epileptogenic and thus exhibit de novo FR after resection, or that resection may modify the electrographic expression of modified/residual epileptic networks. Given these considerations, it is now our practice to ascertain FR both before and after resection. With respect to external validity, we have studied a pediatric cohort with lesional epilepsy whose resections were generally “complete”, with removal of EZs defined by multimodal preoperative noninvasive evaluation. Our findings may not generalize to adults, patients with non-lesional epilepsy, or patients for whom extraoperative ictal ECoG is warranted.
Importantly, whereas many centers often rely upon seizure-onset localization on ECoG, we recorded seizures during ECoG in only five patients. However, even among this limited subset of patients with ictal ECoG, FR and seizure-onset were topographically discordant in four of five patients, suggesting that these electrographic findings offer distinct and perhaps complimentary prognostic meaning. Indeed, in a relatively small study using extraoperative ECoG, although FR and ictal onsets are often co-localized, a minority of patients exhibited distinct FR and seizure-onset zones, and the resection of FR was a better predictor of seizure-freedom than resection of the seizure-onset zone (Cho et al., 2014).
Lastly, although we utilized a rather large cohort for this analysis, many patients underwent extensive resections, including 26 patients with hemispherectomy. As demonstrated in Table 2, these patients had essentially no opportunity to retain cortex that exhibited preoperative FR, and thus contributed little to our assertion that retained FR are independent predictors of early surgical failure. In addition, as we encountered just six patients with incomplete resection of FR, our hazard estimates are somewhat imprecise, as seen in the relatively wide confidence intervals. Accordingly, repetition of this study in a cohort with more conservative resection margins (i.e. smaller resection volume and presumably higher rate of postoperative seizures) might be useful in the effort to more accurately estimate the risk posed by unresected FR.
We believe a subsequent study—i.e. a prospective controlled trial—evaluating the hazard of unresected ictal FR would be of great value. However, we have now exposed several ethical challenges. Foremost, given that all of our patients with incompletely resected FR exhibited early surgical failure, it may not be permissible to randomize a subject to lack of FR removal, especially in cases in which extension of the resection margin confers little additive risk. In addition, whereas these data suggest that a resection should be extended when FR are encountered outside the margins of an EZ defined with standard methods, perhaps resections should be more conservative when FR are detected only within a subportion of a proposed EZ. On the other hand, given the sacrosanct (and evidence-based) belief that lesional tissue and the seizure-onset zone are critical determinants of the EZ, any clinical trial in which patients are assigned to a lack of resection of the seizure-onset zone and/or lesional tissue—supposing a lack of FR identified within the seizure-onset zone or lesion—would be met with great controversy, and appropriately so given the aforementioned limitations of sampling. Indeed, although we suggest resection of FR is necessary to achieve seizure freedom, there is little evidence indicating that FR resection alone is sufficient.
In the absence of a prospective clinical trial “proving” that complete resection of FR improves surgical outcomes, many clinicians are understandably reluctant to use FR characterization on a routine clinical basis, as this would likely yield larger surgical resections. Still, given that multiple independent groups have linked incomplete resection of FR to surgical failure, and now having established the utility of FR localization on an observational basis in the largest and longest study to date, perhaps it is no longer reasonable to ignore these data. Until a carefully designed clinical trial proves otherwise, we believe the judicious use of FR localization to refine the margins of surgical resection is both ethical and feasible. In our program, we will now begin using intraoperative FR localization—mindful of its limitations—to guide surgical resection on a routine clinical basis, especially among those patients for whom extraoperative ECoG is unnecessary. Above all, this and other studies seeking to validate FR as a biomarker of the EZ are a signal to epileptologists—and all practitioners—that epilepsy surgery is a viable means to treat many patients with refractory epilepsy, and that new technologies can be leveraged to mitigate the risk of surgery and further improve epilepsy outcomes.
Highlights.
This is the largest study to evaluate the impact of FR on surgical outcomes.
FR exhibit the best combination of positive and negative predictive value.
Residual FR independently confer greater than 25-fold increased risk of seizures.
Residual slowing independently confers 6-fold risk of postoperative seizures.
The use of FR in guiding epilepsy surgery planning is ready for a clinical trial.
Acknowledgments
Funding
This study was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS 082649).
Abbreviations
- FR
fast ripples
- ECoG
electrocorticography
- EZ
epileptogenic zone
Footnotes
Disclosure of Conflicts of Interest
Dr. Hussain has received research support from the Epilepsy Therapy Project, the Milken Family Foundation, the Hughes Family Foundation, the Elsie and Isaac Fogelman Endowment, Eisai, Lundbeck, Insys Therapeutics, GW Pharmaceuticals, and the NIH (R34MH089299), and has served on the scientific advisory boards of Questcor Pharmaceuticals, Mallinckrodt Pharmaceuticals, and Upsher-Smith Laboratories, Insys Therapeutics and as a consultant to Eisai.
Dr. Mathern serves as Co-Editor in Chief for Epilepsia, and Epilepsia-Open, and is on the editorial boards of Neurology, Epileptic Disorders, Epilepsy & Seizures, and Epilepsy Research, on the Data Management Committee of Neuropace, Inc; and receives research support from the NIH (R01NS038992[PI]), the RE Children’s Project, and is supported by the Davies/Crandall Chair for Epilepsy Research at UCLA. He has received funds to support travel in association with his volunteer work from the International League Against Epilepsy (ILAE).
Dr. Sankar serves on scientific advisory boards for and has received honoraria and funding for travel from Eisai, UCB Pharma, Sunovion, Supernus, Upsher-Smith, Acorda, and Lundbeck Pharma; receives royalties from the publication of Pediatric Neurology, 3rd ed. (Demos Publishing, 2008) and Epilepsy: Mechanisms, Models, and Translational Perspectives (CRC Press, 2011); serves on speakers’ bureaus for and has received speaker honoraria from Eisai, UCB, GlaxoSmithKline, Cyberonics, Supernus, and Lundbeck.
Dr. Wu serves on the professional advisory board for the Tuberous Sclerosis Alliance; has received honoraria from and serves on the scientific advisory board and the speakers’ bureau forNovartis Pharmaceuticals Inc. and Lundbeck; and has received research support from the Tuberous Sclerosis Alliance, Novartis Pharmaceuticals Inc., Today and Tomorrow Children’s Fund, Department of Defense/Congressionally Directed Medical Research Program, and the NIH (P20NS080199 [Co-I], U01NS082320 [Co-I], R01NS082649 [PI]), U54NS092090 [Co-I], and U01 NS092595 [Co-I]).
The remaining authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A, Coull BA. Approximate is Better than “Exact” for Interval Estimation of Binomial Proportions. The American Statistician. 1998;52:119–126. [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC, Rutka JT, Drake JM, Otsubo H. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–1811. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cho JR, Koo DL, Joo EY, Seo DW, Hong SC, Jiruska P, Hong SB. Resection of individually identified high-rate high-frequency oscillations region is associated with favorable outcome in neocortical epilepsy. Epilepsia. 2014;55:1872–1883. doi: 10.1111/epi.12808. [DOI] [PubMed] [Google Scholar]
- Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005;15:266–274. doi: 10.1111/j.1460-9592.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, Guerrini R, Mathern GW. Proposed Criteria for Referral and Evaluation of Children for Epilepsy Surgery: Recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–959. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Devlin AM, Cross JH, Harkness W, Chong WK, Harding B, Vargha-Khadem F, Neville BGR. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126:556–566. doi: 10.1093/brain/awg052. [DOI] [PubMed] [Google Scholar]
- Fallah A, Rodgers SD, Weil AG, Vadera S, Mansouri A, Connolly MB, Major P, Ma T, Devinsky O, Weiner HL, Gonzalez-Martinez JA, Bingaman WE, Najm I, Gupta A, Ragheb J, Bhatia S, Steinbok P, Witiw CD, Widjaja E, Snead OC, Rutka JT. Resective Epilepsy Surgery for Tuberous Sclerosis in Children: Determining Predictors of Seizure Outcomes in a Multicenter Retrospective Cohort Study. Neurosurgery. 2015;77:517–524. doi: 10.1227/NEU.0000000000000875. discussion 524. [DOI] [PubMed] [Google Scholar]
- Fedele T, Ramantani G, Burnos S, Hilfiker P, Curio G, Grunwald T, Krayenbühl N, Sarnthein J. Prediction of seizure outcome improved by fast ripples detected in low-noise intraoperative corticogram. Clinical Neurophysiology. 2017 doi: 10.1016/j.clinph.2017.03.038. (in press) [DOI] [PubMed] [Google Scholar]
- Fedele T, van ’t Klooster M, Burnos S, Zweiphenning W, van Klink N, Leijten F, Zijlmans M, Sarnthein J. Automatic detection of high frequency oscillations during epilepsy surgery predicts seizure outcome. Clinical Neurophysiology. 2016;127:3066–3074. doi: 10.1016/j.clinph.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Palmini A, Andermann F, Dubeau F, Da Costa JC, Felipe Quesney L, Andermann E, Olivier A. Usefulness of focal rhythmic discharges on scalp EEG of patients with focal cortical dysplasia and intractable epilepsy. Electroencephalography and Clinical Neurophysiology. 1996;98:243–249. doi: 10.1016/0013-4694(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Haegelen C, Perucca P, Châtillon CE, Andrade-Valença L, Zelmann R, Jacobs J, Collins DL, Dubeau F, Olivier A, Gotman J. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54:848–857. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AS, Cross JH, Shinnar S, Mathern BW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–155. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon N, Vinters HV, Mathern GW. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology. 2010;74:1768–1775. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SA, Kwong G, Millichap JJ, Mytinger JR, Ryan N, Matsumoto JH, Wu JY, Lerner JT, Sankar R. Hypsarrhythmia assessment exhibits poor interrater reliability: A threat to clinical trial validity. Epilepsia. 2015;56:77–81. doi: 10.1111/epi.12861. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Mathern GW, Sankar R, Wu JY. Prospective and “live” fast ripple detection and localization in the operating room: impact on epilepsy surgery outcomes in children. Epilepsy Res. 2016;127:344–351. doi: 10.1016/j.eplepsyres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakar P, Gotman J, Harvey AS, Palmini A, Tassi L, Schomer D, Dubeau F, Bartolomei F, Yu A, Kršek P, Velis D, Kahane P. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016;57:1735–1747. doi: 10.1111/epi.13515. [DOI] [PubMed] [Google Scholar]
- Kerber K, Dümpelmann M, Schelter B, Le Van P, Korinthenberg R, Schulze-Bonhage A, Jacobs J. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clin Neurophysiol. 2014;125:1339–1345. doi: 10.1016/j.clinph.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Brinkmann BH, Stead SM, Matsumoto J, Kucewicz MT, Marsh WR, Meyer F, Worrell G. Pathological and physiological high-frequency oscillations in focal human epilepsy. J Neurophysiol. 2013;110:1958–1964. doi: 10.1152/jn.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F, Zelmann R, Mari F, Gotman J. Continuous High Frequency Activity: a peculiar SEEG pattern related to specific brain regions. Clin Neurophysiol. 2013;124:1507–1516. doi: 10.1016/j.clinph.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33:569–583. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi T, Akiyama T, Tanaka SI, Mayo E, Mitsutake A, Boelman C, Go C, Snead OC, Drake J, Rutka J, Ochi A, Otsubo H. Interictal high frequency oscillations correlating with seizure outcome in patients with widespread epileptic networks in tuberous sclerosis complex. Epilepsia. 2014;55:1602–1610. doi: 10.1111/epi.12761. [DOI] [PubMed] [Google Scholar]
- Olson DM, Chugani HT, Shewmon DA, Phelps ME, Peacock WJ. Electrocorticographic Confirmation of Focal Positron Emission Tomographic Abnormalities in Children with Intractable Epilepsy. Epilepsia. 1990;31:731–739. doi: 10.1111/j.1528-1157.1990.tb05514.x. [DOI] [PubMed] [Google Scholar]
- Pizzo F, Frauscher B, Ferrari-Marinho T, Amiri M, Dubeau F, Gotman J. Detectability of Fast Ripples (>250 Hz) on the Scalp EEG: A Proof-of-Principle Study with Subdermal Electrodes. Brain Topogr. 2016;29:358–367. doi: 10.1007/s10548-016-0481-7. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, Lerner JT, Sankar R, Shields WD, Engel J, Jr, Fried I, Miyata H, Yong WH, Vinters HV, Mathern GW. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594–1601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RF, Wu C, Lang MJ, Soni P, Williams KA, Boorman DW, Evans JJ, Sperling MR, Sharan AD. Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia. 2016;57:1697–1708. doi: 10.1111/epi.13503. [DOI] [PubMed] [Google Scholar]
- Sperling M. Current Practice of Clinical Electroencephalography. Lippincott Williams & Wilkins; Philadelphia: 2003. Intracranial electroencephalography; pp. 639–680. [Google Scholar]
- van Klink NEC, Van’t Klooster MA, Zelmann R, Leijten FSS, Ferrier CH, Braun KPJ, van Rijen PC, van Putten MJaM, Huiskamp GJM, Zijlmans M. High frequency oscillations in intra-operative electrocorticography before and after epilepsy surgery. Clin Neurophysiol. 2014;125:2212–2219. doi: 10.1016/j.clinph.2014.03.004. [DOI] [PubMed] [Google Scholar]
- van ’t Klooster MA, Leijten FSS, Huiskamp G, Ronner HE, Baayen JC, van Rijen PC, Eijkemans MJC, Braun KPJ, Zijlmans M. High frequency oscillations in the intra-operative ECoG to guide epilepsy surgery (“The HFO Trial”): study protocol for a randomized controlled trial. Trials. 2015a;16:422. doi: 10.1186/s13063-015-0932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Klooster MA, van Klink NEC, Leijten FSS, Zelmann R, Gebbink TA, Gosselaar PH, Braun KPJ, Huiskamp GJM, Zijlmans M. Residual fast ripples in the intraoperative corticogram predict epilepsy surgery outcome. Neurology. 2015b;85:120–128. doi: 10.1212/WNL.0000000000001727. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, Najm IM, So NK. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54:370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Koh S, Sankar R, Mathern GW. Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Res. 2008;82:99–106. doi: 10.1016/j.eplepsyres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010a;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Salamon N, Kirsch HE, Mantle MM, Nagarajan SS, Kurelowech L, Aung MH, Sankar R, Shields WD, Mathern GW. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010b;74:392–398. doi: 10.1212/WNL.0b013e3181ce5d9e. [DOI] [PMC free article] [PubMed] [Google Scholar]