Abstract
Arsenite directly binds to the zinc finger domains of the DNA repair protein poly (ADP ribose) polymerase (PARP)-1, and inhibits PARP-1 activity in the base excision repair (BER) pathway. PARP inhibition by arsenite enhances ultraviolet radiation (UVR)- induced DNA damage in keratinocytes, and the increase in DNA damage is reduced by zinc supplementation. However, little is known about the effects of arsenite and zinc on the zinc finger nucleotide excision repair (NER) protein xeroderma pigmentosum group A (XPA). In this study, we investigated the difference in response to arsenite exposure between XPA and PARP-1, and the differential effectiveness of zinc supplementation in restoring protein DNA binding and DNA damage repair. Arsenite targeted both XPA and PARP-1 in human keratinocytes, resulting in zinc loss from each protein and a pronounced decrease in XPA and PARP-1 binding to chromatin as demonstrated by Chip-on-Western assays. Zinc effectively restored DNA binding of PARP-1 and XPA to chromatin when zinc concentrations were equal to those of arsenite. In contrast, zinc was more effective in rescuing arsenite-augmented direct UVR- induced DNA damage than oxidative DNA damage. Taken together, our findings indicate that arsenite interferes with PARP-1 and XPA binding to chromatin, and that zinc supplementation fully restores DNA binding activity to both proteins in the cellular context. Interestingly, rescue of arsenite- inhibited DNA damage repair by supplemental zinc was more sensitive for DNA damage repaired by the XPA-associated NER pathway than for the PARP-1-dependent BER pathway. This study expands our understanding of arsenite’s role in DNA repair inhibition and co-carcinogenesis.
Keywords: Arsenite, XPA, PARP-1, Zinc, DNA repair
Introduction
DNA damage plays an important role in cancer and aging (Hoeijmakers, 2009), while DNA repair protects the genome from these consequences (Wei, 1998). Nucleotide excision repair (NER) primarily repairs a wide range of bulky DNA adducts, while base excision repair (BER) removes most small base modifications (Rastogi et al., 2010). Zinc finger proteins xeroderma pigmentosum group A (XPA) and poly(ADP-ribose) polymerase 1 (PARP-1) are central regulators of NER and BER, respectively. Moreover, PARP-1 is also implicated in NER through an interaction with XPA which links PARP-1 to the repair of ultraviolet radiation (UVR)-induced photoproducts (King et al., 2012; Robu et al., 2013). We have shown that arsenite can inhibit DNA damage repair by directly replacing the zinc within PARP-1 and XPA zinc finger motifis (Zhou et al., 2011). Moreover, similar effects were observed when cells were treated with the zinc chelator TPEN, resulting in reduced PARP-1 activity and inhibition of DNA repair (Sun et al., 2014). These findings highlight the importance of arsenite’s interaction with zinc-dependent DNA repair proteins.
Zinc is an essential element within numerous proteins involved in cellular defense against oxidative stress and DNA damage repair (Ho et al., 2003; Ho, 2004; Song et al., 2009), and in zinc finger proteins, zinc deficiency results in decreased protein function (Kunzmann et al., 2008). Our previous studies demonstrated that zinc supplementation could restore arsenite-inhibited PARP-1 activity and reduce oxidative DNA damage in human keratinocytes and mouse thymus cells (Qin et al., 2008b; Ding et al., 2009; Xu et al., 2016). Moreover, the beneficial effect of zinc supplementation was found to be due to zinc counteracting the interaction between arsenite and PARP-1 protein, rather than through a global effect on BER or through zinc’s antioxidant properties (Cooper et al., 2013). Taken together, current findings in the literature suggest that zinc supplementation may mitigate the effects of arsenite on impaired PARP-1 function, enhanced DNA damage and inhibition of DNA damage repair responses.
Since arsenite co-exposure increases retention of UVR- induced direct photolesions including 6,4-photoproducts ((6-4)PPs) and cyclobutane pyrimidine dimers (CPDs) in keratinocytes, and zinc alleviates arsenite- induced retention of UVR-induced oxidative and direct DNA damage (Cooper et al., 2013), we hypothesize that arsenite and zinc would affect direct DNA damage levels by regulating the activity and function of XPA in the NER pathway, in a similar manner to that identified for PARP-1 in the BER pathway. In this study, we investigated the potential differential sensitivity to arsenite of XPA versus PARP-1, and the differential sensitivity of zinc in restoring XPA or PARP-1 DNA binding activity and DNA damage repair.
Methods
Chemicals
Sodium meta-arsenite and zinc chloride were purchased from Fluka Chemie (Buchs, Germany), and freshly prepared in phosphate buffered saline (PBS) (Invitrogen, Carlsbad, CA).
Cell culture
Normal neonatal human epidermal keratinocytes (HEKn) and DermaLife K culture medium supplemented with LifeFactors and penicillin/streptomycin were purchased from Lifeline Cell Technology (Oceanside, CA). Cells were cultured in a 5% CO 2-humidified incubator at 37°C.
UV Source
UVR exposures were performed using an Orie l 1000 Watt Solar Ultraviolet Simulator (Oriel Corp., Stratford, CT). This solar simulator produces a high intensity UVR beam in both the UVA (320–400 nm) and UVB (280–320 nm) spectrum with an emission ratio of 14:1 (UVA: UVB). The proportion and intensity of UVA/UVB was measured using a radiospectrometer (Optronics Laboratories, Inc.; Orlando, FL) and exposure time was calculated to give the desired doses.
Antibodies
The following commercially available antibodies were used: anti-(6-4) photoproducts clone KTM50 and anti-thymine dimer clone KTM53 (CPD) (Kamiya Biomedical Company) for immunocytochemistry, anti-8-OHdG (N45.1, Abcam, Cambridge, MA), anti-phospho-H2AX (Cell Signaling Technologies, Danvers, MA), anti-PARP (Cell Signaling; 9542 for immunoprecipitation and western blot), anti- XPA (Abcam; ab85914 for immunoprecipitation and ab2352 for western blotting), anti-GAPDH (Millipore), anti-rabbit and anti- mouse IgG, horseradish peroxidase (HRP)-conjugated antibodies (Promega), and donkey anti- mouse or goat anti-rabbit IgG, Cy3-conjugated antibody (Millipore).
Zinc Finger Protein Isolation by Immunoprecipitation and Measurement of Zinc Content in Protein
Human keratinocytes were treated with varying concentration of arsenite, zinc or both compounds for 24 h. Cells were then harvested in Pierce IP lysis buffer (Thermo Fisher Scientific), sonicated, and centrifuged at 14,000 rpm for 15 min at 4°C to remove cellular debris as described previously (Zhou et al., 2011). Protein (500 μg in 500 μL) was incubated with 2 μL of rabbit polyclonal antibody (PARP-1, Cell Signaling #9542 or XPA, Abcam ab85914) for at least 2 h at 4 °C. Protein A dynabeads (Invitrogen) were added in a 1:1 slurry, and samples were incubated for an additional 2 h at 4 °C. The beads were recovered by centrifugation at 2,000 rpm for 5 min at 4°C and washed five times with 1 mL of wash buffer. To elute protein, the pellets were incubated with 100 μL of 100 mM citric acid (pH 3.0) for 30 min, followed by centrifugation at 2,000 rpm for 5 min at 4 °C. The supernatant was adjusted to pH 7 with 10 M NaOH. Immunoprecipitated protein was incubated with 10 mM H2O2 for 1 h at 4°C to release zinc from proteins. Zinc content was measured by adding 10 μL of 1 mM 4-(2-pyridylazo)resorcinol to 100 μL of protein sample followed by scanning the UV-vis spectra at 350 to 550 nm on a SpectraMax M2 spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA). The absorbance of resorcinol shifts from 411 to 493 nm in the presence of zinc, and the 493 nm peak is recorded and compared with a standard curve for calculation of zinc content in protein samples.
Chip-on-Western
Human keratinocytes were treated with varying concentrations of arsenite, zinc or both compounds for 24 h. Chromatin preparation and collection protocols were followed as previously described (King et al., 2012). Following collection, chromatin suspension was sonicated on ice (90 s) in immunoprecipitation lysis buffer (0.01 M Tris-HCl, pH 8.0, 0.14 M NaCl, 1% Triton X-100, 0.1% deoxycholate, 1% SDS) using a Branson Sonifer (output, 3; duty, 40%; pulsed). The samples were isolated by centrifugation (13,000 rpm at 4°C for 15 min) with the supernatant containing cross- linked chromatin. A 50 μl aliquot of the supernatant was used to determine DNA concentration using the DNeasy blood and tissue kit (Qiagen). For each sample, an equal amount of cross- linked chromatin (40–50 μg) was immunoprecipitated with 0.2 μg of specific antibody (XPA or PARP-1) overnight at 4°C. The immunocomplexes were adsorbed onto protein A Dynabeads (Invitrogen) for 3 h at 4°C. The samples were washed five times in ChIP wash buffer (Santa Cruz), resuspended in loading buffer, and boiled for 10 min at 95 °C before loading onto a 10% polyacrylamide gel followed by western blotting.
Immunocytochemistry
Human keratinocytes were either left untreated, or treated with varying concentrations of arsenite, zinc, or both for 18 h, then exposed to ssUVR (3 kJ/m2) and cultured for an additional 6 h. Cells were fixed with cold MeOH for 10 min. Fixed cells were then permeabilized and DNA was denatured by treatment with 4 N HCl for 10 min followed by pH adjustment with 50 mM Tris (pH 10) for 5 min at room temperature as previously reported (Ding et al., 2009; Cooper et al., 2013). The cells were blocked in 10% normal horse serum in PBS overnight. Anti-(6-4)PP (1:300), CPD (1:200), 8-OHdG (1:400) or pH2AX (1:200) antibody diluted in 2% normal serum/PBS was incubated with cells for 1 h at room temperature. The cells were washed five times with PBS and then incubated with Cy3-conjugated secondary antibody (1:500) for 45 min at room temperature. The cells were washed with PBS three times and mounted with Vectashield plus DAPI (Vector Laboratories). The images were obtained using an Olympus IX70 fluorescence microscope equipped with a DP72 digital camera and cellSens Dimension imaging software. The images used for comparison were acquired with the same exposure time. Five images per group were obtained, and intensity measurements were quantified using Image J (National Institutes of Health).
Statistical Analysis
Statistical analysis was performed using ANOVA and Student’s t-test. Data were presented as means ± S.D from at least 3 independent experiments. A value of P < 0.05 was considered statistically significant.
Results
Comparison of the impact of arsenite exposure and zinc supplementation on zinc content of XPA and PARP-1 protein
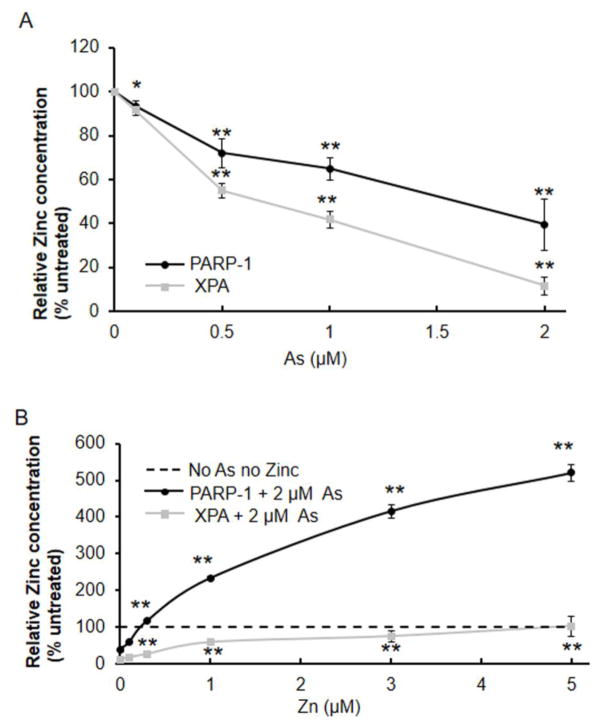
Although we previously reported that arsenite selectively binds with C3H1 (PARP-1) and C4 (XPA) zinc finger proteins (Zhou et al., 2011), little is known about the potential difference in arsenite interaction with each target. As shown in Figure 1A, arsenite displaced zinc from PARP-1 and XPA in a concentration-dependent manner, resulting in decreased zinc content for both proteins. We next compared the concentration dependence for zinc restoration of zinc content in the presence of 2 μM arsenite. Figure 1B shows that zinc supplementation increased the zinc content of both immunoprecipitated PARP-1 and XPA proteins in a zinc concentration-dependent manner. When keratinocytes were treated with 2 μM arsenite and 5 μM zinc, zinc content in XPA was restored to the same level as in untreated cells, whereas the zinc content in PARP-1 exceeded the basal level by about 5 fold. This suggests that in normal keratinocytes the zinc-binding sites in PARP-1 might not be fully occupied.
Fig. 1.
Effects of arsenite and zinc on zinc content in immunoprecipitated PARP-1 and XPA proteins. Human keratinocytes were treated with varying concentrations of arsenite (A), or 2 μM arsenite and varying concentration of zinc (B) for 24 h. PARP-1 or XPA was immunoprecipitated from cell lysates. Zinc content of immunoprecipitated protein was measured by 4-(2-pyridylazo)resorcinol. Statistical comparisons were performed between untreated and treated with arsenite (A) or between arsenite and both (As+Zn) (B). *, p <0.05; **, p <0.01.
Arsenite suppresses the chromatin binding activity of XPA and PARP-1, and zinc supplementation markedly improves DNA binding activity in the presence of arsenite
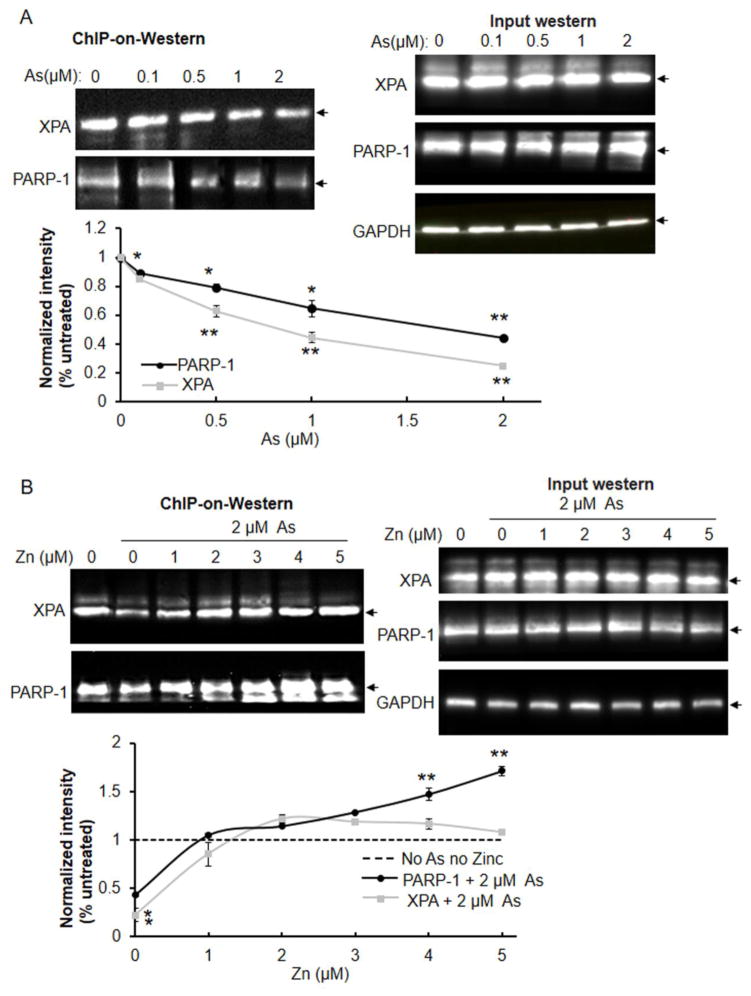
We examined whether arsenite- induced zinc loss would decrease XPA and PARP-1 protein DNA binding function in cells using a modified chromatin-on-western blot approach. Arsenite inhibited chromatin association with PARP-1 and XPA in a concentration-dependent manner, but gave no effects on the expression levels of not only PARP-1 but also XPA (Fig. 2A), suggesting that arsenite serves as a broad inhibitor of excision repair. Zinc supplementation reversed the inhibitory effect of arsenite on the chromatin binding activity of XPA and PARP-1 in a zinc concentration-dependent manner, but had no impacts on the protein levels of both proteins (Fig. 2B). Interestingly, co-treatment with 2 μM zinc restored XPA and PARP-1 binding to control (no arsenite) levels, while increasing the concentration of zinc to 5 μM led to further elevation of chromatin binding by PARP-1, but not by XPA.
Fig. 2.
Effects of arsenite and zinc on the association between XPA or PARP-1 and chromatin DNA. Human keratinocytes were treated with varying concentration of arsenite (A) or 2 μM arsenite and varying concentrations of zinc (B) for 24 h. Cells were fixed and lysed using a modified chromatin immunoprecipitation method (ChIP-on-Western) as described under Methods. PARP-1 or XPA was immunoprecipitated (IP) from chromatin complexes and the proteins were detected by immunoblot. Protein intensity was quantified by Image J. Statistical comparisons were performed between untreated and treated with arsenite (A) or both (As+Zn) (B). The expression levels of both proteins were detected in treated keratinocytes. *, p <0.05; **, p <0.01. Arrow head indicates the target protein.
Direct DNA damage is more sensitive to arsenite and zinc than oxidative DNA damage
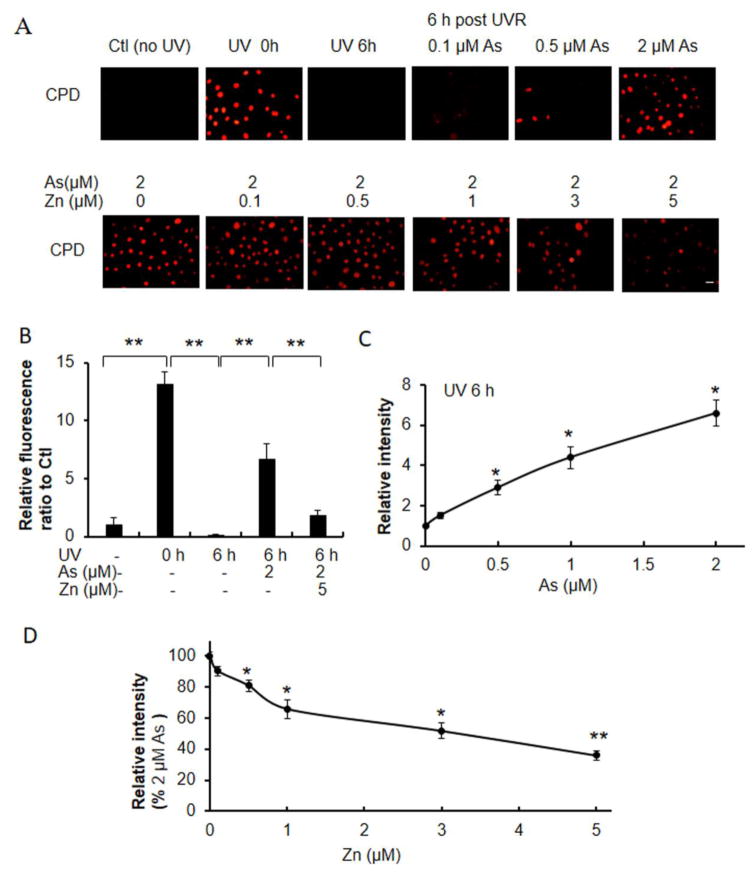
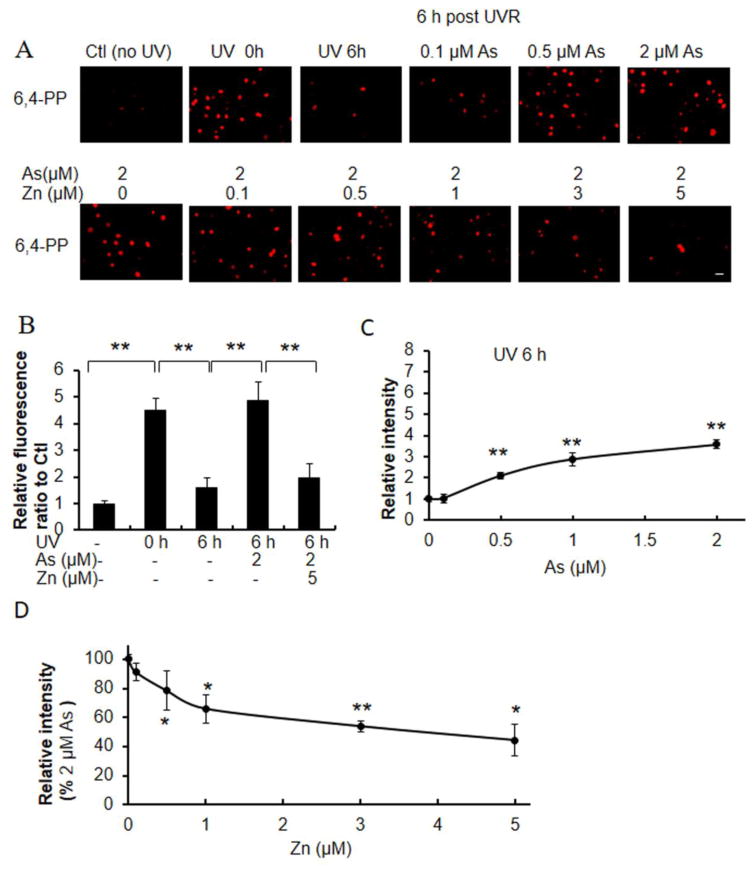
CPDs and (6-4)PPs are direct DNA damage lesions caused by UVR- induced photochemical reactions, that are repaired over time. In comparison to keratinocytes exposed to UVR alone, co-exposure to increasing concentrations of arsenite caused increased retention of UVR- induced CPDs and (6-4)PPs 6 hours after UVR exposure (Figs. 3A and 4A). Keratinocytes treated with 2 μM arsenite retained 50% of CPDs at 6 h post-UVR compared to initial CPDs (Fig. 3B). In contrast only 1.3% of CPDs remained 6h after UVR exposure in the absence of arsenite (Fig. 3B). Importantly, zinc supplementation decreased arsenite- mediated CPD retention in a zinc-concentration dependent manner (Fig 3A, 3D). Treatment of keratinocytes with 2 μM arsenite and 5 μM zinc reduced CPD retention to 14% of the initial damage, or to 28% of that detected with arsenite alone (Figs. 3A, 3D). Similar findings were observed for (6-4)PP. Keratinocytes treated with 2 μM arsenite retained 107% of CPDs at 6 h post-UVR compared to initial CPD levels (Fig. 4B). In contrast 35% of CPDs remained 6h after UVR exposure in the absence of arsenite (Fig. 4B). Importantly, zinc supplementation decreased arsenite-mediated CPD retention in a zinc-concentration dependent manner (Fig 4A, 4D). Treatment of keratinocytes with 2 μM arsenite and 5 μM zinc reduced CPD retention to 44% of the initial damage, or to 40% of that detected with arsenite treatment alone (Figs. 4A, 4D).
Fig. 3.
Effects of zinc on arsenite-dependent and ssUVR-induced CPD formation. HEKn cells were grown on 12-well plates with slide coverslips and treated with varying concentrations of arsenite, 2 μM arsenite and varying concentrations of zinc, or cultured untreated for 18 h. Cells were unexposed or exposed to ssUVR (UVR, 3 kJ/m2), fixed after 0 h or 6 h and stained for CPDs (A). Quantification of relative fluorescence intensity was performed via Image J analysis. (panels B–D). Statistical comparisons were performed between unexposed (Ctl) and exposed to UVR with or without arsenite and zinc (B), between untreated and treated with arsenite for 6 h after UVR (C) and between arsenite and both (As+Zn) (D). *, p <0.05; **, p <0.01. Bar indicates 20 μm.
Fig. 4.
Effects of zinc on arsenite-dependent and ssUVR- induced (6-4)PP formation. HEKn cells were grown on 12-well plates with slide coverslips and treated with varying concentrations of arsenite, or 2 μM arsenite and varying concentrations of zinc or untreated for 18 h. Cells were next unexposed or exposed to ssUVR (UVR, 3 kJ/m2), fixed at 0 h or 6 h and stained for (6-4)PP (A). Quantification of relative fluorescence intensity was performed via Image J analysis (panels B–D). Statistical comparisons were performed between unexposed (Ctl) and exposed to UVR with or without arsenite and zinc (B), between untreated and treated with arsenite for 6 h after UVR (C) and between arsenite and both (As+Zn) (D). *, p <0.05; **, p <0.01. Bar indicates 20 μm.
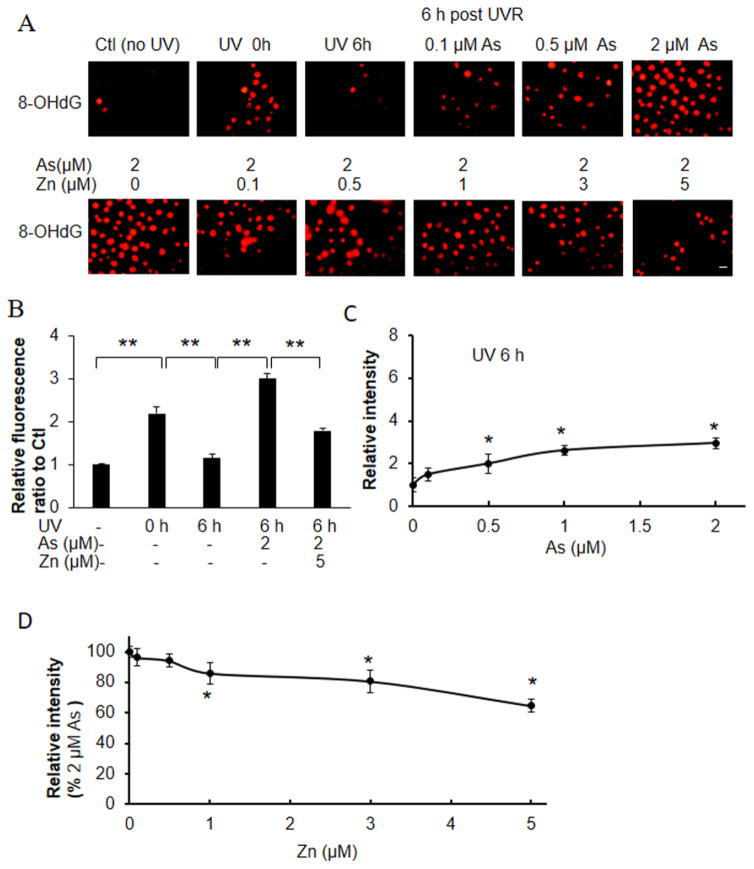
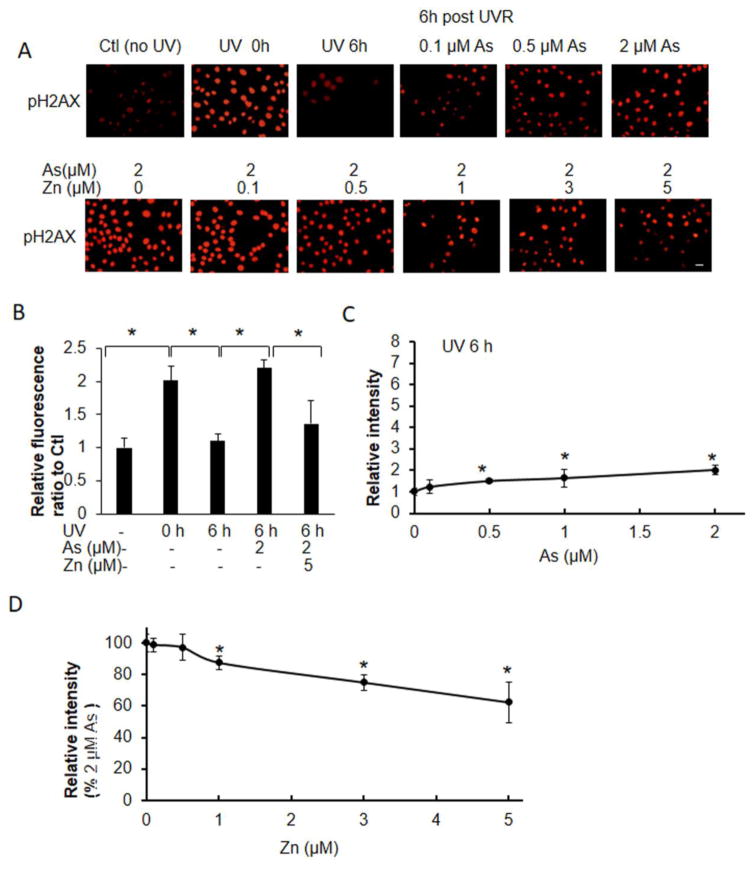
We then analyzed the effect of arsenite and zinc on UVR-induced strand break as detected by pH2AX and oxidative DNA damage as detected by 8-OHdG, which reflect DNA damage mediated through UVR- generated reactive oxygen species (Figs. 5 and 6). Exposure of keratinocytes to UVR induced approximately 2- fold increase in pH2AX and 8-OHdG staining, and this DNA damage returned to basal levels 6 h post radiation (Figs. 5B and 6B). Arsenite increased the retention of pH2AX and 8-OHdG at 6h post-UVR exposure by 143 and 110%, respectively, compared to cells that were not treated with arsenite (Figs. 5C and 6C). Zinc supplementation decreased arsenite-mediated pH2AX and 8-OHdG retention in a zinc-concentration dependent manner (Fig 5A, 5D; Fig 6A, 6D). Treatment of keratinocytes with 2 μM arsenite and 5 μM zinc reduced pH2AX and 8-OHdG retention to 68 and 82% of the initial damage, respectively. The respective levels of pH2AX and 8-OHdG detected in the presence of arsenite and zinc were 61 and 63% of levels detected with arsenite alone (Figs. 5, 6).
Fig. 5.
Effects of zinc on arsenite-dependent and ssUVR- induced 8-OHDG formation. HEKn cells were grown on 12-well plates with slide coverslips and treated with arsenite, zinc, both (As+Zn) or untreated for 18 h. Cells were subsequently unexposed or exposed to ssUVR (UVR, 3 kJ/m2), fixed at 0 h or 6 h and stained for 8-OHDG (A). Quantification of relative fluorescence intensity was performed via Image J analysis (panels B–D). Statistical comparisons were performed between unexposed (Ctl) and exposed to UVR with or without arsenite and zinc (B), between untreated and treated with arsenite for 6 h after UVR (C) and between arsenite and both (As+Zn) (D). *, p <0.05. Bar indicates 20 μm.
Fig. 6.
Effects of zinc on arsenite-dependent and ssUVR- induced pH2AX formation. HEKn cells were grown on 12-well plates with slide coverslips and treated with arsenite, zinc, both (As+Zn) or untreated for 18 h. Cells were subsequently unexposed or exposed to ssUVR (UVR, 3 kJ/m2), fixed at 0 h or 6 h and stained for pH2AX (A). Quantification of relative fluorescence intensity was performed via Image J analysis (panels B–D). Statistical comparisons were performed between unexposed (Ctl) and exposed to UVR with or without arsenite and zinc (B), between untreated and treated with arsenite for 6 h after UVR (C) and between arsenite and both (As+Zn) (D). *, p <0.05. Bar indicates 20 μm.
Discussion
We have reported that arsenite interacts with zinc finger proteins containing C3H1 (PARP-1) or C4 (XPA) motifs (Zhou et al., 2011), but not C2H2 proteins (Zhou et al., 2014). Arsenite enhanced UVR- induced oxidative DNA damage by inhibiting the activity and function of PARP-1 (Sun et al., 2014; Zhou et al., 2016). The present study demonstrates that arsenite displaces zinc and inhibits chromatin association of the NER protein XPA in a similar manner to that of PARP-1. These findings suggest that arsenite may serve as a broad inhibitor of excision repair by targeting the zinc finger motifs of key DNA repair proteins.
Zinc is an essential element in the maintenance of human health. Zinc deficiency has been shown to lead to increased oxidative stress, DNA damage and the development of cancer (Oteiza et al., 2000; Ho et al., 2003; Ho, 2004), while zinc supplementation abolished arsenite enhancement of UVR-stimulated 8-OHdG generation and restored PARP-1 activity (Qin et al., 2008a). Zinc supplementation has also been shown to reverse DNA damage retention and mutagenesis by arsenite in both human cells and in an in vivo mouse model (Cooper et al., 2013). In the present study, we found that zinc could compete with arsenite for the same zinc finger binding site, rescue zinc loss from XPA protein, and restore the DNA binding activity of XPA. Intriguingly, even in the presence of 2 μM arsenite, treatment of normal keratinocytes with 5 μM zinc increased the zinc content of PARP-1 protein by about 5 fold, while PARP-1 activity was increased by about 1.7 fold as compared to untreated control groups. It has previously been shown that zinc demonstrates an extremely low affinity for a peptide based upon the first zinc finger motif of PARP-1, relative to other previously investigated zinc finger motifs (Bossak et al., 2015), suggesting that a significant proportion of cellular PARP-1 may exist wherein the first zinc finger remains in an unbound state. The results of our current study further support the idea that zinc-binding sites in PARP-1 may not be completely occupied in normal keratinocytes under physiological conditions. If such a phenomenon is present, it is possible that the increased cellular concentrations of labile zinc resulting from supplementation could have a particularly strong impact on PARP-1 in the context of rescuing adverse effects of arsenite exposure. Overall, our findings further establish that zinc supplementation might be a potentially effective treatment to mitigate the impact of arsenite exposure through targeting both the BER protein PARP-1 and the NER protein XPA, thus preventing arsenite enhancement of UVR-induced carcinogenesis.
It has been shown that arsenite co-exposure increases the retention of UVR- induced indirect DNA damage including strand break and oxidative lesions, and direct photo-lesions including (6-4)PP and CPD in keratinocytes (Ding et al., 2008; Qin et al., 2008a; Qin et al., 2008b; Ding et al., 2009). Arsenite decreased zinc content in zinc finger targets with a concomitant decrease in DNA binding and disruption of zinc finger protein function, subsequently leading to DNA damage retention (Qin et al., 2008b; Ding et al., 2009). Here for the first time, we show that both XPA and PARP-1 are sensitive targets for arsenite as demonstrated by zinc loss and decreased binding with chromatin DNA. Furthermore, zinc supplementation is more sensitive in rescuing arsenite enhancement of direct DNA damage ((6-4)-PP and CPD) than oxidative DNA damage (strand break and 8-OHdG), despite restoration of the zinc content and binding activity of PARP-1 to beyond basal level. Interestingly, ATM protein at site-specific DSBs required functional NBS1 protein, and two proteins were also required for efficient recruitment of the repair cofactor XRCC4 to DSBs, and for efficient DSB repair (Berkovich et al., 2007). PARP-1 interacts with ATM and binds to double strand DNA breaks, facilitating DNA DSB repair (Aguilar-Quesada et al., 2007). We found that the decreased activity of PARP-1 accounts for an apparently diminished repair of DSB, which is observed within 6 hours as previously described (Cooper et al., 2013), although in an indirect PARP-1- dependent manner. Taken together, these results show that the NER pathway is more sensitive to zinc supplementation, indicating that in addition to XPA, there might be other arsenite-sensitive zinc finger DNA repair targets in the NER pathway which require further study. Moreover, other carcinogenic chromium(VI) leads to the extensive formation of Cr (III)-DNA phosphate adducts and DNA–protein cross- links (DPCs), XPA/NER is important to prevent and remove these formations (Reynolds et al., 2004; Zecevic et al., 2010), showing their common inhibitory roles in genotoxic effects.
In summary, our present study reveals that both the NER protein XPA and the BER protein PARP-1 are sensitive arsenite targets. Moreover, zinc shows differential efficacy in reversing arsenite- induced zinc loss from both proteins and in restoration of their DNA binding activity, thereby leading to differences in reduction of UVR- induced direct and oxidative DNA damage. These findings improve our understanding of arsenite’s impact on DNA damage and co -carcinogenesis by demonstrating that arsenite acts upon distinct specific targets to inhibit excision repair, and that zinc supplementation may show variable contributions to restoring the DNA repair processes dependent upon each of these distinct targets.
Highlights.
Arsenite suppresses the chromatin binding activity of XPA and PARP-1.
Arsenite has greater effect on retention of direct DNA damage such as CPDs and 6.4-PPs.
Arsenite inhibits DNA repair through both BER and NER pathways.
Acknowledgments
The authors would like to acknowledge support from the U.S. National Institutes of Health (R01ES021100, R01ES021100-S1 and R01CA182969) and trainee matching pilot fund #1127 (to Xixi Zhou) through the UNM Comprehensive Cancer Center NCI 2P30 CA118100.
Abbreviations
- PARP-1
poly (ADP-ribose) polymerase 1
- NER
Nucleotide excision repair
- BER
base excision repair
- HEKn
Normal neonatal human epidermal keratinocytes
- XPA
xeroderma pigmentosum group A
- UVR
ultraviolet radiation
- (6-4)PP
6,4-photoproduct
- CPD
cyclobutane pyrimidine dimer
Footnotes
Authorship Contributions
Ke Jian Liu and Laurie G. Hudson conceived and designed the experiments; Xiaofeng Ding performed the experiments; Xixi Zhou, Karen L. Cooper, and Juliana Huestis analyzed the data; Xiaofeng Ding, Ke Jian Liu and Laurie G. Hudson wrote the manuscript.
Conflicts of Interest
The authors declare there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Quesada R, Munoz-Gamez JA, Martin-Oliva D, Peralta A, Valenzuela MT, Matinez-Romero R, Quiles-Perez R, Menissier-de Murcia J, de Murcia G, Ruiz de Almodovar M, Oliver FJ. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol Biol. 2007;8:29. doi: 10.1186/1471-2199-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich E, Monnat RJ, Jr, Kastan MB. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol. 2007;9:683–690. doi: 10.1038/ncb1599. [DOI] [PubMed] [Google Scholar]
- Bossak K, Goch W, Piatek K, Fraczyk T, Poznanski J, Bonna A, Keil C, Hartwig A, Bal W. Unusual Zn(II) Affinities of Zinc Fingers of Poly(ADP-ribose)Polymerase 1 (PARP-1) Nuclear Protein. Chem Res Toxicol. 2015;28:191–201. doi: 10.1021/tx500320f. [DOI] [PubMed] [Google Scholar]
- Cooper KL, King BS, Sandoval MM, Liu KJ, Hudson LG. Reduction of arsenite-enhanced ultraviolet radiation- induced DNA damage by supplemental zinc. Toxicol Appl Pharmacol. 2013;269:81–88. doi: 10.1016/j.taap.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Hudson LG, Sun X, Feng C, Liu KJ. As(III) inhibits ultraviolet radiation-induced cyclobutane pyrimidine dimer repair via generation of nitric oxide in human keratinocytes. Free Radic Biol Med. 2008;45:1065–1072. doi: 10.1016/j.freeradbiomed.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- King BS, Cooper KL, Liu KJ, Hudson LG. Poly(ADP-ribose) contributes to an association between poly(ADP-ribose) polymerase-1 and xeroderma pigmentosum complementation group A in nucleotide excision repair. J Biol Chem. 2012;287:39824–39833. doi: 10.1074/jbc.M112.393504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann A, Dedoussis G, Jajte J, Malavolta M, Mocchegiani E, Burkle A. Effect of zinc on cellular poly(ADP-ribosyl)ation capacity. Exp Gerontol. 2008;43:409–414. doi: 10.1016/j.exger.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Oteiza PI, Clegg MS, Zago MP, Keen CL. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic Biol Med. 2000;28:1091–1099. doi: 10.1016/s0891-5849(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008a;21:1806–1813. doi: 10.1021/tx8001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Timmins GS, Liu KJ. Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol Appl Pharmacol. 2008b;232:41–50. doi: 10.1016/j.taap.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi RP, Richa Kumar A, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Peterson E, Quievryn G, Zhitkovich A. Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J Biol Chem. 2004;279:30419–30424. doi: 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- Robu M, Shah RG, Petitclerc N, Brind’Amour J, Kandan-Kulangara F, Shah GM. Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair. Proc Natl Acad Sci U S A. 2013;110:1658–1663. doi: 10.1073/pnas.1209507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009;139:1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhou X, Du L, Liu W, Liu Y, Hudson LG, Liu KJ. Arsenite binding-induced zinc loss from PARP-1 is equivalent to zinc deficiency in reducing PARP-1 activity, leading to inhibition of DNA repair. Toxicol Appl Pharmacol. 2014;274:313–318. doi: 10.1016/j.taap.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q. Effect of aging on DNA repair and skin carcinogenesis: a minireview of population-based studies. J Investig Dermatol Symp Proc. 1998;3:19–22. [PubMed] [Google Scholar]
- Xu H, Zhou X, Wen X, Lauer FT, Liu KJ, Hudson LG, Aleksunes LM, Burchiel SW. Environmentally Relevant Concentrations of Arsenite Induce Dose-Dependent Differential Genotoxicity Through Poly(ADP-Ribose) Polymerase Inhibition and Oxidative Stress in Mouse Thymus Cells. Toxicol Sci. 2016;149:31–41. doi: 10.1093/toxsci/kfv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic A, Hagan E, Reynolds M, Poage G, Johnston T, Zhitkovich A. XPA impacts formation but not proteasome-sensitive repair of DNA-protein cross-links induced by chromate. Mutagenesis. 2010;25:381–388. doi: 10.1093/mutage/geq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Huestis J, Xu H, Burchiel SW, Hudson LG, Liu KJ. S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite. Oncotarget. 2016;7:80482–80492. doi: 10.18632/oncotarget.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem. 2011;286:22855–22863. doi: 10.1074/jbc.M111.232926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Mobarak C, Gandolfi AJ, Burchiel SW, Hudson LG, Liu KJ. Differential binding of monomethylarsonous acid compared to arsenite and arsenic trioxide with zinc finger peptides and proteins. Chem Res Toxicol. 2014;27:690–698. doi: 10.1021/tx500022j. [DOI] [PMC free article] [PubMed] [Google Scholar]