Abstract
Objectives
To investigate the impact of ascites volume on ovarian cancer outcomes.
Methods
Clinicopathologic features of a cohort of patients with ovarian cancer were obtained from a curated database at a single institution. Progression free survival (PFS) and overall survival (OS) were recorded. Ascites volume at primary surgery was dichotomized at 2000 mL and comparisons for high and low volume ascites were made. Additionally, to elucidate interactions between ascites and ovarian tumor progression, we evaluated the effect of intraperitoneal administrations of cell-free ascites versus saline in a syngeneic mouse model of epithelial ovarian cancer.
Results
Out of 685 patients identified, 58% had ascites present at the time of initial surgery. Considering the volume of ascites continuously, each liter of ascites was associated with shorter PFS (HR=1.12, 95% CI: 1.07–1.17) and OS (HR=1.12, 95%CI: 1.07–1.17). Patients with ascites greater than the median of 2000 mL had significantly shorter PFS (14.5 months vs. 22.7 months; p < 0.001) and OS (27.7 months vs. 42.9 months; p < 0.001). After adjusting for stage, presence of ascites was inversely associated with ability to achieve optimal cytoreductive surgery. Consistent with these correlative results in patients, intraperitoneal administrations of cell-free ascites accelerated ovarian cancer progression in mice.
Conclusions
The volume of ascites at initial diagnosis of ovarian cancer correlated with worse PFS and OS. The effect of large volume on prognosis is likely to be in part related to reduced likelihood for complete resection of tumor (R0). If these findings are confirmed in independent studies, consideration should be made to add the presence of large volume ascites at diagnosis to the staging criteria for ovarian cancer.
Keywords: ovarian cancer, biomarkers, ascites, tumor microenvironment, prognosis
Introduction
More than two-thirds of patients with ovarian cancer die of their disease, making ovarian cancer the most lethal gynecologic malignancy [1]. Age, stage, response to surgery (optimal vs. sub-optimal), and initial platinum-free interval are the strongest predictors of overall clinical outcome [2]. Some clinical factors associated with surgical outcome include pre-operative CA 125, spread of disease on pre-operative imaging, hematologic parameters, extent of disease identified at the time of surgery, and presence or absence of ascites [2–4].
Various factors can influence achieving optimal surgical debulking for ovarian cancer, including the technical ability to remove the tumor as well as biological differences between tumors that have different patterns of spread [5]. Ascites in ovarian cancer results from tumor implants causing disruption in vascular permeability as well as tumor-derived and stromal factors (e.g., pro-inflammatory cytokines and chemokines and products of cellular injury) that lead to recruitment and activation of inflammatory cells and pathologic wound repair responses, including activation of thrombosis and angiogenesis pathways. Additional studies demonstrated distinct proteomic, glycosylation, and metabolic profiles in ascites that can affect tumor cell biology at baseline and in response to therapy [6, 7]. These findings point to both the cellular and soluble constituents of ascites modulating tumor cell biology.
A number of studies have identified the presence of ascites and volume of ascites as negative prognostic factors in patients with ovarian cancer [4, 8–10]. It is unclear from these studies whether ascites is simply a manifestation versus a driver of disease progression. We hypothesized that higher volume ascites would be associated with a higher frequency of sub-optimal debulking at primary surgery and worse clinical outcomes in patients with newly diagnosed advanced ovarian cancer. We found that patients with high volume of ascites at diagnosis of ovarian cancer were less likely to have complete surgical resection and had worse PFS and OS. Consistent with these results, administrations of cell-free ascites worsened tumor progression in syngeneic murine epithelial ovarian cancer. These results support high volume ascites as a negative prognostic factor in patients with newly diagnosed advanced ovarian cancer and that ascites drives tumor progression.
Methods
Patient Selection
We conducted a retrospective analysis of patients with ovarian cancer treated at Roswell Park Cancer Institute (Buffalo, NY). Patients diagnosed with ovarian cancer were identified through the Center for Immunotherapy Gynecologic Cancer Database (CFIGCD), a curated database containing: pre-, intra-, and post-operative clinical information including demographics, comorbidities, surgical procedures performed, surgical outcome (complete or R0, optimal or ≤ 1 cm residual disease, or suboptimal resection with residual disease > 1 cm), and occurrence of post-operative complications; clinicopathologic information, including tumor stage, grade, and histotype; treatment-related information, including chemotherapies received and clinical trial enrollment; laboratory parameters, including CA 125 and hematologic values; human leukocyte antigen (HLA) type; cancer testis (CT) antigen expression status of tumor as well as immunoreactivity to those antigens; and comprehensive survival information, including time to first progression, disease free interval, and overall survival. The database is managed using the REDCap database platform [11]. Patients were entered into the database at the time of diagnosis of ovarian, fallopian tube, or primary peritoneal cancer, or upon referral to our institution for those patients who receive initial therapy at an outside institution. This protocol was approved by the Roswell Park Cancer Institute Institutional Review Board.
Clinical Outcomes
Clinical parameters including age at diagnosis, medical comorbidities, and family history of cancer were obtained from the CFIGCD. The source of data for those variables was the initial history and physical examination stored in the hospital’s electronic medical record (AllScripts; Chicago, IL). Tumor information was obtained from pathology records and confirmed with the hospital’s internal cancer registry. Response to initial treatment was considered complete if after completion of adjuvant chemotherapy there was both complete chemical (as measured by CA 125 < 35 U/mL) and radiographic (as measured by CT scan) resolution of disease. Response to treatment was partial if either the chemical or radiographic parameters did not normalize after the initial adjuvant therapy. The clinical response was considered progressive disease if either chemical or radiographic parameters were increased from the baseline value after initial adjuvant therapy. Progression-free survival was measured from the date of primary cytoreductive surgery until the first evidence of disease recurrence, which was (i) radiographic according to RECIST criteria [12], (ii) serum CA 125 rising to at least two-fold above the upper limit of normal, or (iii) biopsy proven recurrent disease. Overall survival was measured from the date of primary cytoreductive surgery until death from any cause. Patients not experiencing recurrence, progression, or death were censored at the date of last clinical contact.
Determination of Ascites Volume
The study size was determined by the number of patients in the database diagnosed between January 1, 2005 and December 31, 2015 with explicit mention of ascites in their operative report. Patients were excluded from the study if they received neoadjuvant chemotherapy or if they never received surgery. Ascites volume was obtained from the surgeon’s dictation of the operative report, from the nurse’s documentation of specimens removed at the time of surgery, or from the pathologic measurement of ascites volume. If there were discrepancies between documentation, the pathologist’s report was used as the final arbiter of the result. If there was documentation of a paracentesis within 5 days of definitive surgery, that volume was added to the surgical ascites volume. Of patients with ascites, the median volume was 2010 mL. Patients were categorized into three arms: no ascites defined as those with explicit mention in the operative report that ascites was not present or if there was no mention of ascites and no ascites specimen was received by the pathology department (Group A); small volume ascites, defined as less than 2000 mL (Group B); and large volume ascites, defined as ≥ 2000 mL of ascites (Group C).
Murine Ovarian Cancer
We used a syngeneic mouse surface epithelial ovarian cancer (MOSEC; ID8) model to evaluate the effect of ascites on tumor progression [13]. Luciferase-expressing MOSEC (Luc+MOSEC) cells [14] were grown in RPMI 1640 media with heat-inactivated FBS (10%), L-glutamine (2 mM), HEPES (25 mM), sodium pyruvate (1 mM), 2-mercaptoethanol (50 µM), penicillin/ streptomycin (100 µg/ml) and non-essential amino acids. Female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were administered Luc+MOSEC (1.0 × 107 cells in 200 µl PBS) cells via intraperitoneal (IP) injection and were monitored daily by trained animal care staff. Moribund mice were euthanized based on the decisions of animal care staff using pre-specified criteria (abdominal distention, lethargy or inability to ambulate). After sacrifice, ascites was harvested and centrifuged at 500 g for 10 minutes, and cell-free supernatant aliquots were stored at −80°C. This ascites was used in subsequent experiments. A separate group of C57BL/6 mice (n=10 per group) (Jackson Laboratory, Bar Harbor, ME) was administered IP 1.0 × 107 Luc+MOSEC cells resuspended in either PBS or banked cell-free murine ascites that was diluted 1:1 with PBS. Subsequent IP injections of PBS or diluted ascites (200 µl per mouse) were administered on days 4, 7, and 11 in relation to tumor administration in respective group of mice. Tumor burden was measured using IVIS spectrum bioluminescence imaging system (PerkinElmer, Waltham, MA) after IP injection of 100 µl D-Luciferin per mouse (150 mg/ml in PBS; Gold Biotechnology, St. Louis, MO ) on day 18. Day 18 was chosen because it preceded abdominal distension or other signs of disease. Disease was allowed to progress until pre-specified euthanasia criteria were met and moribund mice were euthanized based on the decisions of animal care staff. All mice were maintained under specific pathogen free conditions at the animal care facility at RPCI and used in compliance with all relevant laws and institutional guidelines under a protocol approved by the RPCI Animal Care and Use Committee.
Statistical Analysis
All statistical analyses were performed using the R 3.1.2 statistical computing language. A nominal significance threshold of 0.05 was used unless otherwise specified. Statistical testing included Student’s t-test, χ2 and Fisher’s exact tests, and Kaplan-Meier survival analysis with log-rank testing. The multivariate analysis included stage, categorized as early (I, II, IIIA, or IIIB) or late (IIIC or IV), grade (1 vs. 2/3), debulking status (optimal vs. suboptimal), and platinum-sensitive versus refractory disease. Restricted mean survival curves described by Eng et al. [15] were computed using ascites volume as a continuous variable. In syngeneic murine ovarian cancer, bioluminescence was compared using Mann-Whitney and time to euthanasia was displayed by Kaplan-Meier curves and compared by log-rank analysis.
Results
Patient Characteristics
We reviewed 685 operative reports transcribed between 2005 and 2015 identifying 496 patients with mention of ascites. Patients with no mention of ascites (n=189) or documentation of no ascites (n = 98) were scored as no ascites. Of the 398 cases with ascites, the median volume was 2010 mL (Q1: 150 to Q2:3000 mL). Using the threshold of 2000 mL to divide small and large volume ascites, 141 of 398 (35.4%) had large volume ascites. Clinicopathologic characteristics of these patients are described in Table 1. The average age of diagnosis was 61 years for those in the no and small volume ascites group and 64 years for those in the large volume ascites group, however this difference was not statistically significant (p = 0.20). Patients with stage IIIC and IV disease were more likely to have large volume ascites (p < 0.001) but the grade of tumor did not differ between groups (p = 0.79). Histotype was also evenly distributed between the various ascites groups (p = 0.89); the majority of patients in all three groups had serous cancers. Patients with no ascites had a 33% chance of complete surgical resection (R0), while only 19% of those with small volume ascites had R0 resection, and just 6% of patients with large volume ascites had an R0 resection (p < 0.001 for both Group A vs. Group B and Group A vs. Group C). Similarly, response to initial chemotherapy was significantly different between the three groups, with 78% complete response in the no ascites group, 68% in the small volume group, and only 44% in the large volume group (p < 0.001).
Table 1.
Demographic information
| Ascites Volume (mL) | None | < 2,000 | > 2,000 | ||
|---|---|---|---|---|---|
| n | 287 | 257 | 141 | ||
| Tumor Site | Fallopian | 14 | 11 | 4 | p=0.61 |
| Ovary | 230 | 184 | 98 | p=0.02 | |
| Primary Peritoneal | 30 | 51 | 30 | p=0.002 | |
| Multiple | 13 | 11 | 9 | p=0.62 | |
|
| |||||
| Age at Diagnosis | Mean | 60 | 61 | 64 | p=0.01 |
|
| |||||
| FIGO stage | I/II | 93 | 51 | 7 | p<0.001 |
| IIIA/B | 9 | 8 | 2 | p=0.38 | |
| IIIC | 89 | 145 | 99 | p<0.001 | |
| IV | 18 | 26 | 23 | p=0.04 | |
| Unknown | 78 | 27 | 10 | ||
|
| |||||
| Grade | 1=well differentiated | 36 | 18 | 12 | p=0.06 |
| 2=moderately differentiated | 49 | 39 | 20 | p=0.61 | |
| 3=poorly differentiated | 185 | 190 | 105 | p=0.06 | |
| Unknown | 17 | 10 | 4 | 4 | |
|
| |||||
| Histology | serous | 150 | 168 | 96 | p=0.002 |
| clear cell | 12 | 9 | 8 | p=0.55 | |
| endometrioid | 22 | 9 | 3 | p=0.01 | |
| mixed cell | 27 | 21 | 9 | p=0.53 | |
| mucinous | 19 | 18 | 9 | p=0.99 | |
| Other | 44 | 30 | 11 | ||
| Unknown | 13 | 2 | 5 | ||
|
| |||||
| None | 85/281 (30%) | 47/246 (19%) | 9/138 (7%) | p<0.001 | |
| Residual Disease | Optimal (<1cm) | 188/287 (66%) | 192/257 (75%) | 95/141 (67%) | p=0.06 |
| Unknown | 6 | 11 | 3 | ||
|
| |||||
| Response to | Complete response | 100/127 (79%) | 86/130 (66%) | 32/78 (41%) | p<0.001 |
| Primary Therapy | Unknown/NA | 160 | 127 | 63 | |
Comparison of clinicopathologic information between patients with none (0 mL), low (≤2000 mL), and large (>2000 mL) volume ascites. None includes patients in which there was documentation of no ascites or ascites specimen received in pathology. Comparisons were performed using χ2 and p < 0.05 was the nominal value for significance. Complete response to primary therapy was defined as patients who had no evidence of disease by laboratory and radiographic assessment after adjuvant chemotherapy was completed.
Survival Analysis
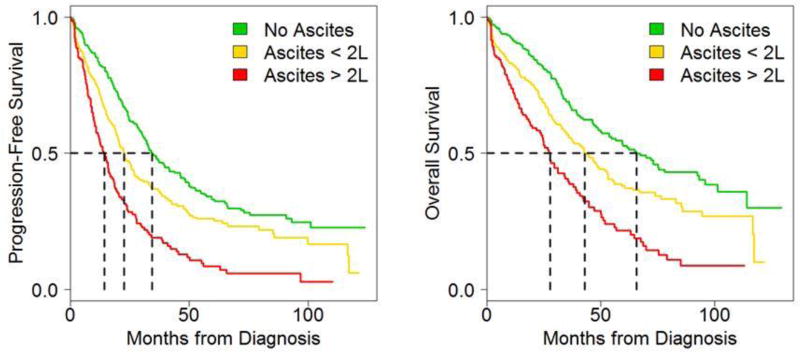
Results from the survival analysis are presented in Table 2 and Figure 1. Median PFS was significantly shorter for patients with large volume ascites (14.5 months), compared with the small volume (22.7 months) and no (34.5 months) ascites cohorts (p < 0.001). The differences are similarly striking for patients with Stage IIIC/IV disease with PFS of 13.6 vs. 17.6 vs. 30.2 months, respectively (p < 0.001). There were similarly disparate outcomes with respect to OS between patients with large volume (27.7 months), small volume (42.9 months), and no ascites (65.8 months). Among Stage IIIC/IV patients, there was a difference of 23.3 months between median OS for those with large volume vs. no ascites (26.7 months vs. 50 months, p < 0.001).
Table 2.
Survival Analysis
| Ascites Volume (mL) | None | < 2,000 | > 2,000 | ||
|---|---|---|---|---|---|
| n | 287 | 257 | 141 | P value | |
| Months PFS | All | 34.5 (30.9–43.8) | 22.7 (20.0–26.8) | 14.5 (11.0–17.7) | p<0.001 |
| Stage < IIIC | 50.1 (34.0–71.8) | 85.9 (49.1-XX.X) | 18.7 (12.6-XX.X) | p<0.001 | |
| Stage IIIC, IV | 30.2 (22.8–34.6) | 17.6 (14.5–21.0) | 13.6 (10.3–16.7) | p<0.001 | |
| Months OS | All | 65.8 (53.6-–3.1) | 42.9 (36.0–52.9) | 27.7 (23.5–35.6) | p<0.001 |
| Stage < IIIC | 101.1 (71.8-XX.X) | NA (85.3-XX.X) | 27.8 (24.5-XX.X) | p=0.009 | |
| Stage IIIC, IV | 50.0 (37.2–62.3) | 30.2 (26.8–40.6) | 26.7 (18.9–34.3) | p<0.001 | |
Comparison of survival between patients with none (0 mL), low (≤2000 mL), and large (>2000 mL) volume ascites. Comparisons were performed using χ2 and p < 0.05 was the nominal value for significance. Data are presented as median (95% CI) months of progression free (PFS) and overall (OS) survival. XX.X indicates no upper estimate exists for the median and NA indicates that 50% of events have not yet occurred for the subgroup. Stage < IIIC includes stages I, II, IIIA, and IIIB.
Figure 1. Volume of ascites at initial diagnosis of ovarian cancer correlates with worse PFS and OS.
Kaplan-Meier analysis of progression free (PFS) and overall (OS) survival, stratified by ascites volume at the time of diagnosis. Patients with no ascites had the best prognosis, while those with ≤ 2000 mL had a better prognosis than those with > 2000 mL
Because ascites was associated with ability to achieve surgical resection, a secondary analysis was performed with stratification based on initial surgical outcome. Table 3 illustrates the impact of ascites on PFS and OS for patients with R0, optimal, and sub-optimal surgical outcomes. When all patients were considered, independent of stage, the median PFS was significantly shorter for optimally cytoreduced patients with large volume ascites (15.8 months) versus those with small volume ascites (15.8 months vs. 25.7 months; p < 0.001) and no ascites (15.8 months vs. 39 months; p < 0.001). The impact was similar for overall survival (34 vs. 53.1 vs. 75.4 months; p < 0.001). Although large volume ascites was less common in early stage disease and there is the possibility of survival advantage based on stage and not ascites volume, when analysis was limited to only those with stage IIIC/IV disease, the differences in survival persisted. Among patients with advanced disease, ascites was universally associated with worse prognosis, independent of the extent of surgical resection (Table 3, right). Additionally, among patients with ascites, large volume correlated with worse OS in patients whose primary surgery was R0 (41.0 vs. median not yet reached; p < 0.001) or optimally cytoreduced (PFS: 15.8 vs. 25.7 months; OS: 34.0 vs. 53.1 months; p < 0.001). In contrast, in patients with sub-optimal cytoreductive surgeries, any ascites was a poor prognostic indicator, but there was no significant difference in survival for high vs. low volume (PFS: 12.2 vs. 15.3 months; OS: 19.9 vs. 23.0 months; p = 0.50 and 0.64, respectively).
Table 3.
Survival Analysis by Surgical Outcome
| All Patients, All Stages | Stage IIIC/IV only | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ascites Volume | None | < 2,000 | > 2,000 | P value | None | < 2,000 | > 2,000 | P value | |
| n | 287 | 257 | 141 | 107 | 171 | 122 | |||
| Months PFS | R0 | 52.7 (33.9–XX.X) | 66.4 (37.1–XX.X) | 23.5 (13.6–XX.X) | p=0.07 | 34.5 (30.9–43.8) | 22.7 (20.0–26.8) | 14.5 (11.0–17.7) | p<0.001 |
| Optimal | 39.0 (32.9–52.4) | 25.7 (22.7–37.1) | 15.8 (12.0–20.7) | p<0.001 | 24.6 (17.2–46.3) | 18.5 (12.2–22.4) | 10.8 (8.5–18.3) | p=0.001 | |
| Sub-optimal | 30.1 (25.1–39.9) | 15.3 (8.13–20.7) | 12.2 (9.02–18.7) | p<0.001 | 30.8 (23.7–36.2) | 19.8 (15.9–23.3) | 13.6 (10.2–17.7) | p<0.001 | |
|
| |||||||||
| Months OS | R0 | 114.0 (65.8-XX.X) | NA (83.0–XX.X) | 41.0 (23.5–XX.X) | p=0.005 | 22.2 (18.3–34.6) | 12.1 (8.13–16.8) | 10.9 (8.7–15.4) | p<0.001 |
| Optimal | 75.4 (61.4–XX.X) | 53.1 (45.0–73.9) | 34.0 (25.4–42.5) | p<0.001 | 65.8 (53.6–93.1) | 42.9 (36.0–52.9) | 27.7 (23.5–35.6) | p<0.001 | |
| Sub-optimal | 47.9 (34.9–73.1) | 23.0 (17.9–35.0) | 19.9 (13.5–27.8) | p<0.001 | 37.2 (26.3–XX.X) | 28.2 (17.9–45.0) | 13.0 (8.72–23.5) | p<0.001 | |
Comparison of progression-free and overall survival between patients with none (0 mL), low (≤2000 mL), and large (>2000 mL) volume ascites. Comparisons between all three groups were performed using χ2 and p < 0.05 was the nominal value for significance. Data are presented as median (95% CI) months of progression free (PFS) and overall (OS) survival. XX.X indicates no upper estimate exists for the median and NA indicates that 50% of events have not yet occurred for the subgroup. R0 is defined as complete surgical resection to no visible disease; optimal is defined as residual disease ≤ 1 cm visible at the end of surgery, and sub-optimal is defined as > 1 cm visible disease at end of surgery.
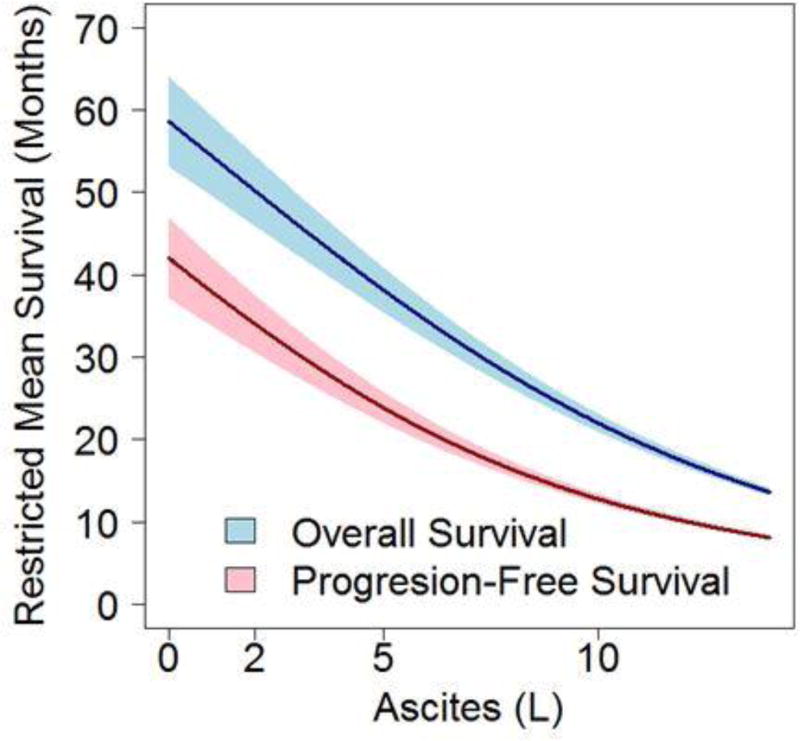
Considering the volume of ascites continuously, each liter of ascites was associated with shorter PFS (HR=1.12, 95% CI: 1.07–1.17) and OS (HR=1.12, 95%CI: 1.07–1.17). Figure 2 plots the restricted mean survival (model-based estimated mean time to progression or death) as a function of ascites volume at surgery. The gap between the curves for PFS and OS remained consistent over a large range of ascites volumes suggesting that ascites volume at initial surgery predicts worse response to adjuvant therapy and shorter survival.
Figure 2. Ascites volume at primary surgery projects predicts progress-free and overall survival.
Modeled survival prediction was analyzed as a function of ascites volume (L). Considering the volume of ascites continuously, each liter of ascites was associated with shorter PFS and OS. The margin between predicted PFS and OS remained static across a large range of ascites volumes.
Effect of Ascites in Syngeneic Murine Epithelial Ovarian Cancer
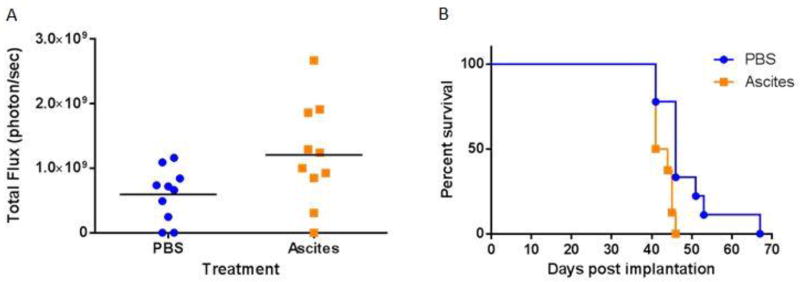
To evaluate whether ascites was a marker of advanced disease versus driving tumor progression, immune intact female mice were administered Luc+MOSEC resuspended in PBS or banked cell-free ascites harvested from a prior cohort of ovarian tumor-bearing mice, and diluted 1:1 in PBS prior to intraperitoneal administration. Subsequent intraperitoneal injections of PBS or ascites were administered on days 4, 7, and 11 in relation to tumor administration. Bioluminescence, a measurement of tumor burden, was modestly but significantly (p=0.036) increased in mice administered cell-free ascites versus PBS (Figure 3A). Consistent with these results, mice treated with cell-free ascites had significantly (p=0.009) shorter time to euthanasia based on pre-specified morbidity criteria (Figure 3B). Taken together, these results point to the administration of cell-free ascites increasing tumor progression in murine ovarian cancer. This experiment was not designed to model the effect of surgery and chemotherapy administered in the clinic.
Figure 3. Intraperitoneal administrations of cell-free ascites accelerate ovarian cancer progression in mice.
Mice were administered intraperitoneal syngeneic epithelial ovarian cancer cells expressing luciferase (Luc+MOSEC), followed by intraperitoneal administration of cell-free ascites or PBS on days 4, 7, and 11. A. Mice administered cell-free ascites had an increased bioluminescent signal on day 18, a marker of greater tumor burden (p =0.034). B. Ascites administration decreased time to morbidity requiring euthanasia (p=0.009). Data are from 10 mice per treatment group and are representative of two separate experiments.
Discussion
Our results point to the volume of ascites at primary surgery as a biomarker of worse clinical outcomes. We found that patients with more than 2 liters of ascites present with higher stage, achieve fewer complete (R0) surgical resections, and experience more failures of first-line therapy. When limiting calculations to only those with advanced (Stage IIIC/IV) disease, those with large volume ascites had significantly shorter PFS and OS when compared with patients with lower volume ascites and no ascites. Consistent with these results in patients, our observations in murine ovarian cancer point to acellular components of ascites driving ovarian cancer progression. If our clinical observations are confirmed in independent studies, consideration should be made to add the presence of large volume ascites at diagnosis to the staging criteria for ovarian cancer.
Prior studies have correlated pre-operative ascites with surgical and perioperative clinical outcomes [10, 16, 17] including the ability to achieve optimal or complete (R0) cytoreduction. The present study confirms the findings of these smaller cohorts that identified ascites as a risk factor for sub-optimal cytoreduction [10], a factor well known to predict worse clinical outcomes [18]. Here we extended those findings regarding the impact of ascites on surgical outcome and long-term clinical outcomes. When patients were stratified by surgical outcome (Table 3), ascites was a significant risk factor for both PFS and OS in advanced stage disease with either optimal or R0 surgical resection. However, although patients with suboptimally cytoreduced disease had a survival detriment if any ascites was present, there was no worse outcome for larger volume disease, suggesting that the residual tumor after surgery remains the most important prognostic factor [18].
In advanced ovarian cancer, solid tumor and ascites are distinct components. While solid tumor implants are composed principally of dense accumulation of tumor with tumor-infiltrating immune cells, ascites is composed principally of inflammatory cells. The critical role of immune surveillance in ovarian cancer was demonstrated by the observation that tumor-infiltrating T cells predicted better outcome [19]. Intraepithelial CD8+ cell accumulation and a high CD8+/Treg ratio were associated with favorable prognosis [20] while increased Treg accumulation predicted worse outcome [21] in patients with advanced ovarian cancer. Tumor cells and tumor-associated macrophages produce CCL22, which mediates trafficking of Tregs to the tumor leading to suppression of tumor-specific T cell immunity [21]. Accumulation of B7-H4-expressing macrophages in the tumor microenvironment impedes T cell responses and correlates with more rapid tumor progression [22]. In pre-treatment ascites of patients with ovarian cancer, the myeloid cell population consists of mature macrophages, immature myeloid cells and granulocytic cells with variable immunosuppressive phenotypes [23, 24]. Cell-free ascites components, including the exosome fraction, can suppress T cell responses [25, 26]. We have also observed that cell-free ascites from patients with newly diagnosed ovarian cancer can induce an immunosuppressive phenotype in normal donor neutrophils [27]. Thus, although ascites is highly inflammatory, it may also create a highly immunosuppressive environment that is an obstacle to anti-tumor T cell immunity.
In addition, specific cytokines within ascites may influence tumor progression. We observed that the combination of high levels of IL-6 and TNF-α in ascites of patients with newly diagnosed epithelial ovarian cancer predicted worse outcome [28]; however, this was a small single-center study, and cytokine profiling from other centers have produced different results [29]. In addition to stimulating thrombocytosis, IL-6 within the tumor microenvironment can have broad effects on tumor, inflammatory, and non-immune stromal cells, including increasing endothelial cell permeability that drives ascites generation [30].
Ascites may promote tumor spread through other mechanisms, including activation of thrombosis. Similar to many other cancers [31, 32], venous thrombosis is common in epithelial ovarian cancer, is associated with more advanced disease, and is predictive of worse survival in all stages of disease [33]. Stone et al. [34] showed that paraneoplastic thrombocytosis in advanced ovarian cancer was driven by IL-6, and correlated with poor prognosis. Thrombosis can accelerate tumor progression through several pathways, including stimulation of tumor cell migration and adhesion to endothelial cells, recruitment of inflammatory cells to the tumor microenvironment, and stimulation of production of VEGF and other pro-angiogenic products that increase metastatic potential [35]. Platelets increased the proliferation of ovarian cancer cells through TGF-β-dependent signaling [36], and in murine ovarian cancer, platelets reduced the efficacy of chemotherapy [37]. Activated thrombin within ascites can facilitate ovarian tumor invasion directly and by cross-signaling to macrophages [38, 39]. Indeed, we observed that ascites of patients with newly diagnosed ovarian cancer contains floating fibrin with abundant neutrophils reflecting active inflammation and tumor cells that we speculate will enhance seeding of serosal surfaces (unpublished results).
Together, these results support a role for ascites in driving ovarian tumor progression through a number of mechanisms including generation of an immunosuppressive environment, promotion of thrombosis and angiogenesis, and alterations in the biology of tumor and stromal cells. However, other ascites factors may play a role in limiting tumor progression. For example, Wong et al. [40] showed that IL-18 primed natural killer (NK) cells isolated from the ascites of patients with ovarian cancer can promote dendritic cell attraction and the conditioning of the tumor microenvironment for the subsequent chemokine-stimulated recruitment of T-effector cells. Therefore, ascites volume as a prognostic marker is almost certainly a “broad brush,” and future studies of cellular and soluble ascites constituents are expected to lead to novel prognostic biomarkers and targets for therapy.
Limitations of this study include the retrospective nature of data ascertainment for the CFIGCD. Although data are collected from the prospectively maintained electronic health record, errors may occur. Specifically, measurement of ascites volume at the time of surgery is inexact, and it is possible that some misclassification occurred between patients in the small and large volume ascites groups. The present study is an important next step in the evaluation of ascites as a driver and biomarker for worse disease, rather than simply a sign of progression. Further studies are needed to confirm the findings identified herein. Future directions of interaction with ascites include targeting specific components of the ascites for therapeutic benefit and potentially use of ascites volume as a tool for triage of patients to surgery versus initial medical management.
Highlights.
Ascites volume at primary surgery was associated with worse clinical outcome
More than 2 liters ascites was associated with primary treatment failures
Cell-free ascites administration accelerated tumor progression in the murine model
Acknowledgments
This work was supported by the Roswell Park Alliance Foundation, NIH grants R01CA188900 (KBM and BHS), 1K01LM012100, T32CA108456, T32CA085183 (KLS), P30CA016056, S10OD016450, and RPCI-UPCI Ovarian Cancer SPORE P50CA159981-01A1. IVIS imaging was performed by the Translational Imaging Shared Resource, which is funded by the National Cancer Institute and is a Roswell Park Cancer Institute Cancer Center Support Grant shared resource.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dinkelspiel HE, et al. Long-term mortality among women with epithelial ovarian cancer. Gynecol Oncol. 2015;138(2):421–8. doi: 10.1016/j.ygyno.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Said H, et al. Predictive factors for irresectability in advanced ovarian cancer. Int J Gynecol Cancer. 2004;14(3):423–30. doi: 10.1111/j.1048-891x.2004.014301.x. [DOI] [PubMed] [Google Scholar]
- 3.Ayhan A, et al. Ascites and epithelial ovarian cancers: a reappraisal with respect to different aspects. Int J Gynecol Cancer. 2007;17(1):68–75. doi: 10.1111/j.1525-1438.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, et al. Clinical significance of ascites in epithelial ovarian cancer. Neoplasma. 2013;60(5):546–52. doi: 10.4149/neo_2013_071. [DOI] [PubMed] [Google Scholar]
- 5.Aust S, Pils D. Epithelial ovarian cancer - more data, more questions? Wien Med Wochenschr. 2014;164(21–22):479–86. doi: 10.1007/s10354-014-0323-8. [DOI] [PubMed] [Google Scholar]
- 6.Biskup K, et al. The ascites N-glycome of epithelial ovarian cancer patients. J Proteomics. 2017;157:33–39. doi: 10.1016/j.jprot.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Shender VO, et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 2014;13(12):3558–71. doi: 10.1074/mcp.M114.041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heintz AP, et al. The treatment of advanced ovarian carcinoma (I): clinical variables associated with prognosis. Gynecol Oncol. 1988;30(3):347–58. doi: 10.1016/0090-8258(88)90249-1. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn W, et al. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer. 2001;92(10):2585–91. doi: 10.1002/1097-0142(20011115)92:10<2585::aid-cncr1611>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Janco JM, et al. Development of a prediction model for residual disease in newly diagnosed advanced ovarian cancer. Gynecol Oncol. 2015;138(1):70–7. doi: 10.1016/j.ygyno.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Godoy HE, et al. Myeloid-derived suppressor cells modulate immune responses independently of NADPH oxidase in the ovarian tumor microenvironment in mice. PLoS One. 2013;8(7):e69631. doi: 10.1371/journal.pone.0069631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil M, et al. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol. 2014;193(10):5327–37. doi: 10.4049/jimmunol.1400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng KH, Schiller E, Morrell K. On representing the prognostic value of continuous gene expression biomarkers with the restricted mean survival curve. Oncotarget. 2015;6(34):36308–18. doi: 10.18632/oncotarget.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong D, et al. Preoperative predictors for residual tumor after surgery in patients with ovarian carcinoma. Oncology. 2007;72(5–6):293–301. doi: 10.1159/000113051. [DOI] [PubMed] [Google Scholar]
- 17.Fotopoulou C, et al. Can complete tumor resection be predicted in advanced primary epithelial ovarian cancer? A systematic evaluation of 360 consecutive patients. Eur J Surg Oncol. 2010;36(12):1202–10. doi: 10.1016/j.ejso.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 20.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 22.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermajer N, et al. PGE2-Dependent CXCL12 Production and CXCR4 Expression Control the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AN, et al. Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer. Oncotarget. 2015 doi: 10.18632/oncotarget.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson-Abelson MR, et al. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14. [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher RJ, Jr, et al. Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singel KLKA, Moysich KB, Odunsi K, Segal BH. Ovarian cancer ascites induces a T cell suppressive phenotype in mature neutrophils: a potential barrier to anti-tumor immunity. Presented at: IMMUNOLOGY 2017, Annual Meeting of the American Association of Immunologists; May 12–17; Washington DC: The American Association of Immunologists, Inc; 2017. Abstract 254. [Google Scholar]
- 28.Kolomeyevskaya N, et al. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-alpha and interleukin-6 in predicting reduced progressionfree survival in epithelial ovarian cancer. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane D, et al. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo CW, et al. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71(2):424–34. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 31.Chew HK, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 32.White RH, et al. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch Intern Med. 2005;165(15):1782–7. doi: 10.1001/archinte.165.15.1782. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez AO, et al. Venous thromboembolism in ovarian cancer. Gynecol Oncol. 2007;105(3):784–90. doi: 10.1016/j.ygyno.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Stone RL, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palumbo JS, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–9. [PubMed] [Google Scholar]
- 36.Cho MS, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–72. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bottsford-Miller J, et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer. Clin Cancer Res. 2015;21(3):602–10. doi: 10.1158/1078-0432.CCR-14-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, et al. Thrombin facilitates invasion of ovarian cancer along peritoneum by inducing monocyte differentiation toward tumor-associated macrophage-like cells. Cancer Immunol Immunother. 2010;59(7):1097–108. doi: 10.1007/s00262-010-0836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naldini A, et al. Identification of thrombin-like activity in ovarian cancer associated ascites and modulation of multiple cytokine networks. Thromb Haemost. 2011;106(4):705–11. doi: 10.1160/TH11-05-0311. [DOI] [PubMed] [Google Scholar]
- 40.Wong JL, et al. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res. 2013;73(15):4653–62. doi: 10.1158/0008-5472.CAN-12-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]