Abstract
Background
We evaluated cognitive function and factors associated with cognitive impairment in a cohort of older homeless adults. We hypothesized that substance use and a history of traumatic brain injury would be associated with cognitive impairment.
Methods
We recruited 350 homeless individuals aged ≥50 years using population-based sampling and conducted structured interviews and neuropsychological testing. We evaluated alcohol use with the Alcohol Use Disorder Identification Test, defining high-severity alcohol use as a total score ≥16 or ≥4 on the alcohol dependency sub-scale. We assessed global cognition with the Modified Mini-Mental State Test (3MS) and processing speed and executive function with the Trail Making Test (TMTB), defining impairment as performing 1.5 standard deviations below the standardized mean. We used multivariable logistic regression to examine the association between alcohol use and cognition.
Results
Participants had a median age of 58 years [IQR 54–61], 76.7% were men, and 79.9% were African American. A quarter (25.1%) of participants met criteria for impairment on the 3MS; 32.9% met criteria for impairment on TMTB. In models adjusted for sociodemographic variables and health conditions, high-severity alcohol use was associated with global cognitive impairment (AOR 2.39, CI 1.19–4.79) and executive dysfunction (AOR 3.09, CI 1.61–5.92).
Conclusions
Older homeless adults displayed a prevalence of cognitive impairment 3–4 times higher than has been observed in general population adults aged 70 and older. Impaired cognition in older homeless adults could impact access to housing programs and the treatment of health conditions, including the treatment of alcohol use disorders.
Keywords: Cognitive impairment, Homeless persons, Alcohol misuse
1. Introduction
The median age of single homeless adults in the United States is rising, and now approaches 50 (Culhane et al., 2013 Hahn et al., 2006). Those born in the latter half of the baby boom (1954–1963) have had an elevated risk of homelessness throughout their lives (Culhane et al., 2013). For older homeless adults, chronic medical conditions, including geriatric syndromes, are causally linked to healthcare utilization and mortality (Brown et al., 2016b; Brown et al., 2012; Garibaldi et al., 2005; Gelberg et al., 1990).
Prior studies have found a high prevalence of cognitive impairment among homeless adults (point estimates range from 4–40%) and impairment occurring at an earlier age than in the general population (Brown et al., 2012; Buhrich et al., 2000; Burra et al., 2009; Depp et al., 2015; Gonzalez et al., 2001; Nishio et al., 2015; Pluck et al., 2011; Spence et al., 2004). However, the majority of these studies relied on samples either recruited from shelter environments, which may not be representative of the homeless population overall, (Burra et al., 2009; Spence et al., 2004) or from specific populations (e.g., persons with mental health conditions) (Bousman et al., 2010; Seidman et al., 1997; Stergiopoulos et al., 2015). Most studies of cognitive function in homeless adults have used global tests of cognition (e.g., Modified Mini Mental Status Exam [MMSE]) (Burra et al., 2009; Depp et al., 2015). Few studies have examined specific domains, such as memory and executive function (Bousman et al., 2010; Brown et al., 2012; Ennis et al., 2014). Executive function is defined as high-level cognitive processing involved in the control and regulation of goal-directed behaviors (Alvarez and Emory, 2006). Studies of homeless adults recruited from shelters identified a high prevalence of executive dysfunction (Brown et al., 2012; Gonzalez et al., 2001; Seidman et al., 1997; Seidman et al., 2003). Preserved executive function is essential to making plans, prioritizing, and completing tasks and thus may be of particular importance to homeless adults attempting to navigate complex social services to address their basic needs (Burra et al., 2009).
There is a poor understanding of the risk factors associated with cognitive impairment in homeless adults. Potential explanations include comorbid conditions such as vascular disease, substance use, traumatic brain injury (TBI), neurodevelopmental disorders, and psychiatric disease (Backer and Howard, 2007). Alcohol misuse and TBI are known causes of cognitive impairment in the general population (Brandt et al., 1983; Gardner et al., 2017), but few studies have explored these risk factors among homeless adults (Seidman et al., 2003; Topolovec-Vranic et al., 2012). We evaluated global cognitive function and executive function in a population-based sample of homeless adults aged ≥50. We chose this age range because of the high prevalence of geriatric conditions occurring in homeless adults 50 and older (Brown et al., 2012, 2013). We examined the relationship between substance use, TBI, and cognitive impairment, hypothesizing that high-risk substance use and a history of traumatic brain injury (TBI) are associated with cognitive impairment.
2. Methods
2.1. Participants
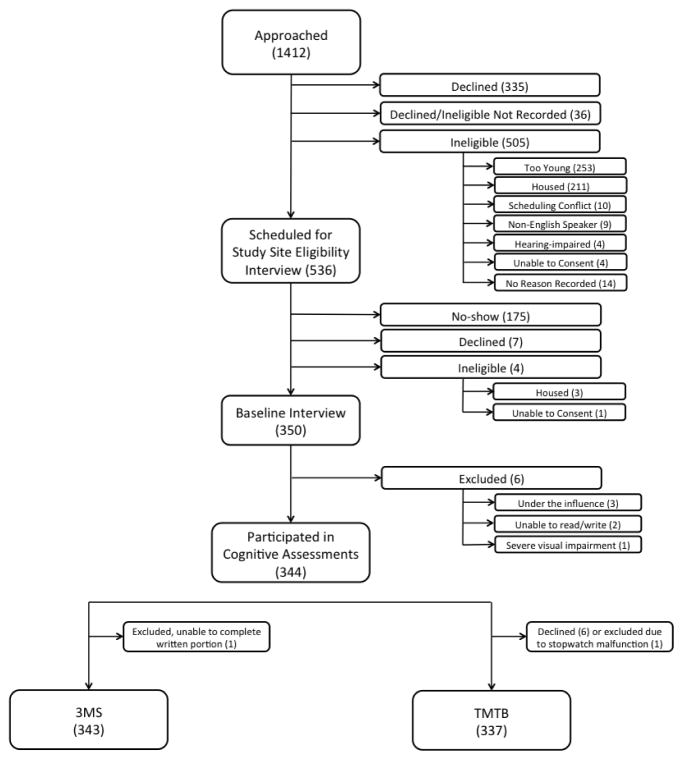
During July 2013–June 2014, we enrolled a population-based sample of 350 homeless adults from overnight shelters, homeless encampments, meal programs, and recycling centers in Oakland, California for the Health Outcomes in People Experiencing Homelessness in Older Middle agE (HOPE HOME) study. This outreach approach expanded on prior methods (Burnam and Koegel, 1988) to include homeless encampments and recycling centers to ensure inclusion of unsheltered adults. We recruited individuals from all overnight homeless shelters in Oakland that served single adults, all free and low-cost meal programs that served homeless persons ≥3 prepared meals a week, a recycling center, and places where unsheltered people stay overnight (Brown et al., 2016a; Lee et al., 2016a). Individuals were eligible to participate if they spoke English, were homeless as defined by the federal Homeless Emergency Assistance and Rapid Transitions to Housing (HEARTH) Act (2010), and were aged ≥50. Participants provided written informed consent. We excluded individuals who could not provide informed consent as demonstrated by an inability to state the goals and risks of participation through a teach-back method (Carpenter et al., 2000). We excluded individuals unable to communicate due to hearing impairment. Research assistants documented possible intoxication with drugs or alcohol for all participants at the time of the interview (Figure 1). Participants received a $25 dollar gift card. The institutional review board at the University of California San Francisco approved this study.
Figure 1. Flow-chart of recruitment of 350 older homeless adults.
This figure shows the number of individuals approached, assessed for eligibility, and enrolled in the study (N=350), noting specific reasons for inability to enroll. Values represent the number of individuals in each group. *Participants who declined after being approached (N=335) declined before being assessed for eligibility. Therefore, the number of participants who were ineligible for the study may have been higher than the numbers presented in this figure.
2.2. Measures
2.2.1. Dependent variables: Neuropsychological Tests
A licensed neuropsychologist (CWJ) trained all research assistants in the administration and scoring of the neuropsychological tests and supervised staff to ensure fidelity.
We measured global cognitive function using the Modified Mini-Mental State Test (3MS), an extended version of the Mini-Mental State Examination (MMSE), which assesses memory, concentration, orientation, and visuospatial functioning (Bland and Newman, 2001; Teng and Chui, 1987). Scores range from 0–100; higher scores indicate better performance. We defined impairment as a score ≤1.5 standard deviations (7th percentile) below age- and education-adjusted norms (Bravo and Hebert, 1997). We used the Trail Making Test part B (TMTB), a measure of task switching (Sanchez-Cubillo et al., 2009b) to test executive function (Reitan, 1958; Sanchez-Cubillo et al., 2009a). The score is the amount of time (in seconds) to complete the test up to a maximum score of 300 seconds (Wong et al., 2016). We excluded participants unable to complete the tests (e.g., interruptions, health conditions). We defined impairment as a score ≤1.5 standard deviations of age- and sex adjusted norms (Heaton et al., 2004).
2.2.2. Independent Variables
2.2.2.1. Demographics
We collected self-reported demographic information including age, sex, and ethnicity (Latino or not Latino), race (American Indian/Alaskan Native, Asian, Black/African American, Native Hawaiian/other Pacific Islander, White, and other), highest educational attainment, occupational status, and history of serving in the military in a combat zone. We classified participants’ highest educational attainment into three categories: less than a high school education, high school or a high school equivalency test (GED), and post-high school education (college or vocational school). We classified occupational status into four categories: jobs involving unskilled labor, semi-skilled labor, clerical or skilled manual labor, and jobs involving executive, managerial or higher level administrative positions.
2.2.2.2. Housing
We asked participants to report the date when they were last stably housed, defined as a non-institutional setting where they stayed for at least 12 months (Burt et al., 1999). Participants were also asked where they stayed each night over the previous 6 months using a residential follow-back calendar (i.e., shelters, unsheltered places etc.) (Tsemberis et al., 2007).
2.2.2.3. Substance Use
We administered the World Health Organization (WHO) Alcohol Use Disorders Identification Test (AUDIT) to assess current risk and severity of alcohol use disorders (Babor et al., 2001). We modified AUDIT by asking about alcohol use in the last six months. The test includes three questions on alcohol consumption, three questions on drinking behavior and possible alcohol dependence, and four questions on drinking consequences. Scores range from 0–40, with higher scores indicating greater alcohol use. A total score ≥ 8 on AUDIT is associated with hazardous or harmful alcohol use (Conigrave et al., 1995); scores ≥8 have good sensitivity and specificity for an alcohol use disorder (Lundin et al., 2015; Rumpf et al., 2002). The WHO classifies scores as 8–15 as risky drinking, 16–19 consistent with high-risk or harmful level of alcohol use, and ≥20 as high-risk with possible alcohol dependence. Scores ≥4 on the alcohol dependence sub-scale suggest risk for alcohol dependence (Babor et al., 2001). We considered a total score of ≥8 as suggestive of hazardous drinking and ≥16 and/or ≥4 on the alcohol dependency sub-scale as suggestive of high-severity alcohol use.
We administered the WHO Alcohol, Smoking and Substance Involvement Screening Test (ASSIST, scores range 0–39) to assess illicit substance use during the six months prior to the interview, classifying current substance risk as moderate (4–26) individually for four substances (marijuana, cocaine, opioids, or amphetamines) (Humeniuk et al., 2010).
2.2.2.4. Mental Health
We assessed mental health conditions in three ways. First, we administered the Center for Epidemiologic Studies Depression Scale (CES-D), a reliable measure of depressive symptoms in homeless populations (Ritchey et al., 1990; Wong, 2000), using a standard cut-off CES-D score of ≥16 as indicative of depressive symptoms (Weissman et al., 1977). Second, we screened participants for post-traumatic stress disorder (PTSD) using the Primary Care PTSD Screen (Prins et al., 2003) using a cut-off score of ≥3 as indicative of possible PTSD. Third, to assess a lifetime history of mental health problems, we asked participants if they had ever experienced significant anxiety, depression, difficulty controlling violent behavior, or hallucinations that were not a result of substance use, or if they had attempted suicide (Burt et al., 1999; McLellan et al., 1980). We included this variable as a participant’s history of having a mental health problem.
2.2.2.5 Health
We dichotomized self-rated health (fair/poor versus good/very good/excellent) (Ware et al., 1996). We asked participants to report whether a clinician had ever told them that they had a stroke, coronary artery disease (CAD), diabetes, or tested positive for human immunodeficiency virus (HIV). To evaluate for a history of moderate to severe traumatic brain injury (TBI), we asked participants to report the number of lifetime head traumas they had experienced and collected detailed information for up to three instances of head trauma that resulted in loss of consciousness or hospitalization (Hwang et al., 2008). We asked participants whether they currently had a regular healthcare provider and whether they had been admitted to the hospital in the last six months.
2.2.2.6. Functional status
Participants reported if they had difficulty performing five activities of daily living (ADLs) (Katz, 1983). To assess difficulty performing six instrumental activities of daily living (IADLs), we used the Brief Instrumental Functioning Scale (BIFs), a test validated in homeless populations (Sullivan et al., 2001). We classified ADL or IADL impairment as difficulty performing ≥1ADL or IADLs.
2.3. Statistical Analyses
2.3.1 Housing Cluster Analysis
Using participants’ self-report of living environments during the past six months, we used cluster analysis with k-medians to classify residential histories of participants (Lee et al., 2016b).
2.3.2 Cognitive Impairment
We used bivariate analyses to compare individuals with and without impairment on the test of global cognition (3MS) using the Chi squared test for categorical variables and the student’s t-test for continuous variables for 3MS and TMTB.
2.3.3 Multivariable Regression
We used multivariable logistic regression to estimate adjusted odds ratios (AOR) and corresponding 95% confidence intervals for two different dependent variables (cognitive impairment assessed by 3MS or TMTB). In both models, we used as predictor variables measures of high-severity alcohol use and TBI and adjusting for sociodemographic variables and health conditions. We conducted model selection using backward stepwise elimination, starting with a model that contained all hypothesized independent variables with a bivariate p-value ≤0.2, and retained independent variables with p-values <0.05. We retained TBI in the final model to evaluate our pre-specified hypotheses despite the absence of a statistically significant association with impairment outcomes in the bivariate analyses. We performed analyses using Stata version 13 (StataCorp, College Station, TX, USA).
3. Results
3.1 Sample Description
Of 350 participants enrolled in the HOPE HOME study, we included data from 343 participants. We excluded one participant who had visual impairment, three participants who were intoxicated during the assessment, two participants who could not read or write, and one participant with limited English fluency. Six individuals declined to complete the TMTB assessment and we excluded TMTB data for an additional participant due to a stopwatch malfunction (n = 336 for TMTB outcome) (Figure 1).
Participants had a median age of 58 years (IQR 54, 61), 76.7% were men, and 79.9% were African American. Participants had a median duration of homelessness of 2.1 years (SD = 8.8); 43.6% first became homeless at age 50 or later. We used cluster analysis to categorize participants into four groups based on their living environments: unsheltered (n = 159, 46.4%), cohabitating with family or friends (n=56, 16.3%), residing in institutional settings (shelters, jails, transitional housing; n=86, 25.1%), and living primarily in rental housing before becoming recently homeless (n=42, 12.2%). One hundred and forty-nine participants (43.4%) reported a history of TBI. Eighty-nine (26.0%) had an AUDIT score suggestive of hazardous alcohol use and 48 (14.0%) had an AUDIT score suggestive of high-severity alcohol use. Forty-three participants (12.5%) reported moderate or greater risk opioid use; 147 (42.9%), 28 (8.2%), and 133 (38.8%) reported moderate or greater risk use of cocaine, amphetamines, and marijuana, respectively. One hundred and thirty four participants (39.1%) reported impairment in one or more ADLs. One hundred and sixty-eight (49.0%) reported impairment in one or more IADLs.
3.2 Neuropsychological Tests
3.2.1 Neuropsychological Test Performance
More than half of participants (n=191, 56.9%) scored within the normal range on both the 3MS and TMTB. One quarter (25.1%) of participants scored in the impaired range on the 3MS and 32.9% scored in the impaired range on the TMTB. Ninety-one participants (27.1%) were unable to complete TMTB in the maximum allotted time. Forty-nine participants (14.6%) were impaired on both 3MS and the TMTB, while 61 (18.2%) participants had impairment only on the TMTB (Table 2).
Table 2.
Cognitive Assessment Scores
| Test | Possible Range of Scores | Observed Range in Sample | Median Test Score | Standard Deviation | Proportion 1.5 SD below reference |
|---|---|---|---|---|---|
| 3MSa | 0–100 | 57–100 | 89 | 8.6 | 25.1 |
| TMTBb | 0–300 | 38–100 | 140 seconds | 92.5 seconds | 32.9 |
3MS: Modified Mini-Mental State Test; Reference mean adjusted for age and education; higher scores indicate better performance
TMTB: Trail Making Test Part B; Reference mean adjusted for age and sex; higher scores indicate worse performance
Participants with impairment on the 3MS were more likely to be African American, have a lower educational attainment, and report a history of work in unskilled labor (Table 1). They reported a longer period since their last stable housing arrangement than those without impairment (7.5 years versus 4.8 years, p = 0.01). Participants with impairment on 3MS were more likely to have an AUDIT score suggestive of hazardous alcohol use (36.1 % versus 22.6%, p = 0.02) or high-severity alcohol use (23.3% versus 10.9%, p = 0.004), but were less likely to have an ASSIST score suggestive of high-risk opioid use (5.8% versus 14.8%, p = 0.04). Individuals with impairment on 3MS were more likely to report impairment in IADLs (67.4% versus 42.8%, p<0.001).
Table 1.
Characteristics of homeless participants with and without cognitive impairment on the Modified Mini-Mental State (3MS) Test (n=343)
| Characteristic | Impairmentn=86 | No Impairmentn=257 | Totaln=343 | p-valuea |
|---|---|---|---|---|
| Age (years), n (%) | 0.6 | |||
| 50–59 | 56 (65.1) | 157 (61.1) | 213 (62.1) | |
| 60–69 | 27 (31.4) | 94 (36.6) | 121 (35.3) | |
| 70+ | 3 (3.5) | 6 (2.3) | 9 (2.6) | |
| Sex, n (%) | ||||
| Male | 65 (75.6) | 198 (77.0) | 263 (76.7) | 0.4 |
| Female | 20 (23.3) | 59 (23.0) | 79 (23.0) | |
| Transgender | 1 (1.2) | 0 | 1 (0.03) | |
| Ethnicity/Race, n (%) | 0.03 | |||
| African American | 77 (89.5) | 197 (76.7) | 274 (79.9) | |
| White | 4 (4.7) | 34 (13.2) | 38 (11.1) | |
| Latino | 4 (4.7) | 11 (4.3) | 15 (4.4) | |
| Other | 1 (1.2) | 15 (5.8) | 16 (4.7) | |
| Highest Education, n (%) | 0.01 | |||
| Less than high school | 31 (36.1) | 56 (21.8) | 87 (25.4) | |
| High School or GED | 20 (23.3) | 54 (21.0) | 74 (21.6) | |
| More than HS (college or vocational) | 35 (40.7) | 147 (57.2) | 182 (53.1) | |
| Usual Occupational Status, n (%) | <0.001 | |||
| Executive, manager, administrative | 1 (1.2) | 17 (6.7) | 18 (5.3) | |
| Clerical or skilled manual | 15 (17.7) | 86 (34.0) | 101 (29.9) | |
| Semi-skilled labor | 27 (31.8) | 92 (36.4) | 119 (35.2) | |
| Unskilled labor | 42 (49.4) | 58 (23.0) | 100 (29.6) | |
| Combat Veteran | 1 (1.2) | 19 (7.4) | 20 (5.8) | 0.03 |
| Housing Status, n (%) | 0.2 | |||
| Renters | 7 (8.1) | 35 (13.6) | 42 (12.2) | |
| Family host | 10 (11.6) | 46 (17.9) | 56 (16.3) | |
| High shelter users, | 23 (26.7) | 63 (24.5) | 86 (25.1) | |
| Unsheltered | 46 (53.5) | 113 (44.0) | 159 (46.4) | |
| Years since stable housing, mean (SD) | 7.5 (10.9) | 4.8 (7.9) | 5.8 (8.8) | 0.01 |
| Healthcare Utilization, n (%) | ||||
| Regular Provider | 37 (43.5) | 142 (55.5) | 179 (52.5) | 0.06 |
| Hospitalized in last 6 months | 19 (22.1) | 42 (16.3) | 61 (17.8) | 0.23 |
| Alcohol Use | ||||
| Years of regular alcohol use, mean (SD)b | 13.4 (16.8) | 11.8 (14.1) | 12.2 (14.8) | 0.4 |
| Hazardous alcohol use, n (%)c | 31 (36.0) | 58 (22.6) | 89 (25.9) | 0.02 |
| High-severity alcohol use, n (%)c | 20 (23.3) | 28 (10.9) | 48 (14.0) | 0.004 |
| Illicit Drug Use, n (%)d | ||||
| Problematic use of marijuana | 29 (33.7) | 104 (40.5) | 133 (38.8) | 0.3 |
| Problematic use of opioids | 5 (5.8) | 38 (14.8) | 43 (12.5) | 0.04 |
| Problematic use of cocaine | 39 (45.4) | 108 (42.0) | 147 (42.9) | 0.6 |
| Problematic use of amphetamine | 5 (5.8) | 23 (9.0 | 28 (8.2) | 0.5 |
| Mental Health, n (%) | ||||
| History of a mental health probleme | 53 (61.6) | 191 (74.6) | 244 (71.3) | 0.02 |
| Depressive symptomsf | 48 (57.1) | 131 (51.2) | 179 (52.6) | 0.3 |
| Post-traumatic stress disorderg | 29 (33.7) | 84 (32.7) | 113 (32.9) | 0.9 |
| Health, n (%) | ||||
| Self-rated poor or fair health | 58 (67.4) | 133 (51.8) | 191 (55.7) | 0.01 |
| History of cerebrovascular accident | 11(12.9) | 26 (10.1) | 37 (10.8) | 0.5 |
| History of diabetes | 12 (14.0) | 36 (14.0) | 48 (14.0) | 1 |
| History of coronary artery disease | 10 (11.6) | 20 (7.8) | 30 (8.8) | 0.3 |
| HIV infectionh | 5 (5.9) | 11 (4.4) | 16 (4.7) | 0.6 |
| History of traumatic brain injuryi | 37 (43.4) | 112 (43.6) | 149 (43.4) | 0.9 |
| History of smoking | 69 (80.2) | 197 (76.7) | 266 (77.6) | 0.5 |
Note. Values reflect excluded missing data. Impairment is defined as score ≤1.5 standard deviations below age- and education adjusted norms.
Categorical variables compared with chi square tests; continuous variables compared using the student's t test
Regular drinking defined as drinking 3 or more times per week to the point of feeling the effects
Hazardous alcohol use defined as Alcohol Use Disorders Identification Test (AUDIT) score ≥ 8; High-risk alcohol use defined as AUDIT score ≥ 16 or a score ≥ 4 on the alcohol dependence subscale
Problematic use of illicit drug defined as Alcohol Smoking Substance Involvement Screening Test (ASSIST) score≥ 4
Mental health problem included self-report of having ever experienced anxiety, depression, difficulty controlling violent behavior, hallucinations not as a result of substance use, or a suicide attempt
Depressive symptoms defined as CES Depression (CES-D) score ≥ 16
Post-traumatic stress disorder (PTSD) defined as Primary Care PTSD Screen score ≥ 3
History of HIV infection
Traumatic brain injury defined as head trauma resulting in loss of consciousness or hospitalization
Participants who scored in the impaired range on TMTB were more likely to have lower educational attainment, to describe their health as poor, and to report a history of a stroke, or coronary artery disease. Participants with impairment on TMTB were more likely to have an AUDIT score suggestive of hazardous alcohol use (33.3% versus 21.2%, p = 0.02) or high-severity alcohol use (22.5% versus 9.3%, p = 0.001). There were no significant differences in self-reported drug use among individuals with and without TMTB impairment. Participants with TMTB impairment were also more likely to report impairment in IADLs (60.0% versus 43.8%, p=0.005).
3.2.2 Multivariable Regression
After adjusting for occupational status, having a regular healthcare provider, and self-reported fair/poor health, high-severity alcohol use was positively associated with global cognitive impairment (AOR 2.39, CI 1.19–4.79) and high-risk opioid use was negatively associated with global impairment (AOR 0.26, CI 0.10–0.73). Only high-severity alcohol use (AOR 3.09, CI 1.61–5.92) and a history of a mental health condition (AOR 0.57, CI 0.34–0.96) were associated with impairment on TMTB (Table 3).
Table 3.
Unadjusted and Adjusted Odds Ratios for Cognitive Impairment on 3MS and Trails B
| Modified Mini-Mental State (n=343) | Trail Making Test Part B (n=336) | |||
|---|---|---|---|---|
| OR (CI) | AOR (CI) | OR (CI) | AOR (CI) | |
| Age (years) | ||||
| 50–59 | REF | REF | ||
| 60–69 | 0.8 (0.5–1.4) | 1.0 (0.6 – 1.5) | ||
| 70+ | 1.4 (0.3 – 5.8) | 1.6 (0.4 – 6.3) | ||
| Sex | ||||
| Male | REF | REF | ||
| Female | 1.0 (0.5 – 1.7) | 0.7 (0.4 – 1.2) | ||
| Race/ethnicity | ||||
| White | REF | REF | ||
| African American | 3.3 (1.1 – 9.7)* | 0.9 (0.4 –1.8) | ||
| Latino | 3.1 (0.7–14.5) | 1.9 (0.6–6.7) | ||
| Other | 0.6 (0.1 – 5.5) | 0.9 (0.3 – 3.1) | ||
| Highest Education | ||||
| Less than high school | REF | REF | ||
| High School or GED | 0.7 (0.3 – 1.3) | 0.7 (0.3 – 1.2) | ||
| More than HS (college or vocational) | 0.4 (0.2 – 0.8)* | 0.5 (0.3 – 0.8)* | ||
| Occupational Status | ||||
| Executive, manager, administrative | REF | REF | REF | |
| Clerical or skilled manual | 3.0 (0.4–24.0) | 2.7 (0.3 – 22.5) | 1.3 (0.4 – 4.4) | |
| Semi-skilled labor | 5.0 (0.6 – 39.2) | 5.1 (0.6 – 41.7) | 1.8 (0.6 – 5.9) | |
| Unskilled labor | 12.3 (1.6 – 96.2)* | 11.9 (1.5 – 96.8)** | 2.0 (0.6 – 6.6) | |
| Combat veteran | 0.2 (0.02 – 1.1) | 0.7 (0.3 – 2.1) | ||
| Housing Status | ||||
| Renters | REF | REF | ||
| Family host | 1.1 (0.4 – 3.1) | 0.8 (0.4 – 2.0) | ||
| High shelter users | 1.8 (0.7 – 4.7) | 1.4 (0.6 – 3.1) | ||
| Unsheltered | 2.0 (0.8 – 4.9) | 0.9 (0.4 – 1.9) | ||
| Years since stable housing | 1.0 (1.0 – 1.1)* | 1.0 (0.98 – 1.0) | ||
| Healthcare Utilization | ||||
| Regular provider | 0.6 (0.4 – 1.0)* | 0.6 (0.3 – 1.0)** | 0.9 (0.6 – 1.4) | |
| Hospitalized in last 6 months | 1.5 (0.8 – 2.6)* | 1.5 (0.9 – 2.7)* | ||
| Alcohol | ||||
| Years of regular alcohol usea | 1.0 (0.99 – 1.0) | 1.0 (1.0 – 1.0)* | ||
| Hazardous alcohol useb | 1.9 (1.1 – 3.3)* | 1.9 (1.1 – 3.1)* | ||
| High-severity alcohol useb | 2.5 (1.3 – 4.7)* | 2.4 (1.2 – 4.8)** | 2.9 (1.5 – 5.4)* | 3.1 (1.6 – 5.9 )** |
| Illicit Drugsc | ||||
| Problematic use of marijuana | 0.8 (0.5 – 1.3) | 1.0 (0.6 – 1.5) | ||
| Problematic use of opioids | 0.4 (0.1 – 0.9)* | 0.3 (0.1 – 0.7)** | 0.9 (0.5 – 1.8) | |
| Problematic use of cocaine | 1.1 (0.7 – 1.9) | 0.7 (0.4 – 1.1)* | ||
| Problematic use of amphetamines | 0.6 (0.2 – 1.7) | 0.9 (0.4 – 2.0) | ||
| Mental Health | ||||
| History of a mental health problemd | 0.6 (0.3 – 0.9)* | 0.6 (0.4 – 1.0)* | 0.6 (0.3–1.0)** | |
| Depressive symptomse | 1.3 (0.8 – 2.1) | 1.1 (0.7 – 1.8) | ||
| Post-traumatic stress disorderf | 1.1 (0.6 – 1.8) | 1.3 (0.8 – 2.0) | ||
| Health | ||||
| Self-rated poor or fair health | 1.9 (1.2 – 3.2)* | 2.2 (1.3 – 3.9)** | 1.4 (0.9 – 2.3)* | |
| History of cerebrovascular accident | 1.3 (0.6 – 2.8) | 1.9 (0.9 – 3.8)* | ||
| History of diabetes | 1.0 (0.5 – 2.0) | 1.0 (0.5 – 1.9) | ||
| History of coronary artery disease | 1.6 (0.7 – 3.5) | 1.8 (0.8 – 3.8)* | ||
| HIV infectiong | 1.4 (0.5 – 4.1) | 1.7 (0.6 – 4.6) | ||
| History of traumatic brain injuryh | 1.0 (0.6–1.6) | 1.0 (0.6 – 1.5) | ||
| History of smoking | 1.2 (0.7 – 2.3) | 1.2 (0.7 – 2.1) | ||
p ≤ 0.2, included in multivariate analysis
p < 0.05 Note. Impairment is defined as performance ≤1.5 standard deviations below adjusted norms.
Regular drinking defined as drinking 3 or more times per week to the point of feeling the effects
Hazardous alcohol use defined as Alcohol Use Disorders Identification Test (AUDIT) score ≥ 8; High-severity alcohol use defined as AUDIT score ≥ 16 or a score ≥ on the alcohol dependence sub-scale
Problematic use of illicit drug defined as Alcohol Smoking Substance Involvement Screening Test (ASSIST) score ≥ 4
Mental health problem included self-report of having ever experienced anxiety, depression, difficulty controlling violent behavior, hallucinations not as a result of substance use, or a suicide attempt
Depressive symptoms defined as CES Depression (CES-D) score ≥ 16
Post-traumatic stress disorder (PTSD) defined as Primary Care PTSD Screen score ≥ 3
HIV: human immunodeficiency virus
Traumatic brain injury defined as head trauma resulting in loss of consciousness or hospitalization
4. Discussion
In a population-based sample of older homeless adults with a median age of 58, we found a prevalence of impairment in global cognitive function (25.1%) and executive function (32.9%) three to four times higher than the reported prevalence in populations more than 10 years older. In a national sample of adults 70 years and older in 2002, 9% demonstrated cognitive impairment on a telephone interview cognitive scale (Langa et al., 2008). Early age of onset of cognitive impairment, defined as the onset of symptoms of impairment before 65, is associated with elevated risk of mortality (Koedam et al., 2008). Our findings are consistent with previous studies that have found a high prevalence of cognitive impairment among homeless adults, but our study is the first to use population-based sampling techniques and focus on older homeless adults. We found that participants with cognitive impairment had experienced a longer duration of homelessness, suggesting that cognitive impairment either might delay exits from homelessness or that a longer duration of homelessness might increase the risk of cognitive impairment. We hypothesize that cognitive impairment might be causally related to housing loss, by diminishing an individual’s ability to access financial resources, family support, and governmental or legal assistance.
Previous studies suggest an association between cognitive impairment and psychiatric disease, substance use, and TBI in homeless adults (Andersen et al., 2014; Seidman et al., 1997; Stergiopoulos et al., 2015). An analysis of a large sample of homeless adults in Canada who had severe mental illness and a mean age of 41 determined that age, education level, speaking a primary language other than English or French, and a history of psychosis explained 20% of the variance in a composite cognitive score. Neither substance use nor TBI were associated with cognitive performance (Stergiopoulos et al., 2015). In comparison, we found that high-severity alcohol use was strongly associated with cognitive impairment, consistent with studies in the general population (Bommersbach et al., 2015; Wilcox et al., 2014) and a single study in homeless adults (Seidman et al., 1997).
Many studies demonstrate that chronic heavy alcohol use is associated with neuropathological damage in the brain (Crews et al., 2005; Kril et al., 1997). Heavy alcohol consumption in older adults has been associated with faster decline in cognition in late middle age, particularly in men (Sabia et al., 2014). A history of alcohol dependence, even when it is not associated with current heavy alcohol use, can be associated with persistent cognitive impairment (Woods et al., 2016). Both alcohol dependence (Bates et al., 2005; Virag et al., 2015) and dependence on illicit substances including opioids, stimulants, and cannabis, are associated with executive dysfunction (Ersche et al., 2006; Lundqvist, 2005; Verdejo-Garcia and Perez-Garcia, 2007). The causal direction between substance dependence and cognitive dysfunction is not clear (Inozemtseva et al., 2016). Individuals with cognitive issues, particularly executive dysfunction, might be unable to regulate their substance use increasing their risk for substance dependence. Alcohol or drug abuse might cause cognitive dysfunction directly through a substance’s toxic effects on the brain. Finally, an individual’s risk for developing substance dependence or cognitive dysfunction may arise from a common source of underlying brain dysfunction in susceptible individuals (Inozemtseva et al., 2016).
In contrast to studies in non-homeless adults, we did not observe a positive association between illicit drug use and cognitive impairment. Individuals with high-risk use of opioids were less likely to have global cognitive dysfunction. Opioid users with more severe cognitive deficits may be at high-risk for unintentional overdose (Darke et al., 2000; Yarborough et al., 2016). We suspect our findings may be related to premature mortality in opioid users with cognitive impairment or due to cessation of opioid use coinciding with the development of cognitive impariment because cognitive deficits may make it more difficult for individuals to access opioids. Individuals with mental health conditions had a lower odds of executive dysfunction and a trend towards a lower odds of impaired global cognition. Studies in the general population demonstrate that cognitive deficits are common in psychiatric disorders (McTeague et al., 2017). We suspect that our findings might be due to premature mortality among individuals with significant mental health conditions and impaired cognition.
The reported prevalence of TBI among people who are homeless is higher than the prevalence described in the general population (Topolovec-Vranic et al., 2012). TBIs are associated with cognitive dysfunction in studies in the general population (Cantor et al., 2014; Cossette et al., 2014; Rabinowitz and Levin, 2014) and a single study in homeless adults (Andersen et al., 2014). Despite a high prevalence of self-reported TBI in our sample, we did not find an association between cognitive impairment and TBI. Our results are similar to the largest study on cognitive impairment in homeless adults with severe mental illness (Stergiopoulos et al., 2015) and other studies completed in homeless individuals (Gonzalez et al., 2001; Pluck et al., 2011; Solliday-McRoy et al., 2004). These studies, like ours, used similar self-reported measures of TBI, which might misclassify clinically significant brain injury.
We observed an association between global cognitive impairment and occupational status. Participants who reported unskilled labor work histories were twelve times more likely to exhibit global cognitive impairment than those who reported skilled or semi-skilled occupations. Occupational status had a stronger relationship with cognitive impairment than a participant’s educational level. Occupational status might be a surrogate variable for non-measured factors, including a person’s long-term socioeconomic status or wealth. Persons with persistent low-income status have been found to have worse performance on a cognitive battery (Zeki Al Hazzouri et al.). Women living in neighborhoods with higher socioeconomic status have better cognitive performance, after adjustment for education and other characteristics. This relationship was only partially explained by vascular risk factors, health behaviors, and psychological factors (Shih et al., 2011). An individual’s occupational history could be a helpful surrogate descriptive variable predicting an individuals’ risk for cognitive impairment, particularly measures of education attainment in years are imperfect measures of qualifications and competencies given a range of educational systems and quality (Connelly et al., 2016).
Cognitive impairment impacts the ability of adults to engage in healthcare or to participate in community programs aimed at housing, employment, or legal aid (Burra et al., 2009; Gabrielian et al., 2015; Montgomery et al., 2015). Similar to other studies, we found high rates of both cognitive and functional impairments. Cognitive and functional impairments in homeless adults are likely to be progressive (Cimino et al., 2015). Homeless adults who lack social support, economic resources, and have a high prevalence of chronic disease might not be able to adapt to cognitive deficits (Fazel et al., 2014; Solarz and Bogat, 1990). Executive function, which includes planning, organizing, social behavior, and impulse control, might be important for navigating social services, medical treatment, and behavioral therapies employed in the treatment of substance use disorders (Arias et al., 2016; Hagen et al., 2016). Persons with executive dysfunction might have difficulty completing multi-step commands, organizing their daily schedule, or participating in interactive treatment models such as cognitive behavioral therapy and self-help programs. In addition, executive dysfunction has been associated with a higher risk of progression of cognitive impairment (Chen et al., 2016), functional decline, and elevated mortality (Johnson et al., 2007).
This analysis has several limitations. Because of the cross-sectional design, we cannot establish causality. We used self-report to measure health conditions including TBI. We did not review participant medical records or perform objective tests for clinical diagnoses. We did not evaluate participants for a substance use disorder; instead, we used a screening tool to identify individuals with self-reported high-risk substance use. It is possible that some of the included participants were under the influence of alcohol during cognitive testing, which could have caused transient worsening of cognitive function.
5. Conclusions
We found a high prevalence of global cognitive and executive function impairment in our population-based sample of older homeless adults. Cognitive impairment was associated with high-severity alcohol use. Our results have several implications. First, policy-makers should consider cognitive impairment when designing supportive housing, intensive case management programming, substance use treatment, and healthcare delivery for older homeless adults. Second, clinicians should screen homeless adults 50 years and older for cognitive impairment, particularly those with an alcohol use disorder. Finally, because executive dysfunction is common in this population, addiction medicine providers will need to employ adapted strategies in the treatment of adults with alcohol misuse. Future research will be helpful in clarifying whether treatment of alcohol misuse leads to improvements in cognitive function and whether specific treatment strategies are more effective in this population.
Highlights.
Cognitive impairment occurs at younger ages than expected among homeless adults
Alcohol misuse, more than other risk factors, is strongly associated with impairment
Impaired cognition may impede the treatment of substance use disorders in homeless adults
Acknowledgments
Role of Funding Source
This study was funded by grants from the National Institute on Aging (NIA): R01AG041860 [Kushel, Ponath, Guzman], K24AG046372 [Kushel, Guzman and Tieu], P30AG15272 [Johnson],and P30AG044281 [Kushel]. These funding sources had no role in the preparation, review, or approval of the manuscript and do not necessarily represent the official views of the NIH or AHRQ. Dr. Hurstak receives fellowship support from National Institute of Health T32HP19025.
The authors gratefully acknowledge their colleagues Angela Allen, Pamela Olsen, Nina Fiellin, Tauni Marin, and Kenneth Perez for their invaluable contributions to the HOPE HOME study. The authors also thank the staff at St. Mary’s Center and the HOPE HOME Community Advisory Board for their guidance and partnership.
Footnotes
Contributors
Drs. Hurstak and Kushel had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kushel, Ponath,
Acquisition of data: Kushel, Ponath, Weyer-Jamora Statistical analysis: Hurstak, Guzman
Analysis and interpretation of data: Hurstak, Johnson, Kushel, Lee, Weyer Jamora
Drafting of the manuscript: Hurstak, Kushel, Johnson, Tieu
Critical revision of the manuscript for important intellectual content: Hurstak, Kushel, Johnson, Tieu
All authors approved of the final manuscript.
Conflicts of Interest
Dr. Kushel is a member of the leadership board of Everyone Home, which seeks to end homelessness in Alameda County, CA. No other conflicts of interest were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Andersen J, Kot N, Ennis N, Colantonio A, Ouchterlony D, Cusimano MD, Topolovec-Vranic J. Traumatic brain injury and cognitive impairment in men who are homeless. Disabil Rehabil. 2014;36:2210–5. doi: 10.3109/09638288.2014.895870. [DOI] [PubMed] [Google Scholar]
- Arias F, Arnsten JH, Cunningham CO, Coulehan K, Batchelder A, Brisbane M, Segal K, Rivera-Mindt M. Neurocognitive, psychiatric, and substance use characteristics in opioid dependent adults. Addict Behav. 2016;60:137–143. doi: 10.1016/j.addbeh.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. World Health Organization; 2001. [Google Scholar]
- Backer TE, Howard EA. Cognitive impairments and the prevention of homelessness: Research and practice review. J Prim Prev. 2007;28:375–388. doi: 10.1007/s10935-007-0100-1. [DOI] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcohol Clin Exp Res. 2005;29:367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland RC, Newman SC. Mild dementia or cognitive impairment: The Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- Bommersbach TJ, Lapid MI, Rummans TA, Morse RM. Geriatric alcohol use disorder: A review for primary care physicians. Mayo Clinic Proc. 2015;90:659–666. doi: 10.1016/j.mayocp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Twamley EW, Vella L, Gale M, Norman SB, Judd P, Everall IP, Heaton RK. Homelessness and neuropsychological impairment: Preliminary analysis of adults entering outpatient psychiatric treatment. J Nerv Ment Dis. 2010;198:790–794. doi: 10.1097/NMD.0b013e3181f97dff. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Bravo G, Hebert R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. Int J Geriatr Psychiatry. 1997;12:1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brown RT, Goodman L, Guzman D, Tieu L, Ponath C, Kushel MB. Pathways to homelessness among older homeless adults: Results from the HOPE HOME Study. PLoS One. 2016a;11:e0155065. doi: 10.1371/journal.pone.0155065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Hemati K, Riley ED, Lee CT, Ponath C, Tieu L, Guzman D, Kushel MB. Geriatric conditions in a population-based sample of older homeless adults. Gerontologist. 2016b doi: 10.1093/geront/gnw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Kiely DK, Bharel M, Mitchell SL. Geriatric syndromes in older homeless adults. J Gen Intern Med. 2012;27:16–22. doi: 10.1007/s11606-011-1848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Kiely DK, Bharel M, Mitchell SL. Factors associated with geriatric syndromes in older homeless adults. J Health Care Poor Underserved. 2013;24:456–468. doi: 10.1353/hpu.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrich N, Hodder T, Teesson M. Prevalence of cognitive impairment among homeless people in inner Sydney. Psychiatr Serv. 2000;51:520–521. doi: 10.1176/appi.ps.51.4.520. [DOI] [PubMed] [Google Scholar]
- Burnam MA, Koegel P. Methodology for obtaining a representative sample of homeless persons - The Los Angeles Skid Row Study. Eval Rev. 1988;12:117–152. [Google Scholar]
- Burra TA, Stergiopoulos V, Rourke SB. A systematic review of cognitive deficits in homeless adults: implications for service delivery. Can J Psychiatry. 2009;54:123–133. doi: 10.1177/070674370905400210. [DOI] [PubMed] [Google Scholar]
- Burt MR U.S. Department of Housing and Urban Development, Interagency Council on the Homeless., Urban Institute. Homelessness: Programs and the people they serve: Findings of the National Survey of Homeless Assistance Providers and Clients: Summary. Washington, DC: 1999. [Google Scholar]
- Cantor J, Ashman T, Dams-O'Connor K, Dijkers MP, Gordon W, Spielman L, Tsaousides T, Allen H, Nguyen M, Oswald J. Evaluation of the short-term executive plus intervention for executive dysfunction after traumatic brain injury: A randomized controlled trial with minimization. Arch Phys Med Rehabil. 2014;95:1–9. e3. doi: 10.1016/j.apmr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- Chen Y, Denny KG, Harvey D, Farias ST, Mungas D, DeCarli C, Beckett L. Progression from normal cognition to mild cognitive impairment in a diverse clinic-based and community-based elderly cohort. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.07.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino T, Steinman MA, Mitchell SL, Miao Y, Bharel M, Barnhart CE, Brown RT. The course of functional impairment in older homeless adults: Disabled on the street. JAMA Intern Med. 2015;175:1237–1239. doi: 10.1001/jamainternmed.2015.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave KM, Saunders JB, Reznik RB. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction. 1995;90:1479–1485. doi: 10.1046/j.1360-0443.1995.901114796.x. [DOI] [PubMed] [Google Scholar]
- Connelly R, Gayle V, Lambert PS. A review of educational attainment measures for social survey research. Method Innov. 2016;9:2059799116638001. [Google Scholar]
- Cossette I, Ouellet MC, McFadyen BJ. A preliminary study to identify locomotor-cognitive dual tasks that reveal persistent executive dysfunction after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95:1594–1597. doi: 10.1016/j.apmr.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: Changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Culhane D, Metraux S, Byrne T, Steno M, Bainbridge J. The age structure of contemporary homelessness: Evidence and implications for public policy. Anal Soc Issues Public Policy. 2013;13:1–17. [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95:687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- Department of Housing and Urban Development. CFR parts 91 581, and 283. HUD; Washington, D.C: 2011. Homeless Emergency Assistance and Rapid Transition to Housing: Defining “Homeless”. [Google Scholar]
- Depp CA, Vella L, Orff HJ, Twamley EW. A quantitative review of cognitive functioning in homeless adults. J Nerv Ment Dis. 2015;203:126–131. doi: 10.1097/NMD.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis N, Roy S, Topolovec-Vranic J. Memory impairment among people who are homeless: A systematic review. Memory. 2014:1–19. doi: 10.1080/09658211.2014.921714. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: Descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet (London, England) 2014;384:1529–1540. doi: 10.1016/S0140-6736(14)61132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielian S, Bromley E, Hellemann GS, Kern RS, Goldenson NI, Danley ME, Young AS. Factors affecting exits from homelessness among persons with serious mental illness and substance use disorders. J Clin Psychiatry. 2015;76:e469–476. doi: 10.4088/JCP.14m09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Langa KM, Yaffe K. Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study. PLoS Med. 2017;14:e1002246. doi: 10.1371/journal.pmed.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi B, Conde-Martel A, O'Toole TP. Self-reported comorbidities, perceived needs, and sources for usual care for older and younger homeless adults. J Gen Intern Med. 2005;20:726–730. doi: 10.1111/j.1525-1497.2005.0142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg L, Linn LS, Mayer-Oakes SA. Differences in health status between older and younger homeless adults. J Am Geriatr Soc. 1990;38:1220–1229. doi: 10.1111/j.1532-5415.1990.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez EA, Dieter JN, Natale RA, Tanner SL. Neuropsychological evaluation of higher functioning homeless persons: A comparison of an abbreviated test battery to the mini-mental state exam. J Nerv Ment Dis. 2001;189:176–181. doi: 10.1097/00005053-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Hagen E, Erga AH, Hagen KP, Nesvag SM, McKay JR, Lundervold AJ, Walderhaug E. Assessment of executive function in patients with substance use disorder: A comparison of inventory- and performance-based assessment. J Subst Abuse Treat. 2016;66:1–8. doi: 10.1016/j.jsat.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults (HRB) Psychological Assessment Resources 2004 [Google Scholar]
- Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro M. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for use in primary care. World Health Organization; Geneva: 2010. [Google Scholar]
- Hwang SW, Colantonio A, Chiu S, Tolomiczenko G, Kiss A, Cowan L, Redelmeier DA, Levinson W. The effect of traumatic brain injury on the health of homeless people. CMAJ. 2008;179:779–784. doi: 10.1503/cmaj.080341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inozemtseva O, Perez-Solis L, Matute E, Juarez J. Differential improvement of executive functions during abstinence in cocaine-dependent patients: A longitudinal study. Subst Use Misuse. 2016:1–13. doi: 10.1080/10826084.2016.1178293. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- Koedam EL, Pijnenburg YA, Deeg DJ, Baak MM, van der Vlies AE, Scheltens P, van der Flier WM. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26:147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Mangurian C, Tieu L, Ponath C, Guzman D, Kushel M. Childhood adversities associated with poor adult mental health outcomes in older homeless adults: Results from the HOPE HOME Study. Am J Geriatr Psychiatry. 2016a;25:107–17. doi: 10.1016/j.jagp.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Guzman D, Ponath C, Tieu L, Riley E, Kushel M. Residential patterns in older homeless adults: Results of a cluster analysis. Soc Sci Med. 2016b;153:131–140. doi: 10.1016/j.socscimed.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A, Hallgren M, Balliu N, Forsell Y. The use of alcohol use disorders identification test (AUDIT) in detecting alcohol use disorder and risk drinking in the general population: Validation of AUDIT using schedules for clinical assessment in neuropsychiatry. Alcohol Clin Exp Res. 2015;39:158–165. doi: 10.1111/acer.12593. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.16040400. appiajp201716040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AE, Dichter ME, Thomasson AM, Roberts CB, Byrne T. Disparities in housing status among veterans with general medical, cognitive, and behavioral health conditions. Psychiatr Serv. 2015;66:317–320. doi: 10.1176/appi.ps.201400014. [DOI] [PubMed] [Google Scholar]
- Nishio A, Yamamoto M, Horita R, Sado T, Ueki H, Watanabe T, Uehara R, Shioiri T. Prevalence of mental illness, cognitive disability, and their overlap among the homeless in Nagoya, Japan. PLoS One. 2015;10:e0138052. doi: 10.1371/journal.pone.0138052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluck G, Lee KH, David R, Macleod DC, Spence SA, Parks RW. Neurobehavioural and cognitive function is linked to childhood trauma in homeless adults. Br J Clin Psychol. 2011;50:33–45. doi: 10.1348/014466510X490253. [DOI] [PubMed] [Google Scholar]
- Prins A, Ouimette P, Kimerling R, Cameron RP, Hugelshofer DS, Shaw-Hegwer J, Thrailkill A, Gusman FD, Sheikh JI. The primary care PTSD screen (PC-PTSD): Development and operating characteristics. Primary Care Psychiatry. 2003;9:9–14. [Google Scholar]
- Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37:1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percep Motor Skills. 1958;8:271–276. [Google Scholar]
- Ritchey FJ, La Gory M, Fitzpatrick KM, Mullis J. A comparison of homeless, community-wide, and selected distressed samples on the CES-Depression Scale. Am J Public Health. 1990;80:1384–1386. doi: 10.2105/ajph.80.11.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpf HJ, Hapke U, Meyer C, John U. Screening for alcohol use disorders and at-risk drinking in the general population: psychometric performance of three questionnaires. Alcohol Alcohol. 2002;37:261–268. doi: 10.1093/alcalc/37.3.261. [DOI] [PubMed] [Google Scholar]
- Sabia S, Elbaz A, Britton A, Bell S, Dugravot A, Shipley M, Kivimaki M, Singh-Manoux A. Alcohol consumption and cognitive decline in early old age. Neurology. 2014;82:332–339. doi: 10.1212/WNL.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsycholog Soc. 2009a;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009b;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Caplan BB, Tolomiczenko GS, Turner WM, Penk WE, Schutt RK, Goldfinger SM. Neuropsychological function in homeless mentally ill individuals. J Nerv Ment Dis. 1997;185:3–12. doi: 10.1097/00005053-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Schutt RK, Caplan B, Tolomiczenko GS, Turner WM, Goldfinger SM. The effect of housing interventions on neuropsychological functioning among homeless persons with mental illness. Psychiatr Serv. 2003;54:905–908. doi: 10.1176/appi.ps.54.6.905. [DOI] [PubMed] [Google Scholar]
- Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, Eibner C, Denburg NL, Ockene J, Messina CR, Espeland MA. Neighborhood socioeconomic status and cognitive function in women. Am J Public Health. 2011;101:1721–1728. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solarz A, Bogat GA. When social support fails: The homeless. J Community Psychol. 1990;18:79–96. [Google Scholar]
- Solliday-McRoy C, Campbell TC, Melchert TP, Young TJ, Cisler RA. Neuropsychological functioning of homeless men. J Nerv Ment Dis. 2004;192:471–478. doi: 10.1097/01.nmd.0000131962.30547.26. [DOI] [PubMed] [Google Scholar]
- Spence S, Stevens R, Parks R. Cognitive dysfunction in homeless adults: A systematic review. J R Soc Med. 2004;97:375–379. doi: 10.1258/jrsm.97.8.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos V, Cusi A, Bekele T, Skosireva A, Latimer E, Schutz C, Fernando I, Rourke SB. Neurocognitive impairment in a large sample of homeless adults with mental illness. Acta Psychiatr Scand. 2015;131:256–68. doi: 10.1111/acps.12391. [DOI] [PubMed] [Google Scholar]
- Sullivan G, Dumenci L, Burnam A, Koegel P. Validation of the brief instrumental functioning scale in a homeless population. Psychiatr Serv (Washington, DC) 2001;52:1097–1099. doi: 10.1176/appi.ps.52.8.1097. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Topolovec-Vranic J, Ennis N, Colantonio A, Cusimano MD, Hwang SW, Kontos P, Ouchterlony D, Stergiopoulos V. Traumatic brain injury among people who are homeless: A systematic review. BMC Public Health. 2012;12:1059. doi: 10.1186/1471-2458-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsemberis S, McHugo G, Williams V, Hanrahan P, Stefancic A. Measuring homelessness and residential stability: The residential time-line follow-back inventory. J Community Psychol. 2007;35:29–42. [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Virag M, Janacsek K, Horvath A, Bujdoso Z, Fabo D, Nemeth D. Competition between frontal lobe functions and implicit sequence learning: evidence from the long-term effects of alcohol. Exp Brain Res. 2015;233:2081–2089. doi: 10.1007/s00221-015-4279-8. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: Deficits and clinical relevance. Rev Neurosci. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Bertoux M, Savage G, Hodges JR, Piguet O, Hornberger M. Comparison of prefrontal atrophy and episodic memory performance in dysexecutive Alzheimer's disease and behavioral-variant frontotemporal dementia. J Alzheimers Dis. 2016;51:889–903. doi: 10.3233/JAD-151016. [DOI] [PubMed] [Google Scholar]
- Wong YL. Measurement properties of the Center for Epidemiologic Studies-Depression Scale in a homeless population. Psycholog Assess. 2000;12:69–76. doi: 10.1037//1040-3590.12.1.69. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, de la Monte S, Monti PM, Cohen RA. Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcohol Clin Exp Res. 2016;40:2435–44. doi: 10.1111/acer.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough BJ, Stumbo SP, Janoff SL, Yarborough MT, McCarty D, Chilcoat HD, Coplan PM, Green CA. Understanding opioid overdose characteristics involving prescription and illicit opioids: A mixed methods analysis. Drug Alcohol Depend. 2016;167:49–56. doi: 10.1016/j.drugalcdep.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Elfassy T, Sidney S, Jacobs D, Pérez Stable EJ, Yaffe K. Sustained economic hardship and cognitive function: The coronary artery risk development in young adults study. Am J Prev Med. 2017;52:1–9. doi: 10.1016/j.amepre.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]